Myocardial Perfusion Imaging with Cardiovascular Magnetic Resonance in Nonischemic Cardiomyopathies: An In-Depth Review of Techniques and Clinical Applications
Abstract
1. Introduction
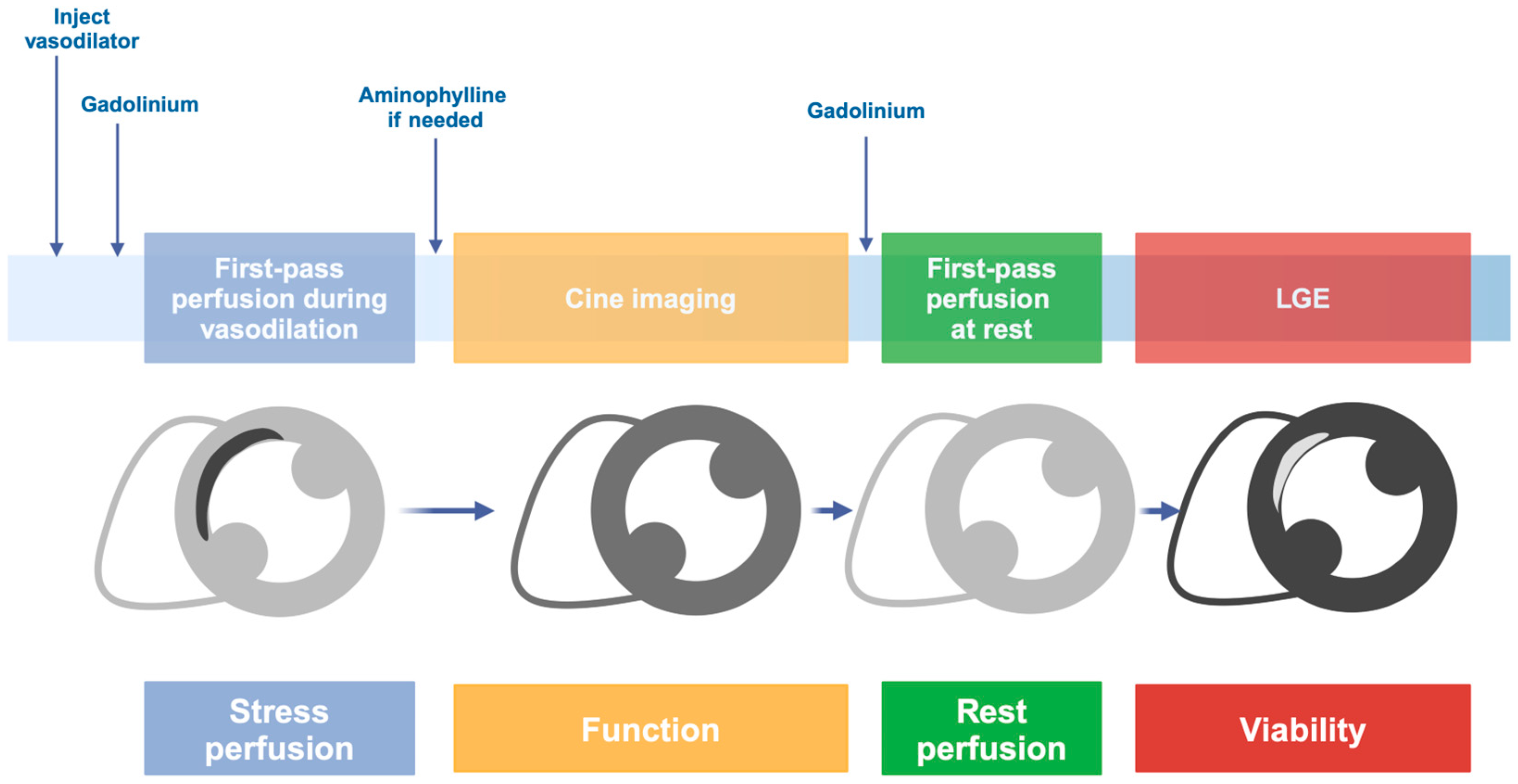
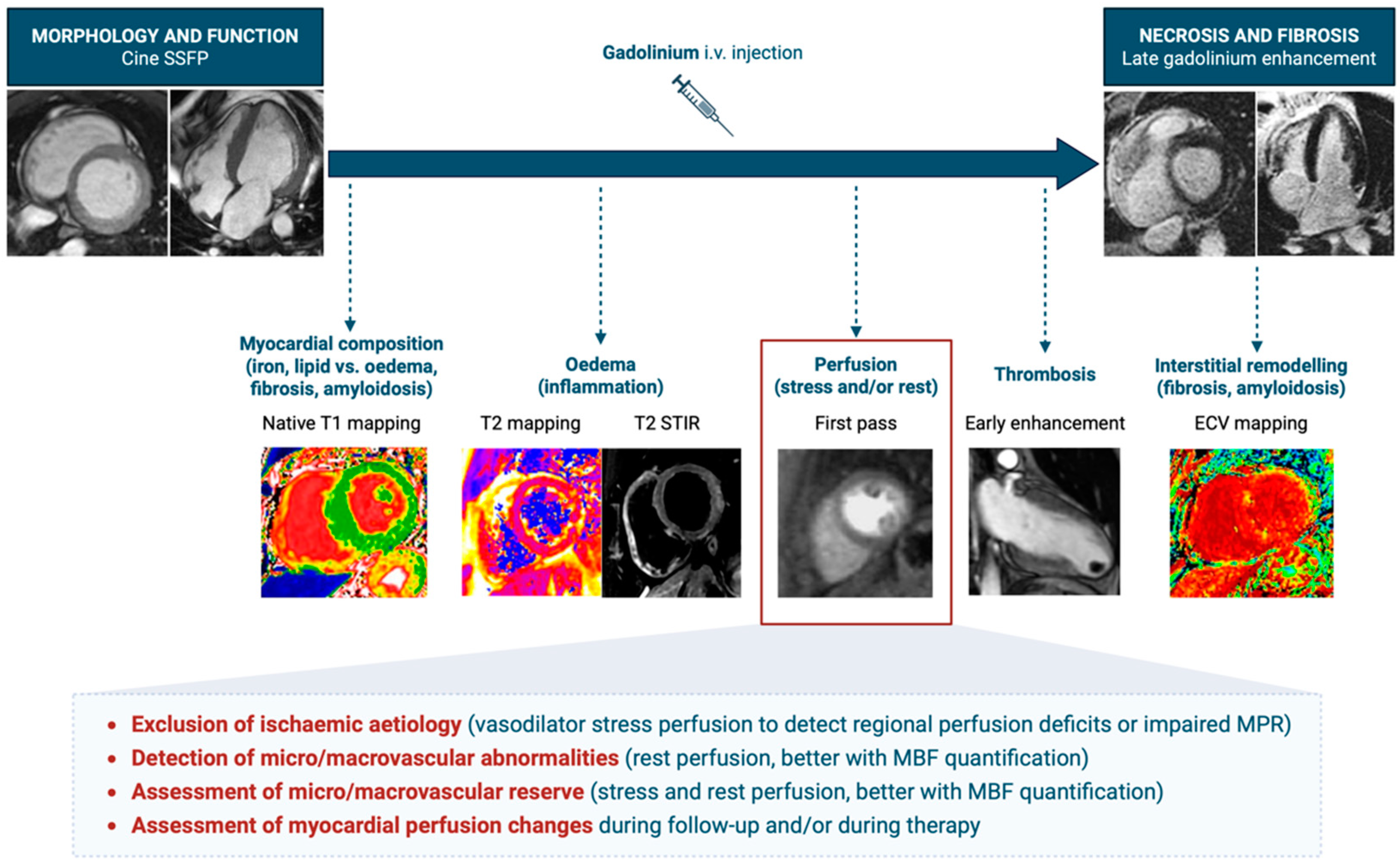
2. Stress Cardiac Magnetic Resonance
2.1. Stress CMR with Vasodilators
2.2. Stress CMR with Dobutamine
2.3. Contraindications and Safety Considerations
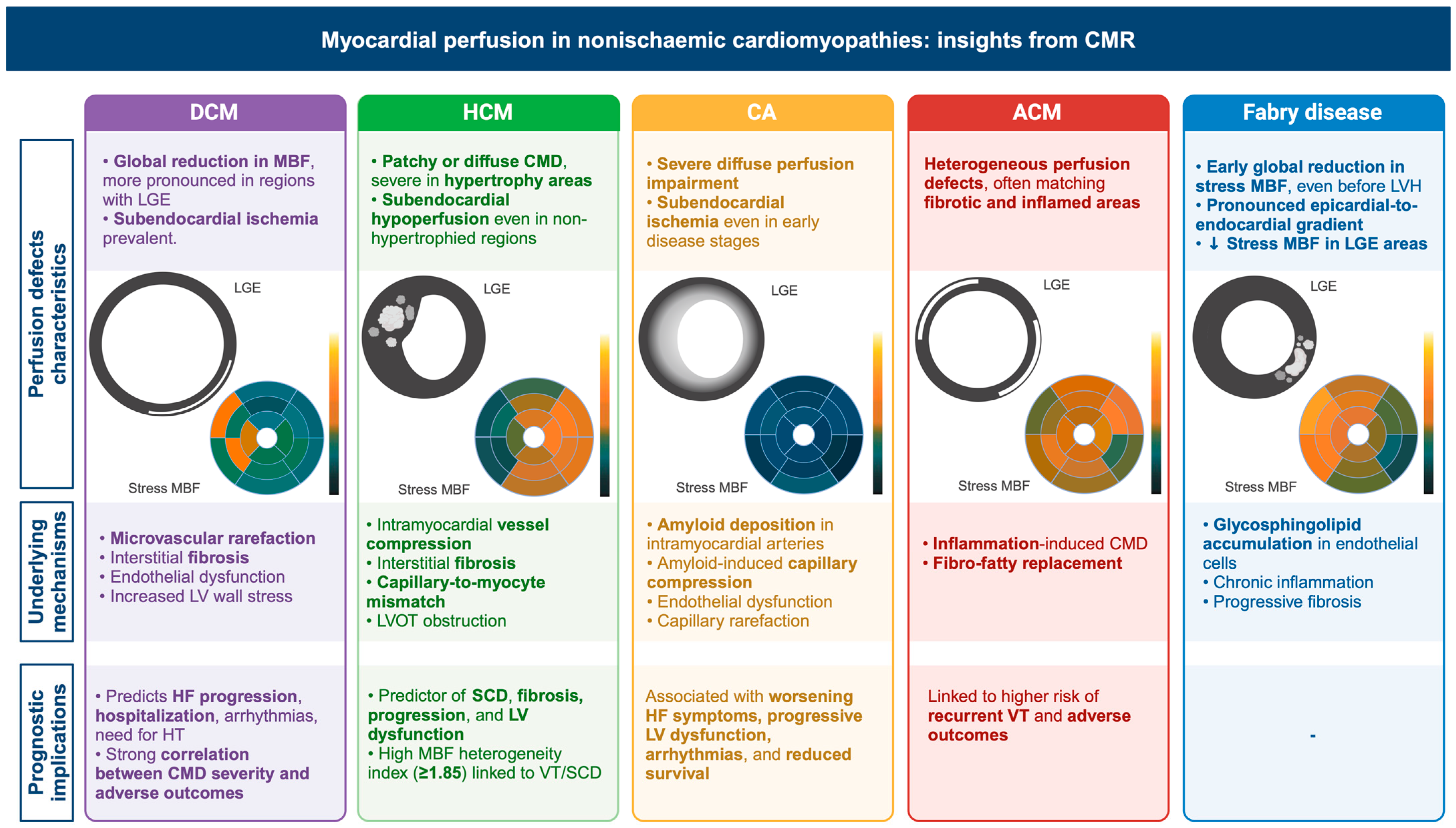
3. Dilated Cardiomyopathy
3.1. Non-CMR Studies on Myocardial Perfusion in DCM
3.2. CMR Studies on Myocardial Perfusion in DCM
| Author, Year [Ref] | Patients | Stressor | Quantitative (Yes/No) | Main Finding | Prevalence of Perfusion Defects | Distribution of Perfusion Defects |
|---|---|---|---|---|---|---|
| Dilated cardiomyopathy | ||||||
| Bell SP, 2013 [46] | 13 NIDCM pts/15 controls in CMR and PET study | adenosine | No |
| All | Circumferential subendocardial perfusion defects (hypoperfusion) |
