Phospholipase Cζ, the Molecular Spark of Fertilization and Male Infertility: Insights from Bench to Bedside
Abstract
1. Introduction
2. Molecular and Functional Overview
2.1. Structure, Biochemical Features, Expression and Localization of PLCζ
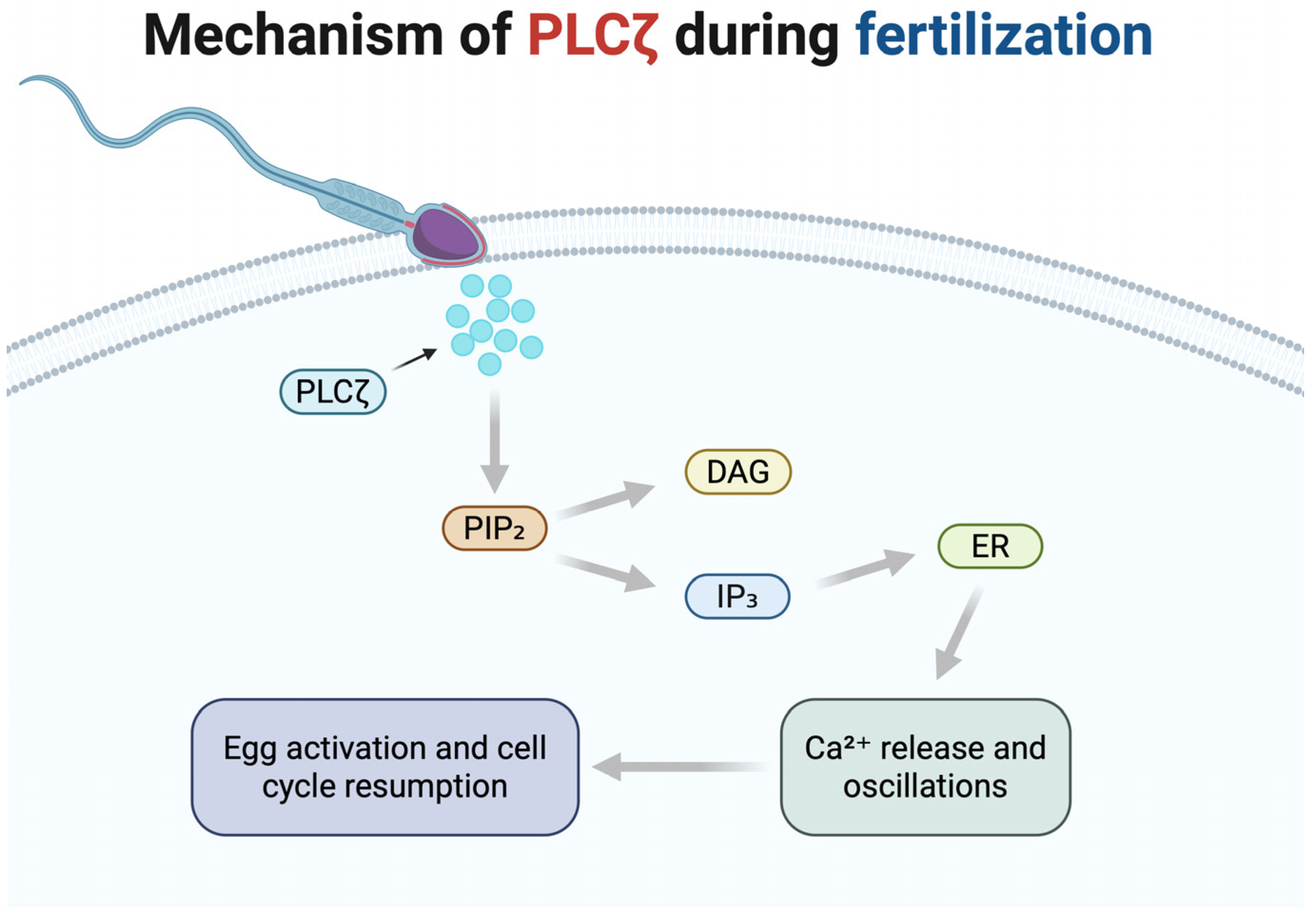
2.2. Mechanism of Action During Fertilization
3. Clinical Relevance
3.1. PLCζ Deficiency and Male Infertility
3.2. Broader Impacts on Assisted Reproductive Technologies and on Reproductive Outcomes
4. Diagnostic Strategies
5. Therapeutic Approaches
5.1. Assisted Oocyte Activation (AOA)
5.2. Emerging Treatments: PLCζ Protein/mRNA Therapy and Novel Interventions
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Sigman, M.; Collura, B.; De Jonge, C.J.; Eisenberg, M.L.; Lamb, D.J.; Mulhall, J.P.; Niederberger, C.; Sandlow, J.I.; Sokol, R.Z.; et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil. Steril. 2021, 115, 54–61. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Jones, C.; Meng, X.; Coward, K. Sperm factors and egg activation: Phospholipase C zeta (PLCZ1) and the clinical diagnosis of oocyte activation deficiency. Reproduction 2022, 164, F53–F66. [Google Scholar] [CrossRef]
- Cheung, S.; Xie, P.; Parrella, A.; Keating, D.; Rosenwaks, Z.; Palermo, G.D. Identification and treatment of men with phospholipase Cζ–defective spermatozoa. Fertil. Steril. 2020, 114, 535–544. [Google Scholar] [CrossRef]
- Meng, X.; Jones, C.; Melo, P.; Ross, C.; Mounce, G.; Child, T.; Coward, K. Antigen unmasking does not improve the visualization of phospholipase C zeta in human spermatozoa. Asian J. Androl. 2022, 24, 345–352. [Google Scholar] [CrossRef]
- Rahimizadeh, P.; Topraggaleh, T.R.; Nasr-Esfahani, M.H.; Ziarati, N.; Mirshahvaladi, S.; Esmaeili, V.; Seifi, S.; Eftekhari-Yazdi, P.; Shahverdi, A. The alteration of PLCζ protein expression in unexplained infertile and asthenoteratozoospermic patients: A potential effect on sperm fertilization ability. Mol. Reprod. Dev. 2020, 87, 115–123. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jellerette, T.; Salicioni, A.M.; Lee, H.C.; Yoo, M.S.; Coward, K.; Parrington, J.; Grow, D.; Cibelli, J.B.; Visconti, P.E.; et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J. Clin. Investig. 2008, 118, 3671–3681. [Google Scholar] [CrossRef]
- Amdani, S.N.; Yeste, M.; Jones, C.; Coward, K. Phospholipase C zeta (PLCζ) and male infertility: Clinical update and topical developments. Adv. Biol. Regul. 2016, 61, 58–67. [Google Scholar] [CrossRef]
- Saunders, C.M.; Larman, M.G.; Parrington, J.; Cox, L.J.; Royse, J.; Blayney, L.M.; Swann, K.; Lai, F.A. PLCζ: A sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 2002, 129, 3533–3544. [Google Scholar] [CrossRef]
- Nomikos, M.; Kashir, J.; Swann, K.; Lai, F.A. Sperm PLCζ: From structure to Ca2+ oscillations, egg activation and therapeutic potential. FEBS Lett. 2013, 587, 3609–3616. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Swann, K.; Lai, F.A. Starting a new life: Sperm PLC-zeta mobilizes the Ca2+ signal that induces egg activation and embryo development: An essential phospholipase C with implications for male infertility. Bioessays 2012, 34, 126–134. [Google Scholar] [CrossRef]
