Epicardial Adipose Tissue: A Multimodal Imaging Diagnostic Perspective
Abstract
1. Introduction
2. Anatomy and Distribution of Epicardial Adipose Tissue
3. Physiology of EAT
4. The Interplay Between Epicardial Adipose Tissue and Atherosclerosis Development
5. Non-Invasive Imaging
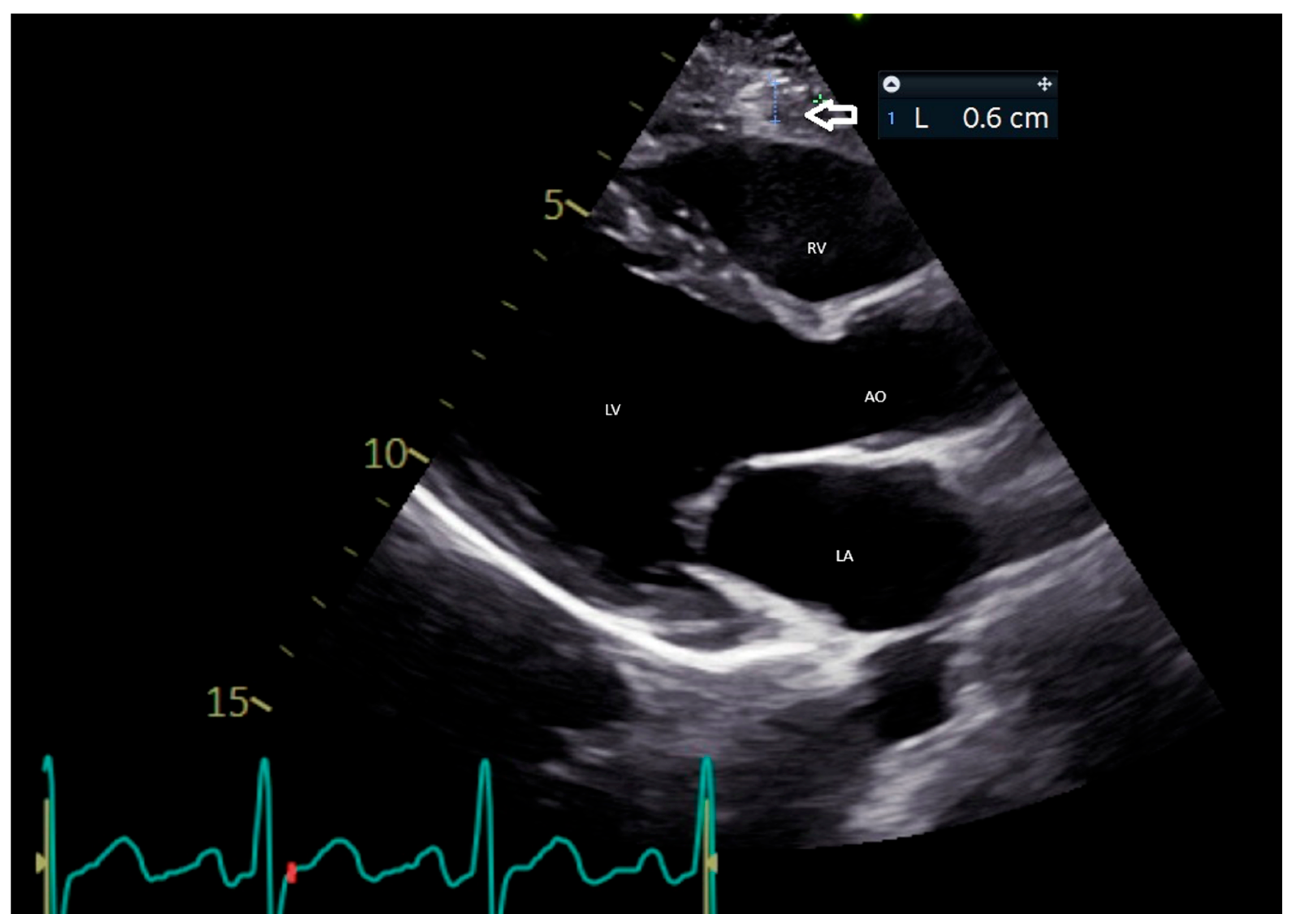
5.1. Echocardiographic Evaluation of EAT
5.2. CMR Evaluation of EAT
5.3. CCT Evaluation of EAT
5.4. PCAT Assessment Through CT
6. EAT and Treatment Strategies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASCVD | atherosclerotic cardiovascular disease |
| BMI | body mass index; |
| CABG | coronary artery bypass grafting; |
| CAD | coronary artery disease; |
| CCTA | cardiac computed tomography angiography; |
| CMR | cardiac magnetic resonance; |
| CT | computed tomography; |
| EAT | epicardial adipose tissue; |
| FAI | fat attenuation index; |
| GLP-1 RA | glucagon-like peptide-1 receptor agonist; |
| NCCT | non-contrast computed tomography; |
| PAT | pericardial adipose tissue; |
| PCAT | pericoronary adipose tissue; |
| PET | positron emission tomography; |
| PVAT | perivascular adipose tissue; |
| RCA | right coronary artery; |
| SGLT-2i | sodium-dependent glucose transporter 2 inhibitor; |
| TTE | transthoracic echocardiography. |
References
- Romano, A.D.; La Marca, A.; Villani, R.; Sangineto, M.; Manuppelli, V.; Brunetti, N.D.; Vendemiale, G.; Serviddio, G. Exploring the Relationship between Epicardial Fat Thickness and Coronary Revascularization: Implications for Cardiovascular Health. J. Clin. Med. 2023, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Dhore-Patil, A.; Urina-Jassir, D.; Samson, R.; Le Jemtel, T.H.; Oparil, S. Epicardial Adipose Tissue Thickness and Preserved Ejection Fraction Heart Failure. Curr. Hypertens. Rep. 2024, 26, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Shambu, S.K.; Desai, N.; Sundaresh, N.; Babu, M.S.; Madhu, B.; Gona, O.J. Study of Correlation between Epicardial Fat Thickness and Severity of Coronary Artery Disease. Indian Heart J. 2020, 72, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Lin, A.; Dey, D.; Baggiano, A.; Fusini, L.; Muscogiuri, G.; Pontone, G. Epicardial Fat and Coronary Artery Disease: Role of Cardiac Imaging. Atherosclerosis 2021, 321, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Penso, M.; Carerj, M.L.; Giacari, C.M.; Volpe, A.; Fusini, L.; Baggiano, A.; Mushtaq, S.; Annoni, A.; Cannata, F.; et al. DEep LearnIng-Based QuaNtification of Epicardial Adipose Tissue Predicts MACE in Patients Undergoing Stress CMR. Atherosclerosis 2024, 397, 117549. [Google Scholar] [CrossRef]
- Grodecki, K.; Lin, A.; Razipour, A.; Cadet, S.; McElhinney, P.A.; Chan, C.; Pressman, B.D.; Julien, P.; Maurovich-Horvat, P.; Gaibazzi, N.; et al. Epicardial Adipose Tissue Is Associated with Extent of Pneumonia and Adverse Outcomes in Patients with COVID-19. Metabolism 2021, 115, 154436. [Google Scholar] [CrossRef]
- Napoli, G.; Pergola, V.; Basile, P.; De Feo, D.; Bertrandino, F.; Baggiano, A.; Mushtaq, S.; Fusini, L.; Fazzari, F.; Carrabba, N.; et al. Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and Acute Coronary Syndromes. J. Clin. Med. 2023, 12, 7212. [Google Scholar] [CrossRef]