| Dass S, 2015 [49] | 14 NIDCM pts/12 controls in CMR, OS-(BOLD) CMR, spectroscopy MR | adenosine | No |
| - | Circumferential perfusion defects |
| Gulati A, 2019 [50] | 65 NIDCM pts/45 controls | adenosine | yes |
| - | Circumferential perfusion defects |
| Slivnick JA, 2022 [47] | 41 NIDCM pts/58 controls | adenosine | No |
| 56% (by MPRI) 27% (visually) | Subendocardial stress perfusion defects |
| Javed W, 2023 [48] | 160 NIDCM pts | adenosine | Yes | Reduced MPR predicted MACE | 42/160 pts (26%) with MPR < 2.06 | Subendocardial perfusion defects |
| Hypertrophic cardiomyopathy | ||||||
| Petersen SE, 2007 [56] | 35 HCM pts/14 controls | adenosine | yes |
| - | Predominatly subendocardial perfusion defects |
| Ismail TF, 2014 [57] | 35 HCM pts | adenosine | yes | Pixel-wise quantitative CMR perfusion differentiates HCM pts with non-severe from severe localized MVD (potential myocardial ischemia) |
| Predominantly subendocardial perfusion defects |
| Xu H, 2014 [58] | 42 HCM pts/14 controls | - | No |
