Dynamic Lipid–Glycaemic Index and Inflammation—Endothelial Shifts and Fetal Aortic Wall Thickening: A Repeated-Measures Gestational Phenotyping Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Legal and Ethical Considerations
2.2. Data Collection
2.3. Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Analysis of Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.Y.; Lee, S.M.; Kwon, G.E.; Kim, B.J.; Koo, J.N.; Oh, I.H.; Kim, S.M.; Shin, S.; Kim, W.; Joo, S.K.; et al. Maternal dyslipidemia and altered cholesterol metabolism in early pregnancy as a risk factor for small for gestational age neonates. Sci. Rep. 2021, 11, 21066. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.; Feingold, K.R. Effect of Pregnancy on Lipid Metabolism and Lipoprotein Levels. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: Buzzards Bay, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK498654/ (accessed on 1 May 2025).
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, X.; Cai, C.; Li, R.; Gao, Y.; Yang, L.; Wu, C.; Dong, H.; Pang, X.; Bai, D.; et al. Associations between Maternal Lipid Profiles and Pregnancy Complications: A Prospective Population-Based Study. Am. J. Perinatol. 2021, 38, 834–840. [Google Scholar] [CrossRef]
- Ouidir, M.; Zeng, X.; Workalemahu, T.; Shrestha, D.; Grantz, K.L.; Mendola, P.; Zhang, C.; Tekola-Ayele, F. Early pregnancy dyslipidemia is associated with placental DNA methylation at loci relevant for cardiometabolic diseases. Epigenomics 2020, 12, 921–934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Enquobahrie, D.A.; Williams, M.A.; Butler, C.L.; Frederick, I.O.; Miller, R.S.; Luthy, D.A. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am. J. Hypertens. 2004, 17, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Skilton, M.R.; Celermajer, D.S.; Cosmi, E.; Crispi, F.; Gidding, S.S.; Raitakari, O.T.; Urbina, E.M. Natural History of Atherosclerosis and Abdominal Aortic Intima-Media Thickness: Rationale, Evidence, and Best Practice for Detection of Atherosclerosis in the Young. J. Clin. Med. 2019, 8, 1201. [Google Scholar] [CrossRef]