- Hara, T.; Sata, M. Pericoronary Adipose Tissue: Potential for Pathological Diagnosis and Therapeutic Applications. Cardiovasc. Interv. Ther. 2025. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting Human Coronary Inflammation by Imaging Perivascular Fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Kim, H.W.; Shi, H.; Winkler, M.A.; Lee, R.; Weintraub, N.L. Perivascular Adipose Tissue and Vascular Perturbation/Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2569–2576. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, S.W.; Lee, J.S.; Lee, S.K.; Abbott, R.; Lee, K.Y.; Lim, H.E.; Sung, K.-C.; Cho, G.-Y.; Koh, K.K.; et al. Association of Pericardial Adipose Tissue with Left Ventricular Structure and Function: A Region-specific Effect? Cardiovasc. Diabetol. 2021, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.T.; Park, J.; Ghemigian, K.; Mayrhofer, T.; Puchner, S.B.; Liu, T.; Fleg, J.L.; Udelson, J.E.; Truong, Q.A.; Ferencik, M.; et al. Epicardial and Paracardial Adipose Tissue Volume and Attenuation–Association with High-Risk Coronary Plaque on Computed Tomographic Angiography in the ROMICAT II Trial. Atherosclerosis 2016, 251, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tan, Y.; Deng, M.; Shan, W.; Zheng, W.; Zhang, B.; Cui, J.; Feng, L.; Shi, L.; Zhang, M.; et al. Epicardial Adipose Tissue, Metabolic Disorders, and Cardiovascular Diseases: Recent Advances Classified by Research Methodologies. MedComm 2023, 4, e413. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial Adipose Tissue in Contemporary Cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Bambace, C.; Telesca, M.; Zoico, E.; Sepe, A.; Olioso, D.; Rossi, A.; Corzato, F.; Di Francesco, V.; Mazzucco, A.; Santini, F.; et al. Adiponectin Gene Expression and Adipocyte Diameter: A Comparison between Epicardial and Subcutaneous Adipose Tissue in Men. Cardiovasc. Pathol. 2011, 20, e153–e156. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Li, M.; Liu, Y.; Shi, Y.; Zhang, J. Plasticity of Adipose Tissues: Interconversion among White, Brown, and Beige Fat and Its Role in Energy Homeostasis. Biomolecules 2024, 14, 483. [Google Scholar] [CrossRef]
- Tarsitano, M.G.; Pandozzi, C.; Muscogiuri, G.; Sironi, S.; Pujia, A.; Lenzi, A.; Giannetta, E. Epicardial Adipose Tissue: A Novel Potential Imaging Marker of Comorbidities Caused by Chronic Inflammation. Nutrients 2022, 14, 2926. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Adhikari, B.K.; Chen, L.; Liu, W.; Wang, Y.; Zhang, H. The Role of Epicardial Adipose Tissue Dysfunction in Cardiovascular Diseases: An Overview of Pathophysiology, Evaluation, and Management. Front. Endocrinol. 2023, 14, 1167952. [Google Scholar] [CrossRef]
- Doukbi, E.; Soghomonian, A.; Sengenès, C.; Ahmed, S.; Ancel, P.; Dutour, A.; Gaborit, B. Browning Epicardial Adipose Tissue: Friend or Foe? Cells 2022, 11, 991. [Google Scholar] [CrossRef]