- Yu, Y.; Nomikos, M.; Theodoridou, M.; Nounesis, G.; Lai, F.A.; Swann, K. PLCzeta causes Ca2+ oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P(2). Mol. Biol. Cell. 2012, 23, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Mulgrew-Nesbitt, A.; Pallavi, P.; Mihalyne, G.; Zaitseva, I.; Swann, K.; Lai, F.A.; Murray, D.; McLaughlin, S. Binding of phosphoinositide-specific phospholipase C-ζ (PLC-ζ) to phospholipid membranes: Potential role of an unstructured cluster of basic residues. J. Biol. Chem. 2007, 282, 16644–16653. [Google Scholar] [CrossRef]
- Kuroda, K.; Ito, M.; Shikano, T.; Awaji, T.; Yoda, A.; Takeuchi, H.; Kinoshita, K.; Miyazaki, S. The role of X/Y linker region and N-terminal EF-hand domain in nuclear translocation and Ca2+ oscillation-inducing activities of phospholipase Cζ, a mammalian egg-activating factor. J. Biol. Chem. 2006, 281, 27794–27805. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Elgmati, K.; Theodoridou, M.; Calver, B.L.; Nounesis, G.; Swann, K.; Lai, F.A. Phospholipase Cζ binding to PtdIns(4,5)P2 requires the XY-linker region. J. Cell. Sci. 2011, 124, 2582–2590. [Google Scholar] [CrossRef]
- Sanders, J.R.; Ashley, B.; Moon, A.; Woolley, T.E.; Swann, K. PLCζ Induced Ca2+ Oscillations in Mouse Eggs Involve a Positive Feedback Cycle of Ca2+ Induced InsP3 Formation from Cytoplasmic PIP2. Front. Cell. Dev. Biol. 2018, 6, 36. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Carnevale, E.M. Phospholipase C Zeta 1 (PLCZ1): The Function and Potential for Fertility Assessment and In Vitro Embryo Production in Cattle and Horses. Vet. Sci. 2023, 10, 698. [Google Scholar] [CrossRef]
- Rengaraj, D.; Kim, D.K.; Zheng, Y.H.; Lee, S.I.; Kim, H.; Han, J.Y. Testis-specific novel transcripts in chicken: In situ localization and expression pattern profiling during sexual development. Biol. Reprod. 2008, 79, 413–420. [Google Scholar] [CrossRef]
- Yoneda, A.; Kashima, M.; Yoshida, S.; Terada, K.; Nakagawa, S.; Sakamoto, A.; Hayakawa, K.; Suzuki, K.; Ueda, J.; Watanabe, T. Molecular cloning, testicular postnatal expression, and oocyte-activating potential of porcine phospholipase Cζ. Reproduction 2006, 132, 393–401. [Google Scholar] [CrossRef]
- Kaewmala, K.; Uddin, M.J.; Cinar, M.U.; Grosse-Brinkhaus, C.; Jonas, E.; Tesfaye, D.; Phatsara, C.; Tholen, E.; Looft, C.; Schellander, K. Investigation into association and expression of PLCz and COX-2 as candidate genes for boar sperm quality and fertility. Reprod. Domest. Anim. 2012, 47, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, M.; Yu, Y.; Xu, W.; Tse, M.Y.; Pang, S.C.; Yi, Y.J.; Sutovsky, P.; Oko, R. The testicular and epididymal expression profile of PLCζin mouse and human does not support its role as a sperm-borne oocyte activating factor. PLoS ONE 2012, 7, e33496. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Medrano, L.; Aizpurua, J.; Gomez-Torres, M.J. Phospholipase C Zeta in Human Spermatozoa: A Systematic Review on Current Development and Clinical Application. Int. J. Mol. Sci. 2024, 25, 1344. [Google Scholar] [CrossRef] [PubMed]