- Cosmi, E.; Visentin, S.; Fanelli, T.; Mautone, A.J.; Zanardo, V. Aortic intima media thickness in fetuses and children with intrauterine growth restriction. Obstet. Gynecol. 2009, 114, 1109–1114. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujii, T.; Iwasaki, J.; Nakano, Y.; Sakurai, M.; Miura, F.; Dobashi, K.; Mizuno, K.; Itabashi, K. Abdominal aortic intima-media thickness in preschool children born preterm. Pediatr. Cardiol. 2014, 35, 121–125. [Google Scholar] [CrossRef]
- Bartels, A.; O’Donoghue, K. Cholesterol in pregnancy: A review of the literature. Obstet. Med. 2011, 4, 147–151. [Google Scholar] [CrossRef]
- Varcoe, T.J.; Darby, J.R.T.; Holman, S.L.; Bradshaw, E.L.; Kuchel, T.; Vaughan, L.; Seed, M.; Wiese, M.D.; Morrison, J.L. Fetal cardiovascular response to acute hypoxia during maternal anesthesia. Physiol. Rep. 2020, 8, e14365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blackmore, H.L.; Ozanne, S.E. Maternal diet-induced obesity and offspring cardiovascular health. J. Dev. Orig. Health Dis. 2013, 4, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr. Pharm. Biotechnol. 2014, 15, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Maritz, G.S.; Cock, M.L.; Louey, S.; Suzuki, K.; Harding, R. Fetal growth restriction has long-term effects on postnatal lung structure in sheep. Pediatr. Res. 2004, 55, 287–295. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Medbury, H.J.; Williams, H.; Lee, V.W. Lipid profiling in maternal and fetal circulations in preeclampsia and fetal growth restriction-a prospective case control observational study. BMC Pregnancy Childbirth 2020, 20, 61. [Google Scholar] [CrossRef]