- Lin, A.; Dey, D.; Wong, D.T.L.; Nerlekar, N. Perivascular Adipose Tissue and Coronary Atherosclerosis: From Biology to Imaging Phenotyping. Curr. Atheroscler. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Waksman, R.; Merdler, I.; Case, B.C.; Waksman, O.; Porto, I. Targeting Inflammation in Atherosclerosis: Overview, Strategy and Directions. EuroIntervention 2024, 20, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Kawabe, J.-I.; Hasebe, N. Role of the Vasa Vasorum and Vascular Resident Stem Cells in Atherosclerosis. BioMed Res. Int. 2014, 2014, 701571. [Google Scholar] [CrossRef]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial Adipose Tissue and Cardiovascular Diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Antoniades, C. The Role of Adipose Tissue in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 2019, 16, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Dey, D.; Marwick, T.H.; Nerlekar, N. Pericoronary Adipose Tissue as a Marker of Cardiovascular Risk: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 913–923. [Google Scholar] [CrossRef]
- Wall, C.; Huang, Y.; Le, E.P.V.; Ćorović, A.; Uy, C.P.; Gopalan, D.; Ma, C.; Manavaki, R.; Fryer, T.D.; Aloj, L.; et al. Pericoronary and Periaortic Adipose Tissue Density Are Associated with Inflammatory Disease Activity in Takayasu Arteritis and Atherosclerosis. Eur. Heart J. Open 2021, 1, oeab019. [Google Scholar] [CrossRef]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary Adventitial and Perivascular Adipose Tissue Inflammation in Patients With Vasospastic Angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef]
- Mazurek, T.; Kobylecka, M.; Zielenkiewicz, M.; Kurek, A.; Kochman, J.; Filipiak, K.J.; Mazurek, K.; Huczek, Z.; Królicki, L.; Opolski, G. PET/CT Evaluation of 18F-FDG Uptake in Pericoronary Adipose Tissue in Patients with Stable Coronary Artery Disease: Independent Predictor of Atherosclerotic Lesions’ Formation? J. Nucl. Cardiol. 2017, 24, 1075–1084. [Google Scholar] [CrossRef]
- Cundari, G.; Marchitelli, L.; Pambianchi, G.; Catapano, F.; Conia, L.; Stancanelli, G.; Catalano, C.; Galea, N. Imaging Biomarkers in Cardiac CT: Moving beyond Simple Coronary Anatomical Assessment. Radiol. Med. 2024, 129, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.; Jayabaskaran, J.; Ruban, J.; Goh, R.; Chin, Y.H.; Kong, G.; Ng, C.H.; Lin, C.; Loong, S.; Muthiah, M.D.; et al. Epicardial Adipose Tissue Assessed by Computed Tomography and Echocardiography Are Associated With Adverse Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2023, 16, e015159. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, G.; van Veldhuisen, D.J.; Gorter, T.M.; Ophuis, B.; Saucedo-Orozco, H.; van Empel, V.P.M.; Willems, T.P.; Geelhoed, B.; Rienstra, M.; Westenbrink, B.D. The Value of Echocardiographic Measurement of Epicardial Adipose Tissue in Heart Failure Patients. ESC Heart Fail. 2022, 9, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Willens, H.J.; Barbaro, G.; Sharma, A.M. Threshold Values of High-Risk Echocardiographic Epicardial Fat Thickness. Obesity 2008, 16, 887–892. [Google Scholar] [CrossRef]
- Natale, F.; Tedesco, M.A.; Mocerino, R.; de Simone, V.; Di Marco, G.M.; Aronne, L.; Credendino, M.; Siniscalchi, C.; Calabrò, P.; Cotrufo, M.; et al. Visceral Adiposity and Arterial Stiffness: Echocardiographic Epicardial Fat Thickness Reflects, Better than Waist Circumference, Carotid Arterial Stiffness in a Large Population of Hypertensives. Eur. J. Echocardiogr. 2009, 10, 549–555. [Google Scholar] [CrossRef]