- Bedford-Guaus, S.J.; McPartlin, L.A.; Xie, J.; Westmiller, S.L.; Buffone, M.G.; Roberson, M.S. Molecular cloning and characterization of phospholipase C zeta in equine sperm and testis reveals species-specific differences in expression of catalytically active protein. Biol. Reprod. 2011, 85, 78–88. [Google Scholar] [CrossRef]
- Kashir, J.; Buntwal, L.; Nomikos, M.; Calver, B.L.; Stamatiadis, P.; Ashley, P.; Vassilakopoulou, V.; Sanders, D.; Knaggs, P.; Livaniou, E.; et al. Antigen unmasking enhances visualization efficacy of the oocyte activation factor, phospholipase C zeta, in mammalian sperm. Mol. Hum. Reprod. 2017, 23, 54–67. [Google Scholar] [CrossRef]
- Heytens, E.; Parrington, J.; Coward, K.; Young, C.; Lambrecht, S.; Yoon, S.Y.; Fissore, R.A.; Hamer, R.; Deane, C.M.; Ruas, M.; et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCζ) in spermatozoa from infertile men. Hum. Reprod. 2009, 24, 2417–2428. [Google Scholar] [CrossRef]
- Young, C.; Grasa, P.; Coward, K.; Davis, L.C.; Parrington, J. Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil. Steril. 2009, 91, 2230–2242. [Google Scholar] [CrossRef]
- Ramadan, W.M.; Kashir, J.; Jones, C.; Coward, K. Oocyte activation and phospholipase C zeta (PLCζ): Diagnostic and therapeutic implications for assisted reproductive technology. Cell. Commun. Signal 2012, 10, 12. [Google Scholar] [CrossRef]
- Mejia-Flores, I.; Chiquete-Felix, N.; Palma-Lara, I.; Uribe-Carvajal, S.; de Lourdes Juarez-Mosqueda, M. During capacitation in bull spermatozoa, actin and PLC-ζ undergo dynamic interactions. Zygote 2017, 25, 558–566. [Google Scholar] [CrossRef]
- Neri, Q.V.; Lee, B.; Rosenwaks, Z.; Machaca, K.; Palermo, G.D. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell. Calcium 2014, 55, 24–37. [Google Scholar] [CrossRef]
- Nomikos, M.; Kashir, J.; Lai, F.A. The role and mechanism of action of sperm PLC-ζ in mammalian fertilisation. Biochem. J. 2017, 474, 3659–3673. [Google Scholar] [CrossRef] [PubMed]
- Ducibella, T.; Huneau, D.; Angelichio, E.; Xu, Z.; Schultz, R.M.; Kopf, G.S.; Fissore, R.; Madoux, S.; Ozil, J.P. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev. Biol. 2002, 250, 280–291. [Google Scholar] [CrossRef]
- Swann, K.; Lai, F.A. PLCζ and the initiation of Ca2+ oscillations in fertilizing mammalian eggs. Cell. Calcium 2013, 53, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bedford-Guaus, S.J.; Yoon, S.Y.; Fissore, R.A.; Choi, Y.H.; Hinrichs, K. Microinjection of mouse phospholipase C zeta complementary RNA into mare oocytes induces long-lasting intracellular calcium oscillations and embryonic development. Reprod. Fertil. Dev. 2008, 20, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Malcuit, C.; Knott, J.G.; He, C.; Wainwright, T.; Parys, J.B.; Robl, J.M.; Fissore, R.A. Fertilization and inositol 1,4,5-trisphosphate (IP3)-induced calcium release in type-1 inositol 1,4,5-trisphosphate receptor down-regulated bovine eggs. Biol. Reprod. 2005, 73, 2–13. [Google Scholar] [CrossRef]