- Li, G.; Kong, L.; Zhang, L.; Fan, L.; Su, Y.; Rose, J.C.; Zhang, W. Early Pregnancy Maternal Lipid Profiles and the Risk of Gestational Diabetes Mellitus Stratified for Body Mass Index. Reprod. Sci. 2015, 22, 712–717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paulo, M.S.; Abdo, N.M.; Bettencourt-Silva, R.; Al-Rifai, R.H. Gestational Diabetes Mellitus in Europe: A Systematic Review and Meta-Analysis of Prevalence Studies. Front. Endocrinol. 2021, 12, 691033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, W.Y.; Lin, S.L.; Hou, R.L.; Chen, X.Y.; Han, T.; Jin, Y.; Tang, L.; Zhu, Z.W.; Zhao, Z.Y. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: A population-based study from China. BMC Pregnancy Childbirth 2016, 16, 60. [Google Scholar] [CrossRef]
- Quaresima, P.; Fesslova, V.; Farina, A.; Kagan, K.O.; Candiani, M.; Morelli, M.; Crispi, F.; Cavoretto, P.I. How to do a fetal cardiac scan. Arch. Gynecol. Obstet. 2023, 307, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Kodinariya, T.; Makwana, P. Review on Determining of Cluster in K-means Clustering. Int. J. Adv. Res. Comput. Sci. Manag. Stud. 2013, 1, 90–95. [Google Scholar]
- Barnard, C.R.; Peters, M.; Sindler, A.L.; Farrell, E.T.; Baker, K.R.; Palta, M.; Stauss, H.M.; Dagle, J.M.; Segar, J.; Pierce, G.L.; et al. Increased aortic stiffness and elevated blood pressure in response to exercise in adult survivors of prematurity. Physiol. Rep. 2020, 8, e14462. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Liberati, M.; Thilaganathan, B. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension 2012, 60, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Bahado-Singh, R.O.; Akolekar, R.; Mandal, R.; Dong, E.; Xia, J.; Kruger, M.; Wishart, D.S.; Nicolaides, K. First-trimester metabolomic detection of late-onset preeclampsia. Am. J. Obstet. Gynecol. 2013, 208, 58.e1–58.e7. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lu, R.; Yu, G.; Rahman, M.L.; Chen, L.; Zhu, Y.; Tsai, M.Y.; Fiehn, O.; Chen, Z.; Zhang, C. Longitudinal lipidomic profiles during pregnancy and associations with neonatal anthropometry: Findings from a multiracial cohort. eBioMedicine 2023, 98, 104881. [Google Scholar] [CrossRef]