- Le Jemtel, T.H.; Samson, R.; Ayinapudi, K.; Singh, T.; Oparil, S. Epicardial Adipose Tissue and Cardiovascular Disease. Curr. Hypertens. Rep. 2019, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Nerlekar, N.; Baey, Y.-W.; Brown, A.J.; Muthalaly, R.G.; Dey, D.; Tamarappoo, B.; Cameron, J.D.; Marwick, T.H.; Wong, D.T. Poor Correlation, Reproducibility, and Agreement Between Volumetric Versus Linear Epicardial Adipose Tissue Measurement: A 3D Computed Tomography Versus 2D Echocardiography Comparison. JACC Cardiovasc. Imaging 2018, 11, 1035–1036. [Google Scholar] [CrossRef]
- Antonini-Canterin, F.; Pellegrinet, M.; Marinigh, R.; Favretto, G. Role of Cardiovascular Ultrasound in the Evaluation of Obese Subjects. J. Cardiovasc. Echogr. 2014, 24, 67–71. [Google Scholar] [CrossRef]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef]
- Carerj, M.L.; Restelli, D.; Poleggi, C.; Di Bella, G.; Zito, C.; Manganaro, R.; Piccione, M.C.; Trimarchi, G.; Farina, A.; Micari, A.; et al. The Role of Imaging in Cardiovascular Prevention: A Comprehensive Review. J. Cardiovasc. Echogr. 2025, 35, 8. [Google Scholar] [CrossRef]
- Duca, F.; Mascherbauer, K.; Donà, C.; Koschutnik, M.; Binder, C.; Nitsche, C.; Halavina, K.; Beitzke, D.; Loewe, C.; Bartko, P.; et al. Association of Epicardial Adipose Tissue on Magnetic Resonance Imaging with Cardiovascular Outcomes: Quality over Quantity? Obesity 2024, 32, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Malahfji, M.; Al-Mallah, M. Epicardial Adipose Tissue on Cardiac MRI and Cardiovascular Risk: Another Reason to Watch What You “EAT”. Atherosclerosis 2024, 397, 118523. [Google Scholar] [CrossRef]
- Schulz, A.; Gronwald, J.; Hagedorn, C.; Lange, T.; Stiermaier, T.; Evertz, R.; Backhaus, S.; Kowallick, J.; Hasenfuß, G.; Thiele, H.; et al. CMR-Derived Epicardial Adipose Tissue Predicts Risk of Major Adverse Cardiac Events Following Myocardial Infarction Independent of Obesity and LV Function. J. Cardiovasc. Magn. Reson. 2025, 27, 101364. [Google Scholar] [CrossRef]
- Cristobal-Huerta, A.; Torrado-Carvajal, A.; Malpica, N.; Luaces, M.; Hernandez-Tamames, J.A. Automated Quantification of Epicardial Adipose Tissue in Cardiac Magnetic Resonance Imaging. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 7308–7311. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Gorter, T.M.; van Empel, V.P.M.; Hemels, M.E.W.; Hazebroek, E.J.; van Veldhuisen, S.L.; Willems, T.P.; Rienstra, M.; Westenbrink, B.D. Importance of Epicardial Adipose Tissue Localization Using Cardiac Magnetic Resonance Imaging in Patients with Heart Failure with Mid-Range and Preserved Ejection Fraction. Clin. Cardiol. 2021, 44, 987–993. [Google Scholar] [CrossRef]
- Nogajski, Ł.; Mazuruk, M.; Kacperska, M.; Kurpias, M.; Mączewski, M.; Nowakowski, M.; Mączewski, M.; Michałowska, I.; Leszek, P.; Paterek, A. Epicardial Fat Density Obtained with Computed Tomography Imaging—More Important than Volume? Cardiovasc. Diabetol. 2024, 23, 389. [Google Scholar] [CrossRef]
- Marwan, M.; Koenig, S.; Schreiber, K.; Ammon, F.; Goeller, M.; Bittner, D.; Achenbach, S.; Hell, M.M. Quantification of Epicardial Adipose Tissue by Cardiac CT: Influence of Acquisition Parameters and Contrast Enhancement. Eur. J. Radiol. 2019, 121, 108732. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Mahmood, A.; Flynn, M.G.; Alexander, T. Coronary Artery Calcium Scoring in Asymptomatic Patients. HCA Healthc. J. Med. 2023, 4, 341–352. [Google Scholar] [CrossRef]