- Yu, Y.; Saunders, C.M.; Lai, F.A.; Swann, K. Preimplantation development of mouse oocytes activated by different levels of human phospholipase C zeta. Hum. Reprod. 2008, 23, 365–373. [Google Scholar] [CrossRef]
- Kashir, J.; Jones, C.; Lee, H.C.; Rietdorf, K.; Nikiforaki, D.; Durrans, C.; Ruas, M.; Tee, S.T.; Heindryckx, B.; Galione, A.; et al. Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum. Reprod. 2011, 26, 3372–3387. [Google Scholar] [CrossRef]
- Nozawa, K.; Satouh, Y.; Fujimoto, T.; Oji, A.; Ikawa, M. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci. Rep. 2018, 8, 1315. [Google Scholar] [CrossRef]
- Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F.A.; Nomikos, M. Essential Role of Sperm-Specific PLC-ζ in Egg Activation and Male Factor Infertility: An Update. Front. Cell. Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef]
- Abdulsamad, H.M.R.; Murtaza, Z.F.; AlMuhairi, H.M.; Bafleh, W.S.; AlMansoori, S.A.; AlQubaisi, S.A.; Hamdan, H.; Kashir, J. The Therapeutic and Diagnostic Potential of Phospholipase C Zeta, Oocyte Activation, and Calcium in Treating Human Infertility. Pharmaceuticals 2023, 16, 441. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, Y.; Deng, K.; Shen, J.; Cui, Y.; Liu, J.; Yang, X.; Diao, F. Mutations in PLCZ1 induce male infertility associated with polyspermy and fertilization failure. J. Assist. Reprod. Genet. 2023, 40, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Fan, Y.; Wang, F.; Yan, Z.; Li, M.; Ouyang, J.; Wu, L.; Yin, M.; Zhao, J.; Kuang, Y.; et al. Novel mutations in PLCZ1 cause male infertility due to fertilization failure or poor fertilization. Hum. Reprod. 2020, 35, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Torra-Massana, M.; Cornet-Bartolome, D.; Barragan, M.; Durban, M.; Ferrer-Vaquer, A.; Zambelli, F.; Rodriguez, A.; Oliva, R.; Vassena, R. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum. Reprod. 2019, 34, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Cardona Barberan, A.; Reddy Guggilla, R.; Colenbier, C.; Van der Velden, E.; Rybouchkin, A.; Stoop, D.; Leybaert, L.; Coucke, P.; Symoens, S.; Boel, A.; et al. High rate of detected variants in male PLCZ1 and ACTL7A genes causing failed fertilization after ICSI. Hum. Reprod. Open 2024, 2024, hoae057. [Google Scholar] [CrossRef]
- Li, Q.; Guo, J.; Huang, G.; Wu, N.; Chen, S.; Dai, J.; Zhang, X.; Zhang, G.; Zhi, W.; Yan, J.; et al. Novel PLCZ1 compound heterozygous mutations indicate gene dosage effect involved in total fertilisation failure after ICSI. Reproduction 2024, 168, e230466. [Google Scholar] [CrossRef]
- Lee, H.C.; Arny, M.; Grow, D.; Dumesic, D.; Fissore, R.A.; Jellerette-Nolan, T. Protein phospholipase C Zeta1 expression in patients with failed ICSI but with normal sperm parameters. J. Assist. Reprod. Genet. 2014, 31, 749–756. [Google Scholar] [CrossRef]