- Rumbold, A.R.; Crowther, C.A.; Haslam, R.R.; Dekker, G.A.; Robinson, J.S.; ACTS Study Group. Vitamins C and E and the risks of preeclampsia and perinatal complications. N. Engl. J. Med. 2006, 354, 1796–1806. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, B.; Xi, Y.; Bai, Y. Triglyceride-glucose index: A promising biomarker for predicting risks of adverse pregnancy outcomes in Hangzhou, China. Prev. Med. Rep. 2024, 41, 102683. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zou, S.; Xu, Y.; Di, R.; Gu, H.; Wang, Z.; Wei, X.; Yang, C.; Zhang, G. Is there any association between early-trimester triglyceride–glucose index and adverse pregnancy outcomes? Front. Endocrinol. 2023, 14, 1093991. [Google Scholar] [CrossRef]
- Visentin, S.; Lapolla, A.; Londero, A.P.; Cosma, C.; Dalfrà, M.; Camerin, M.; Faggian, D.; Plebani, M.; Cosmi, E. Adiponectin levels are reduced while markers of systemic inflammation and aortic remodelling are increased in intrauterine growth restricted mother–child couples. Biomed Res. Int. 2014, 2014, 401595. [Google Scholar] [CrossRef]
- Mannaerts, D.; Faes, E.; Goovaerts, I.; Stoop, T.; Cornette, J.; Gyselaers, W.J.; Spaanderman, M.E.A.; Van Craenenbroeck, E.M.; Jacquemyn, Y. Flow-mediated dilation and peripheral arterial tonometry are disturbed in preeclampsia and reflect different aspects of endothelial function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R518–R525. [Google Scholar] [CrossRef]
- Arogbokun, O.; Rosen, E.; Keil, A.P.; Milne, G.L.; Barrett, E.; Nguyen, R.; Bush, N.R.; Swan, S.H.; Sathyanarayana, S.; Ferguson, K.K. Maternal oxidative stress biomarkers in pregnancy and child growth from birth to age 6. J. Clin. Endocrinol. Metab. 2021, 106, 1427–1436. [Google Scholar] [CrossRef]