- Stocker, T.J.; Deseive, S.; Leipsic, J.; Hadamitzky, M.; Chen, M.Y.; Rubinshtein, R.; Heckner, M.; Bax, J.J.; Fang, X.-M.; Grove, E.L.; et al. Reduction in Radiation Exposure in Cardiovascular Computed Tomography Imaging: Results from the PROspective Multicenter Registry on radiaTion Dose Estimates of Cardiac CT angIOgraphy iN Daily Practice in 2017 (PROTECTION VI). Eur. Heart J. 2018, 39, 3715–3723. [Google Scholar] [CrossRef]
- Cho, I.; Al’Aref, S.J.; Berger, A.; Ó Hartaigh, B.; Gransar, H.; Valenti, V.; Lin, F.Y.; Achenbach, S.; Berman, D.S.; Budoff, M.J.; et al. Prognostic Value of Coronary Computed Tomographic Angiography Findings in Asymptomatic Individuals: A 6-Year Follow-up from the Prospective Multicentre International CONFIRM Study. Eur. Heart J. 2018, 39, 934–941. [Google Scholar] [CrossRef]
- Williams, M.G.L.; Liang, K.; De Garate, E.; Spagnoli, L.; Fiori, E.; Dastidar, A.; Benedetto, U.; Biglino, G.; Johnson, T.W.; Luscher, T.; et al. Peak Troponin and CMR to Guide Management in Suspected ACS and Nonobstructive Coronary Arteries. JACC Cardiovasc. Imaging 2022, 15, 1578–1587. [Google Scholar] [CrossRef]
- Andreini, D.; Magnoni, M.; Conte, E.; Masson, S.; Mushtaq, S.; Berti, S.; Canestrari, M.; Casolo, G.; Gabrielli, D.; Latini, R.; et al. Coronary Plaque Features on CTA Can Identify Patients at Increased Risk of Cardiovascular Events. JACC Cardiovasc. Imaging 2020, 13, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Hell, M.M.; Achenbach, S.; Schuhbaeck, A.; Klinghammer, L.; May, M.S.; Marwan, M. CT-Based Analysis of Pericoronary Adipose Tissue Density: Relation to Cardiovascular Risk Factors and Epicardial Adipose Tissue Volume. J. Cardiovasc. Comput. Tomogr. 2016, 10, 52–60. [Google Scholar] [CrossRef]
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcao-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial Adipose Tissue Volume Assessed by Computed Tomography and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Nerlekar, N.; Brown, A.J.; Muthalaly, R.G.; Talman, A.; Hettige, T.; Cameron, J.D.; Wong, D.T.L. Association of Epicardial Adipose Tissue and High-Risk Plaque Characteristics: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006379. [Google Scholar] [CrossRef]
- Tachibana, M.; Miyoshi, T.; Osawa, K.; Toh, N.; Oe, H.; Nakamura, K.; Naito, T.; Sato, S.; Kanazawa, S.; Ito, H. Measurement of Epicardial Fat Thickness by Transthoracic Echocardiography for Predicting High-Risk Coronary Artery Plaques. Heart Vessel. 2016, 31, 1758–1766. [Google Scholar] [CrossRef]
- Eisenberg, E.; McElhinney, P.A.; Commandeur, F.; Chen, X.; Cadet, S.; Goeller, M.; Razipour, A.; Gransar, H.; Cantu, S.; Miller, R.J.H.; et al. Deep Learning-Based Quantification of Epicardial Adipose Tissue Volume and Attenuation Predicts Major Adverse Cardiovascular Events in Asymptomatic Subjects. Circ. Cardiovasc. Imaging 2020, 13, e009829. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Massaro, J.M.; Rosito, G.A.; Levy, D.; Murabito, J.M.; Wolf, P.A.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Association of Pericardial Fat, Intrathoracic Fat, and Visceral Abdominal Fat with Cardiovascular Disease Burden: The Framingham Heart Study. Eur. Heart J. 2009, 30, 850–856. [Google Scholar] [CrossRef]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J. Am. Heart Assoc. 2019, 8, e013104. [Google Scholar] [CrossRef]