- Chithiwala, Z.H.; Lee, H.C.; Hill, D.L.; Jellerette-Nolan, T.; Fissore, R.; Grow, D.; Dumesic, D.A. Phospholipase C-zeta deficiency as a cause for repetitive oocyte fertilization failure during ovarian stimulation for in vitro fertilization with ICSI: A case report. J. Assist. Reprod. Genet. 2015, 32, 1415–1419. [Google Scholar] [CrossRef]
- Cheung, S.; Parrella, A.; Tavares, D.; Keating, D.; Xie, P.; Rosenwaks, Z.; Palermo, G.D. Single-center thorough evaluation and targeted treatment of globozoospermic men. J. Assist. Reprod. Genet. 2021, 38, 2073–2086. [Google Scholar] [CrossRef]
- Tavalaee, M.; Nasr-Esfahani, M.H. Expression profile of PLCζ, PAWP, and TR-KIT in association with fertilization potential, embryo development, and pregnancy outcomes in globozoospermic candidates for intra-cytoplasmic sperm injection and artificial oocyte activation. Andrology 2016, 4, 850–856. [Google Scholar] [CrossRef]
- Tavalaee, M.; Nomikos, M.; Lai, F.A.; Nasr-Esfahani, M.H. Expression of sperm PLCζand clinical outcomes of ICSI-AOA in men affected by globozoospermia due to DPY19L2 deletion. Reprod. Biomed. Online 2018, 36, 348–355. [Google Scholar] [CrossRef]
- Tavalaee, M.; Kiani-Esfahani, A.; Nasr-Esfahani, M.H. Relationship between phospholipase C-zeta, semen parameters, and chromatin status. Syst. Biol. Reprod. Med. 2017, 63, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Azad, N.; Nazarian, H.; Ghaffari Novin, M.; Masteri Farahani, R.; Piryaei, A.; Heidari, M.H.; Abdollahpour Alitappeh, M. Oligoasthenoteratozoospermic (OAT) men display altered phospholipase C zeta (PLCζ) localization and a lower percentage of sperm cells expressing PLCζand post-acrosomal sheath WW domain-binding protein (PAWP). Bosn. J. Basic Med. Sci. 2018, 18, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Tavalaee, M.; Kiani-Esfahani, A.; Nasr-Esfahani, M.H. Relationship between Potential Sperm Factors Involved in Oocyte Activation and Sperm DNA Fragmentation with Intra-Cytoplasmic Sperm Injection Clinical Outcomes. Cell. J. 2017, 18, 588–596. [Google Scholar] [CrossRef]
- Janghorban-Laricheh, E.; Ghazavi-Khorasgani, N.; Tavalaee, M.; Zohrabi, D.; Abbasi, H.; Nasr-Esfahani, M.H. An association between sperm PLCζlevels and varicocele? J. Assist. Reprod. Genet. 2016, 33, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Che, J.F.; Wu, H.X.; Zeng, S.C.; Wu, Y.R.; Dai, J.; Cheng, D.H.; Gong, F.; Lu, G.X.; Lin, G.; Dai, C. Defects in phospholipase C zeta cause polyspermy and low fertilization after conventional IVF: Not just ICSI failure. Asian J. Androl. 2024, 26, 175–182. [Google Scholar] [CrossRef]
- Tong, K.Y.; Liu, W.W.; Sun, L.W.; Liu, D.Y.; Xiang, Y.Z.; Li, C.; Chai, L.W.; Chen, K.; Huang, G.N.; Li, J.Y. Novel PLCZ1 mutation caused polyspermy during in vitro fertilization. Asian J. Androl. 2024, 26, 389–395. [Google Scholar] [CrossRef]
- Kashir, J.; Mistry, B.V.; Rajab, M.A.; BuSaleh, L.; Abu-Dawud, R.; Ahmed, H.A.; Alharbi, S.; Nomikos, M.; AlHassan, S.; Coskun, S.; et al. The mammalian sperm factor phospholipase C zeta is critical for early embryo division and pregnancy in humans and mice. Hum. Reprod. 2024, 39, 1256–1274. [Google Scholar] [CrossRef]