- Nield, L.E.; Manlhiot, C.; Magor, K.; Freud, L.; Chinni, B.; Ims, A.; Melamed, N.; Nevo, O.; Van Mieghem, T.; Weisz, D.; et al. Machine learning to predict outcomes of fetal cardiac disease: A pilot study. Pediatr. Cardiol. 2025, 46, 895–901. [Google Scholar] [CrossRef] [PubMed]
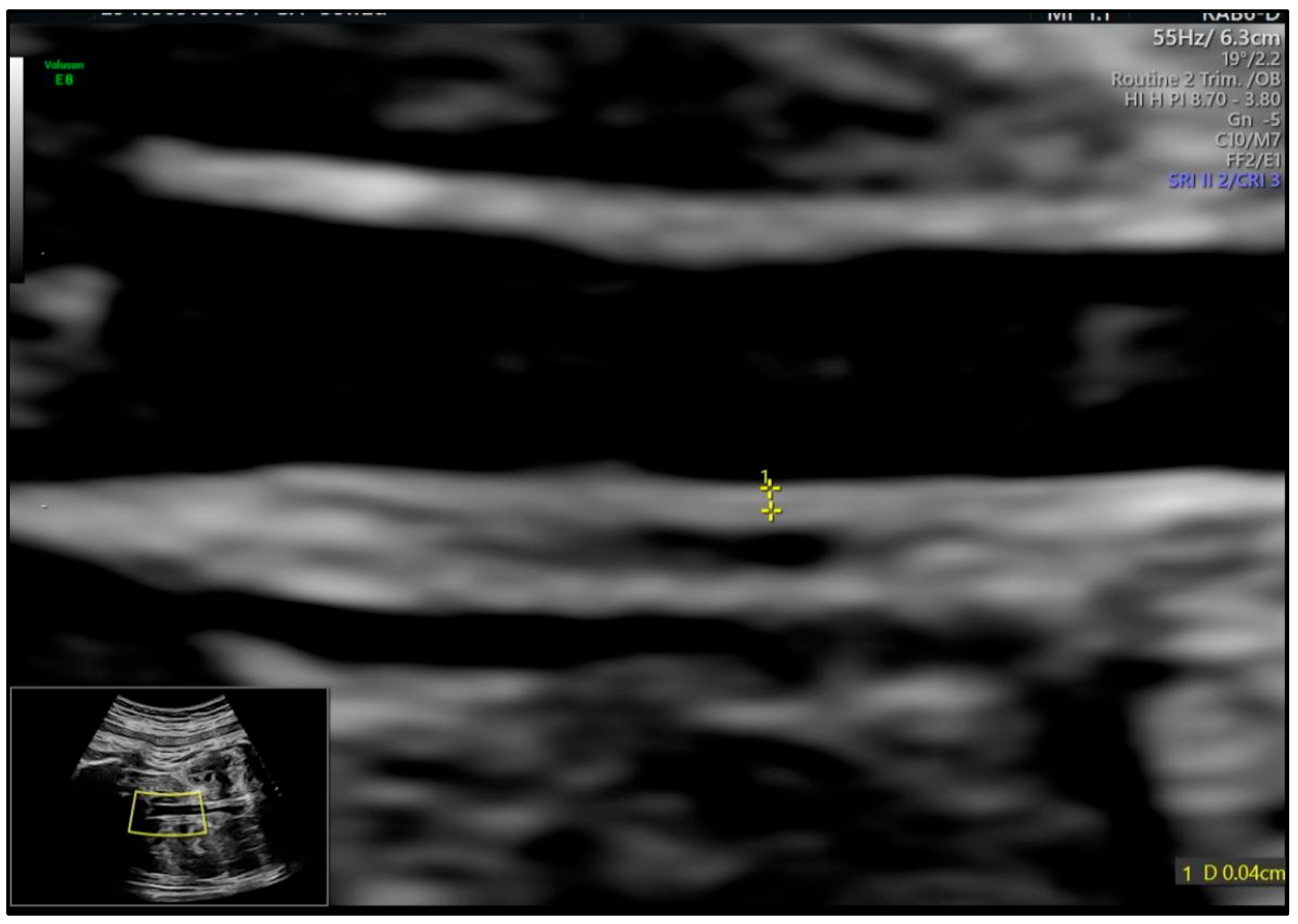
| Variable | Mean ± SD or n (%) |
|---|---|
| Age (years) | 30.7 ± 4.1 |
| Pre-pregnancy BMI (kg/m2) | 28.2 ± 3.8 |
| Gravidity (median [IQR]) | 2 [1–3] |
| Parity (median [IQR]) | 1 [1–2] |
| Smoking Status (n, %) | 12 (13.3%) |
| Cesarean History (n, %) | 15 (16.7%) |
| Parameter | Visit-1 24–26 Weeks | Visit-2 32–34 Weeks | Δ (V2–V1) | p |
|---|---|---|---|---|
| TG (mg/dL) | 138.6 ± 14.1 | 166.9 ± 15.2 | +28.3 ± 10.4 | <0.001 |
| LDL-C (mg/dL) | 124.7 ± 16.4 | 136.1 ± 17.0 | +11.4 ± 6.7 | 0.002 |
| HDL-C (mg/dL) | 44.7 ± 3.1 | 42.5 ± 3.0 | −2.2 ± 2.4 | 0.012 |
| TyG index | 8.45 ± 0.18 | 8.66 ± 0.21 | +0.21 ± 0.07 | <0.001 |
| hsCRP (mg/L) | 0.65 ± 0.23 | 1.05 ± 0.34 | +0.40 ± 0.19 | <0.001 |
| IL-6 (pg/mL) | 3.8 ± 0.9 | 5.1 ± 1.1 | +1.3 ± 0.7 | <0.001 |
| MDA (µmol/L) | 2.34 ± 0.44 | 2.89 ± 0.51 | +0.55 ± 0.29 | <0.001 |
| NOx (µmol/L) | 32.1 ± 4.8 | 28.7 ± 5.1 | −3.4 ± 3.2 | 0.004 |
| FMD (%) | 10.4 ± 2.1 | 8.6 ± 2.3 | −1.86 ± 0.45 | <0.001 |
| Carotid IMT (mm) | 0.56 ± 0.05 | 0.58 ± 0.06 | +0.02 ± 0.03 | 0.041 |
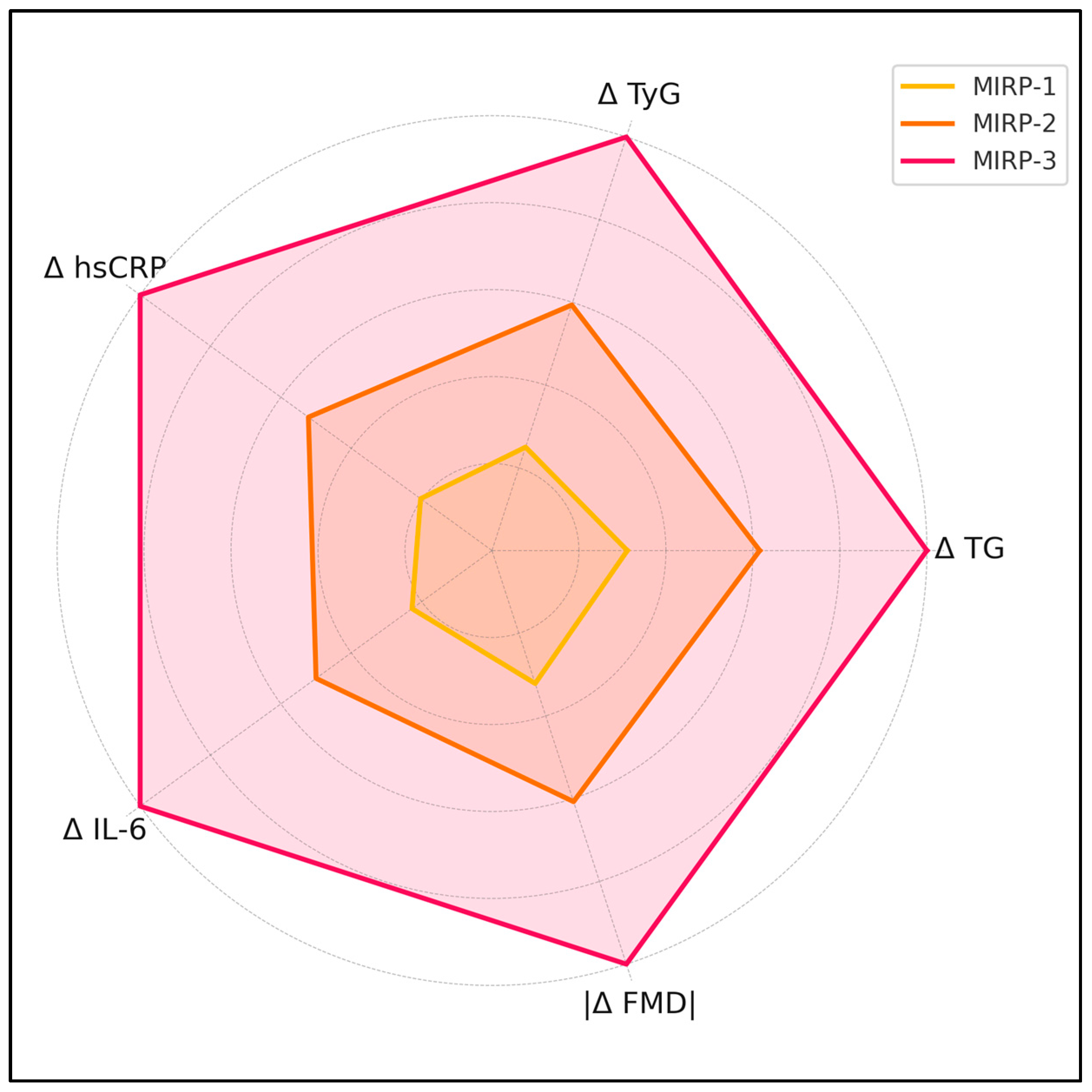
| Variable | MIRP-1 (“Stable”) | MIRP-2 (“Metabolic”) | MIRP-3 (“Metabolic + Inflammatory”) | p (ANOVA/KW) |
|---|---|---|---|---|
| Δ TG (mg/dL) | +13.6 ± 6.1 | +26.9 ± 8.9 | +43.7 ± 11.5 | <0.001 |
| Δ TyG | +0.08 ± 0.04 | +0.19 ± 0.05 | +0.32 ± 0.06 | <0.001 |
| Δ hsCRP (mg/L) | +0.14 ± 0.09 | +0.36 ± 0.13 | +0.69 ± 0.18 | <0.001 |
| Δ IL-6 (pg/mL) | +0.5 ± 0.4 | +1.1 ± 0.5 | +2.2 ± 0.7 | <0.001 |
| Δ FMD (%) | −0.9 ± 0.6 | −1.7 ± 0.5 | −2.8 ± 0.7 | <0.001 |
| Outcome | MIRP-1 | MIRP-2 | MIRP-3 | p |
|---|---|---|---|---|