- Ding, X.; Terzopoulos, D.; Diaz-Zamudio, M.; Berman, D.S.; Slomka, P.J.; Dey, D. Automated Pericardium Delineation and Epicardial Fat Volume Quantification from Noncontrast CT. Med. Phys. 2015, 42, 5015–5026. [Google Scholar] [CrossRef]
- Commandeur, F.; Goeller, M.; Razipour, A.; Cadet, S.; Hell, M.M.; Kwiecinski, J.; Chen, X.; Chang, H.-J.; Marwan, M.; Achenbach, S.; et al. Fully Automated CT Quantification of Epicardial Adipose Tissue by Deep Learning: A Multicenter Study. Radiol. Artif. Intell. 2019, 1, e190045. [Google Scholar] [CrossRef]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J. Modulation of the Interleukin-6 Signalling Pathway and Incidence Rates of Atherosclerotic Events and All-Cause Mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Napoli, G.; Mushtaq, S.; Basile, P.; Carella, M.C.; De Feo, D.; Latorre, M.D.; Baggiano, A.; Ciccone, M.M.; Pontone, G.; Guaricci, A.I. Beyond Stress Ischemia: Unveiling the Multifaceted Nature of Coronary Vulnerable Plaques Using Cardiac Computed Tomography. J. Clin. Med. 2024, 13, 4277. [Google Scholar] [CrossRef]
- Hassan, M.; Said, K.; Rizk, H.; ElMogy, F.; Donya, M.; Houseni, M.; Yacoub, M. Segmental Peri-Coronary Epicardial Adipose Tissue Volume and Coronary Plaque Characteristics. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1169–1177. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, D.; Han, X.; Han, S.; Zhang, W.; Zang, Z.; Gai, C.; Rong, R.; Gao, T. The Role of Perivascular Adipose Tissue-Secreted Adipocytokines in Cardiovascular Disease. Front. Immunol. 2023, 14, 1271051. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Nerlekar, N.; Yuvaraj, J.; Fernandes, K.; Jiang, C.; Nicholls, S.J.; Dey, D.; Wong, D.T.L. Pericoronary Adipose Tissue Computed Tomography Attenuation Distinguishes Different Stages of Coronary Artery Disease: A Cross-Sectional Study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Dey, D.; Cadet, S.; Lee, S.-E.; Otaki, Y.; Huynh, P.T.; Doris, M.K.; Eisenberg, E.; Yun, M.; Jansen, M.A.; et al. Peri-Coronary Adipose Tissue Density Is Associated With 18F-Sodium Fluoride Coronary Uptake in Stable Patients With High-Risk Plaques. JACC Cardiovasc. Imaging 2019, 12, 2000–2010. [Google Scholar] [CrossRef]
- Goeller, M.; Tamarappoo, B.K.; Kwan, A.C.; Cadet, S.; Commandeur, F.; Razipour, A.; Slomka, P.J.; Gransar, H.; Chen, X.; Otaki, Y.; et al. Relationship between Changes in Pericoronary Adipose Tissue Attenuation and Coronary Plaque Burden Quantified from Coronary Computed Tomography Angiography. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 636–643. [Google Scholar] [CrossRef]
- Lin, A.; Nerlekar, N.; Rajagopalan, A.; Yuvaraj, J.; Modi, R.; Mirzaee, S.; Munnur, R.K.; Seckington, M.; Doery, J.C.; Seneviratne, S.; et al. Remnant Cholesterol and Coronary Atherosclerotic Plaque Burden Assessed by Computed Tomography Coronary Angiography. Atherosclerosis 2019, 284, 24–30. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Tzolos, E.; Williams, M.C.; Dey, D.; Berman, D.; Slomka, P.; Newby, D.E.; Dweck, M.R. Noninvasive Coronary Atherosclerotic Plaque Imaging. JACC Cardiovasc. Imaging 2023, 16, 1608–1622. [Google Scholar] [CrossRef]