- Crafa, A.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E.; Cannarella, R. Globozoospermia: A Case Report and Systematic Review of Literature. World J. Men’s Health 2023, 41, 49–80. [Google Scholar] [CrossRef]
- Wang, T.; Cao, B.; Cai, Y.; Chen, S.; Wang, B.; Yuan, Y.; Zhang, Q. Plcz1 Deficiency Decreased Fertility in Male Mice Which Is Associated with Sperm Quality Decline and Abnormal Cytoskeleton in Epididymis. Int. J. Mol. Sci. 2022, 24, 314. [Google Scholar] [CrossRef]
- Kashir, J. Increasing associations between defects in phospholipase C zeta and conditions of male infertility: Not just ICSI failure? J. Assist. Reprod. Genet. 2020, 37, 1273–1293. [Google Scholar] [CrossRef]
- Dai, J.; Dai, C.; Guo, J.; Zheng, W.; Zhang, T.; Li, Y.; Lu, C.; Gong, F.; Lu, G.; Lin, G. Novel homozygous variations in PLCZ1 lead to poor or failed fertilization characterized by abnormal localization patterns of PLCζin sperm. Clin. Genet. 2020, 97, 347–351. [Google Scholar] [CrossRef]
- Nazarian, H.; Azad, N.; Nazari, L.; Piryaei, A.; Heidari, M.H.; Masteri-Farahani, R.; Karimi, M.; Ghaffari-Novin, M. Effect of Artificial Oocyte Activation on Intra-Cytoplasmic Sperm Injection Outcomes in Patients with Lower Percentage of Sperm Containing Phospholipase Czeta: A Randomized Clinical Trial. J. Reprod. Infertil. 2019, 20, 3–9. [Google Scholar] [PubMed]
- Durban, M.; Barragan, M.; Colodron, M.; Ferrer-Buitrago, M.; De Sutter, P.; Heindryckx, B.; Vernaeve, V.; Vassena, R. PLCζ disruption with complete fertilization failure in normozoospermia. J. Assist. Reprod. Genet. 2015, 32, 879–886. [Google Scholar] [CrossRef]
- Yelumalai, S.; Yeste, M.; Jones, C.; Amdani, S.N.; Kashir, J.; Mounce, G.; Da Silva, S.J.; Barratt, C.L.; McVeigh, E.; Coward, K. Total levels, localization patterns, and proportions of sperm exhibiting phospholipase C zeta are significantly correlated with fertilization rates after intracytoplasmic sperm injection. Fertil. Steril. 2015, 104, 561–568.e4. [Google Scholar] [CrossRef]
- Ferrer-Vaquer, A.; Barragan, M.; Freour, T.; Vernaeve, V.; Vassena, R. PLCζ sequence, protein levels, and distribution in human sperm do not correlate with semen characteristics and fertilization rates after ICSI. J. Assist. Reprod. Genet. 2016, 33, 747–756. [Google Scholar] [CrossRef]
- Khakpour, S.; Sadeghi, E.; Tavalaee, M.; Bahadorani, M.; Nasr-Esfahani, M.H. Zeta method: A noninvasive method based on membrane charge for selecting spermatozoa expressing high level of phospholipaseCζ. Andrologia 2019, 51, e13249. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Jones, C.; Mounce, G.; Ramadan, W.M.; Lemmon, B.; Heindryckx, B.; de Sutter, P.; Parrington, J.; Turner, K.; Child, T.; et al. Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil. Steril. 2013, 99, 107–117.e3. [Google Scholar] [CrossRef]