| Δ Abdominal-aorta IMT (mm) | +0.07 ± 0.03 | +0.11 ± 0.04 | +0.17 ± 0.05 | <0.001 |
| Δ LV GLS (points) | +1.8 ± 1.1 | +2.6 ± 1.3 | +4.4 ± 1.6 | 0.002 |
| Δ LV Tei-index | +0.01 ± 0.02 | +0.03 ± 0.03 | +0.06 ± 0.04 | 0.004 |
| Δ fPWV (m/s) | +0.12 ± 0.14 | +0.29 ± 0.18 | +0.52 ± 0.21 | 0.001 |
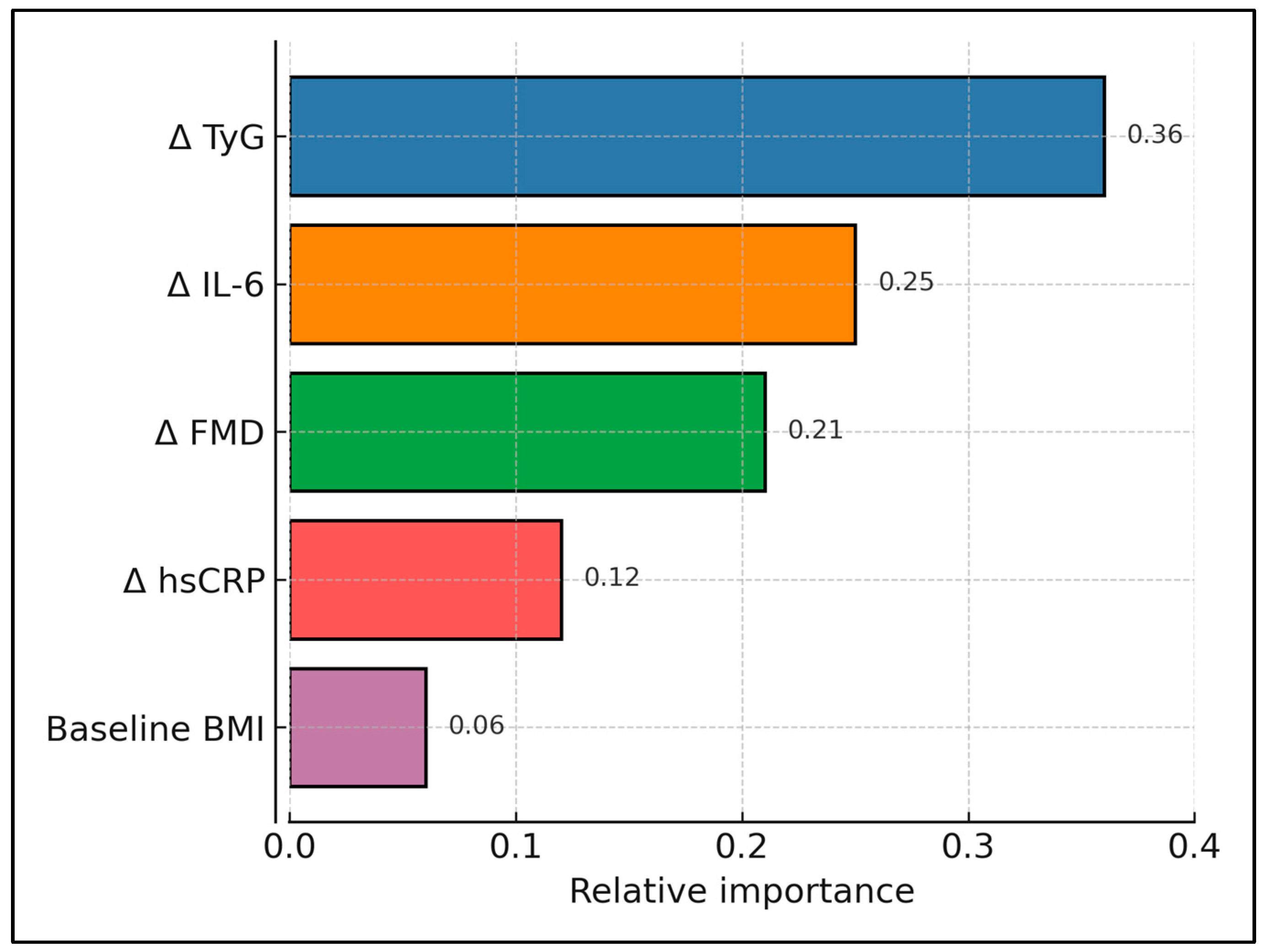
| Predictor | β (mm Per Unit) | 95% CI | p |
|---|---|---|---|
| Δ TyG | 0.054 | 0.031–0.077 | <0.001 |
| Δ IL-6 (pg/mL) | 0.009 | 0.004–0.014 | 0.001 |
| Δ FMD (%) | −0.007 | −0.011–−0.003 | 0.002 |
| Baseline BMI | 0.001 | −0.001–0.003 | 0.326 |
| Maternal age | −0.000 | −0.002–0.001 | 0.482 |
| Random intercept SD | 0.018 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muresan, M.C.; Belovan, B.; Sîrbu, I.; Popa, Z.L.; Citu, C.; Sas, I.; Ratiu, A. Dynamic Lipid–Glycaemic Index and Inflammation—Endothelial Shifts and Fetal Aortic Wall Thickening: A Repeated-Measures Gestational Phenotyping Study. Medicina 2025, 61, 964. https://doi.org/10.3390/medicina61060964
Muresan MC, Belovan B, Sîrbu I, Popa ZL, Citu C, Sas I, Ratiu A. Dynamic Lipid–Glycaemic Index and Inflammation—Endothelial Shifts and Fetal Aortic Wall Thickening: A Repeated-Measures Gestational Phenotyping Study. Medicina. 2025; 61(6):964. https://doi.org/10.3390/medicina61060964
Chicago/Turabian StyleMuresan, Maria Cezara, Biliana Belovan, Ioan Sîrbu, Zoran Laurentiu Popa, Cosmin Citu, Ioan Sas, and Adrian Ratiu. 2025. "Dynamic Lipid–Glycaemic Index and Inflammation—Endothelial Shifts and Fetal Aortic Wall Thickening: A Repeated-Measures Gestational Phenotyping Study" Medicina 61, no. 6: 964. https://doi.org/10.3390/medicina61060964
APA StyleMuresan, M. C., Belovan, B., Sîrbu, I., Popa, Z. L., Citu, C., Sas, I., & Ratiu, A. (2025). Dynamic Lipid–Glycaemic Index and Inflammation—Endothelial Shifts and Fetal Aortic Wall Thickening: A Repeated-Measures Gestational Phenotyping Study. Medicina, 61(6), 964. https://doi.org/10.3390/medicina61060964