- Antoniades, C.; Tousoulis, D.; Vavlukis, M.; Fleming, I.; Duncker, D.J.; Eringa, E.; Manfrini, O.; Antonopoulos, A.S.; Oikonomou, E.; Padró, T.; et al. Perivascular Adipose Tissue as a Source of Therapeutic Targets and Clinical Biomarkers. Eur. Heart J. 2023, 44, 3827–3844. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-Invasive Detection of Coronary Inflammation Using Computed Tomography and Prediction of Residual Cardiovascular Risk (the CRISP CT Study): A Post-Hoc Analysis of Prospective Outcome Data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Yuki, H.; Sugiyama, T.; Suzuki, K.; Kinoshita, D.; Niida, T.; Nakajima, A.; Araki, M.; Dey, D.; Lee, H.; McNulty, I.; et al. Coronary Inflammation and Plaque Vulnerability: A Coronary Computed Tomography and Optical Coherence Tomography Study. Circ. Cardiovasc. Imaging 2023, 16, E014959. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.N.; Gomez-Perez, L.; Zimin, V.N.; Makhlouf, M.H.E.; Al-Kindi, S.; Wilson, D.L.; Lee, J. Pericoronary Adipose Tissue Radiomics from Coronary Computed Tomography Angiography Identifies Vulnerable Plaques. Bioengineering 2023, 10, 360. [Google Scholar] [CrossRef]
- Lin, A.; Nerlekar, N.; Munnur, R.K.; Kataoka, Y.; Andrews, J.; Dey, D.; Nicholls, S.J.; Wong, D.T.L. Cholesterol Crystal-Induced Coronary Inflammation: Insights from Optical Coherence Tomography and Pericoronary Adipose Tissue Computed Tomography Attenuation. J. Cardiovasc. Comput. Tomogr. 2020, 14, 277–278. [Google Scholar] [CrossRef]
- Salazar, J.; Luzardo, E.; Mejías, J.C.; Rojas, J.; Ferreira, A.; Rivas-Ríos, J.R.; Bermúdez, V. Epicardial Fat: Physiological, Pathological, and Therapeutic Implications. Cardiol. Res. Pract. 2016, 2016, 1291537. [Google Scholar] [CrossRef]
- Christensen, R.H.; Wedell-Neergaard, A.-S.; Lehrskov, L.L.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses From a Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 778–787. [Google Scholar] [CrossRef]
- Chacińska, M.; Zabielski, P.; Książek, M.; Szałaj, P.; Jarząbek, K.; Kojta, I.; Chabowski, A.; Błachnio-Zabielska, A.U. The Impact of OMEGA-3 Fatty Acids Supplementation on Insulin Resistance and Content of Adipocytokines and Biologically Active Lipids in Adipose Tissue of High-Fat Diet Fed Rats. Nutrients 2019, 11, 835. [Google Scholar] [CrossRef]
- van Schinkel, L.D.; Sleddering, M.A.; Lips, M.A.; Jonker, J.T.; de Roos, A.; Lamb, H.J.; Jazet, I.M.; Pijl, H.; Smit, J.W.A. Effects of Bariatric Surgery on Pericardial Ectopic Fat Depositions and Cardiovascular Function. Clin. Endocrinol. 2014, 81, 689–695. [Google Scholar] [CrossRef]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin Monotherapy Significantly Decreases Epicardial Adipose Tissue Thickness in Newly Diagnosed Type 2 Diabetes Patients. Rev. Port. Cardiol. 2019, 38, 419–423. [Google Scholar] [CrossRef]