- Kashir, J.; Konstantinidis, M.; Jones, C.; Lemmon, B.; Lee, H.C.; Hamer, R.; Heindryckx, B.; Deane, C.M.; De Sutter, P.; Fissore, R.A.; et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum. Reprod. 2012, 27, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, J.; Kong, S.; Li, C.; Zhang, Z.; He, X.; Wu, H.; Tang, D.; Zha, X.; Tan, Q.; et al. A homozygous nonsense mutation of PLCZ1 cause male infertility with oocyte activation deficiency. J. Assist. Reprod. Genet. 2020, 37, 821–828. [Google Scholar] [CrossRef]
- Kashir, J.; Heindryckx, B.; Jones, C.; De Sutter, P.; Parrington, J.; Coward, K. Oocyte activation, phospholipase C zeta and human infertility. Hum. Reprod. Update 2010, 16, 690–703. [Google Scholar] [CrossRef]
- Vanden Meerschaut, F.; Nikiforaki, D.; Heindryckx, B.; De Sutter, P. Assisted oocyte activation following ICSI fertilization failure. Reprod. Biomed. Online 2014, 28, 560–571. [Google Scholar] [CrossRef]
- Kashir, J.; Ganesh, D.; Jones, C.; Coward, K. Oocyte activation deficiency and assisted oocyte activation: Mechanisms, obstacles and prospects for clinical application. Hum. Reprod. Open 2022, 2022, hoac003. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Wang, M.; Yang, Q.Y.; Hu, S.Q.; Zhu, L.X.; Jin, L. Risk of birth defects in children conceived by artificial oocyte activation and intracytoplasmic sperm injection: A meta-analysis. Reprod. Biol. Endocrinol. 2020, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Ebner, T.; Montag, M.; Oocyte Activation Study, G.; Montag, M.; Van der Ven, K.; Van der Ven, H.; Ebner, T.; Shebl, O.; Oppelt, P.; Hirchenhain, J.; et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: A prospective multicentre study. Reprod. Biomed. Online 2015, 30, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Versieren, K.; Heindryckx, B.; Lierman, S.; Gerris, J.; De Sutter, P. Developmental competence of parthenogenetic mouse and human embryos after chemical or electrical activation. Reprod. Biomed. Online 2010, 21, 769–775. [Google Scholar] [CrossRef]
- Vanden Meerschaut, F.; Nikiforaki, D.; De Roo, C.; Lierman, S.; Qian, C.; Schmitt-John, T.; De Sutter, P.; Heindryckx, B. Comparison of pre- and post-implantation development following the application of three artificial activating stimuli in a mouse model with round-headed sperm cells deficient for oocyte activation. Hum. Reprod. 2013, 28, 1190–1198. [Google Scholar] [CrossRef]
- Swann, K. The soluble sperm factor that activates the egg: PLCζ and beyond. Reproduction 2020, 160, V9–V11. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Hao, Y.; Panhwar, F.; Chen, Z.; Zou, W.; Ji, D.; Chen, B.; Zhou, P.; Zhao, G.; et al. Effects of trehalose vitrification and artificial oocyte activation on the development competence of human immature oocytes. Cryobiology 2017, 74, 43–49. [Google Scholar] [CrossRef]
- Tesarik, J.; Rienzi, L.; Ubaldi, F.; Mendoza, C.; Greco, E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil. Steril. 2002, 78, 619–624. [Google Scholar] [CrossRef]
- Mansour, R.; Fahmy, I.; Tawab, N.A.; Kamal, A.; El-Demery, Y.; Aboulghar, M.; Serour, G. Electrical activation of oocytes after intracytoplasmic sperm injection: A controlled randomized study. Fertil. Steril. 2009, 91, 133–139. [Google Scholar] [CrossRef]
- Nikiforaki, D.; Vanden Meerschaut, F.; de Roo, C.; Lu, Y.; Ferrer-Buitrago, M.; de Sutter, P.; Heindryckx, B. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil. Steril. 2016, 105, 798–806.e2. [Google Scholar] [CrossRef] [PubMed]
- Toth, S.; Huneau, D.; Banrezes, B.; Ozil, J.P. Egg activation is the result of calcium signal summation in the mouse. Reproduction 2006, 131, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Thanassoulas, A.; Aliyev, E.; Swann, K.; Naija, A.; Yalcin, H.C.; Lai, F.A.; Nomikos, M. Development of Recombinant PLC-Zeta Protein as a Therapeutic Intervention for the Clinical Treatment of Oocyte Activation Failure. Biomedicines 2024, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, R.; Yu, Y.; Nomikos, M.; Lai, F.A.; Swann, K. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Czeta. Mol. Hum. Reprod. 2015, 21, 783–791. [Google Scholar] [CrossRef]
- Escoffier, J.; Lee, H.C.; Yassine, S.; Zouari, R.; Martinez, G.; Karaouzene, T.; Coutton, C.; Kherraf, Z.E.; Halouani, L.; Triki, C.; et al. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum. Mol. Genet. 2016, 25, 878–891. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef]
- Javadian-Elyaderani, S.; Ghaedi, K.; Tavalaee, M.; Rabiee, F.; Deemeh, M.R.; Nasr-Esfahani, M.H. Diagnosis of genetic defects through parallel assessment of PLCζ and CAPZA3 in infertile men with history of failed oocyte activation. Iran J. Basic Med. Sci. 2016, 19, 281–289. [Google Scholar]

| Method | Key Principle/ Procedure | Main Observations/Points | References |
|---|---|---|---|
| Immunofluorescence (IF) |
|
| [23,25,46,62,64] |
| Western Blot/Protein Quantification (e.g., Flow Cytometry) |
|
| [47,65,66] |
| Functional Oocyte-Activation Tests (e.g., Mouse Oocyte Activation Test) |
|
| [5,63,67] |
| Genetic Testing (PLCZ1 Gene Sequencing) |
|
| [26,42,43,44,45,68,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Kyrgiafini, M.-A.; Mamuris, Z.; Dimitriadis, F.; Zachariou, A.; Chrisofos, M.; Sofikitis, N. Phospholipase Cζ, the Molecular Spark of Fertilization and Male Infertility: Insights from Bench to Bedside. Medicina 2025, 61, 963. https://doi.org/10.3390/medicina61060963
Kaltsas A, Kyrgiafini M-A, Mamuris Z, Dimitriadis F, Zachariou A, Chrisofos M, Sofikitis N. Phospholipase Cζ, the Molecular Spark of Fertilization and Male Infertility: Insights from Bench to Bedside. Medicina. 2025; 61(6):963. https://doi.org/10.3390/medicina61060963
Chicago/Turabian StyleKaltsas, Aris, Maria-Anna Kyrgiafini, Zissis Mamuris, Fotios Dimitriadis, Athanasios Zachariou, Michael Chrisofos, and Nikolaos Sofikitis. 2025. "Phospholipase Cζ, the Molecular Spark of Fertilization and Male Infertility: Insights from Bench to Bedside" Medicina 61, no. 6: 963. https://doi.org/10.3390/medicina61060963
APA StyleKaltsas, A., Kyrgiafini, M.-A., Mamuris, Z., Dimitriadis, F., Zachariou, A., Chrisofos, M., & Sofikitis, N. (2025). Phospholipase Cζ, the Molecular Spark of Fertilization and Male Infertility: Insights from Bench to Bedside. Medicina, 61(6), 963. https://doi.org/10.3390/medicina61060963