- Sardu, C.; D’Onofrio, N.; Torella, M.; Portoghese, M.; Loreni, F.; Mureddu, S.; Signoriello, G.; Scisciola, L.; Barbieri, M.; Rizzo, M.R.; et al. Pericoronary Fat Inflammation and Major Adverse Cardiac Events (MACE) in Prediabetic Patients with Acute Myocardial Infarction: Effects of Metformin. Cardiovasc. Diabetol. 2019, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide Causes Large and Rapid Epicardial Fat Reduction. Obesity 2017, 25, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.F.; de Oliveira, S.F.; Higuchi, M.d.L.; Favarato, D.; Dallan, L.A.d.O.; da Luz, P.L. Synergistic Anti-Inflammatory Effect: Simvastatin and Pioglitazone Reduce Inflammatory Markers of Plasma and Epicardial Adipose Tissue of Coronary Patients with Metabolic Syndrome. Diabetol. Metab. Syndr. 2014, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Barchuk, M.; Lobo, M.; Nogueira, J.P. Effect of Glucagon-like Peptide-1 (GLP-1) Analogues on Epicardial Adipose Tissue: A Meta-Analysis. Diabetes Metab. Syndr. 2022, 16, 102562. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Paoli, M.; Rodney, M.; Balladares, N.; Contreras, M.; D’Marco, L.; Iacobellis, G. Effect of Sitagliptin on Epicardial Fat Thickness in Subjects with Type 2 Diabetes and Obesity: A Pilot Study. Endocrine 2016, 51, 448–455. [Google Scholar] [CrossRef]
- Braha, A.; Timar, B.; Diaconu, L.; Lupusoru, R.; Vasiluta, L.; Sima, A.; Vlad, A.; Munteanu, M.; Albai, A.; Cipu, D.; et al. Dynamics of Epicardiac Fat and Heart Function in Type 2 Diabetic Patients Initiated with SGLT-2 Inhibitors. Diabetes Metab. Syndr. Obes. 2019, 12, 2559–2566. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Parisi, V.; Moschetta, D.; Valerio, V.; Conte, M.; Massaiu, I.; Bozzi, M.; Celeste, F.; Leosco, D.; Iaccarino, G.; et al. Efficacy of Cardiometabolic Drugs in Reduction of Epicardial Adipose Tissue: A Systematic Review and Meta-Analysis. Cardiovasc. Diabetol. 2023, 22, 23. [Google Scholar] [CrossRef]


| Imaging Methods | Measurements | Advantages | Disadvantages |
|---|---|---|---|
| Echocardiography | Thickness |
|
|
| Cardiac Magnetic Resonance | Thickness Area Volume |
|
|
| Cardiac Computed Tomography | Thickness Area Volume |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimarchi, G.; Carerj, M.L.; Zito, C.; Bella, G.D.; Taverna, G.; Cusmà Piccione, M.; Crea, P.; Lo Giudice, S.; Buonpane, A.; Bonanni, M.; et al. Epicardial Adipose Tissue: A Multimodal Imaging Diagnostic Perspective. Medicina 2025, 61, 961. https://doi.org/10.3390/medicina61060961
Trimarchi G, Carerj ML, Zito C, Bella GD, Taverna G, Cusmà Piccione M, Crea P, Lo Giudice S, Buonpane A, Bonanni M, et al. Epicardial Adipose Tissue: A Multimodal Imaging Diagnostic Perspective. Medicina. 2025; 61(6):961. https://doi.org/10.3390/medicina61060961
Chicago/Turabian StyleTrimarchi, Giancarlo, Maria Ludovica Carerj, Concetta Zito, Gianluca Di Bella, Giovanni Taverna, Maurizio Cusmà Piccione, Pasquale Crea, Stefania Lo Giudice, Angela Buonpane, Michela Bonanni, and et al. 2025. "Epicardial Adipose Tissue: A Multimodal Imaging Diagnostic Perspective" Medicina 61, no. 6: 961. https://doi.org/10.3390/medicina61060961
APA StyleTrimarchi, G., Carerj, M. L., Zito, C., Bella, G. D., Taverna, G., Cusmà Piccione, M., Crea, P., Lo Giudice, S., Buonpane, A., Bonanni, M., Restelli, D., Paradossi, U., Monteleone, A., Micari, A., & Carerj, S. (2025). Epicardial Adipose Tissue: A Multimodal Imaging Diagnostic Perspective. Medicina, 61(6), 961. https://doi.org/10.3390/medicina61060961