| 3 pts (12%) in obstructive HCM group | Predominantly subendocardial perfusion defects |
| Chiribiri A, 2015 [59] | 80 HCM pts | - | No |
| 24 pts (30%) with rest perfusion defets in visual analysis | Predominantly subendocardial perfusion defects |
| Villa ADM, 2016 [60] | 30 HCM pts | adenosine | yes |
| Visual analysis:
|
|
| Tezuka D, 2018 [61] | 81 pts (37 HCM, 24 LVH, 20 normal controls) in stress CMR | adenosine | No |
| - | Predominantly subendocardial perfusion defects |
| Kim EK, 2020 [62] | 115 HCM pts | adenosine | No | Stress perfusion defects associated with NSVT, higher LV mass index, apical aneurysms | 42% of pts (visual analysis) |
|
| Camaioni C, 2020 [63] | 101 HCM pts/30 controls | adenosine | yes |
| 79% of pts with perfusion defects |
|
| Raman B, 2021 [64] | 103 HCM/32 controls in stress CMR/OS-(BOLD) CMR | adenosine | yes |
| - | Predominantly subendocardial perfusion defects |
| Hughes RK, 2021 [65] | 50 Genotype + LVH- pts/28 controls | adenosine | yes | Impaired myocardial perfusion in HCM mutation carriers even in the absence of significant LVH or scarring | 9 pts (20%) of carriers with visual perfusion defects in perfusion mapping | Predominantly subendocardial perfusion defects |
| Das A, 2022 [66] | 20 HCM pts in stress CMR/ 10 cDTI controls | adenosine | yes | Lower MPR and cardiomyocyte disarray in HCM | - | Mainly subendocardial perfusion defects |
| Garcia Brás P, 2023 [67] | 75 HCM pts CMR and strain echo study | regadenoson | No | Impaired myocardial work significantly correlated with MVD (with higher predictive power than GLS); independently of LGE, LV obstruction and hypertrophy | 90.7% of pts with perfusion defects |
|
| Cardiac amyloidosis | ||||||
| Li R, 2016 [68] | 32 AL amyloidosis pts/25 healthy controls | - | No |
| - |
|
| Kotecha T, 2019 [69] | 86 amyloidosis pts/20 healthy volunteers | adenosine | yes |
| - | Subendocardial/diffuse perfusion impairment |
| Ioannou A, 2022 [70] | 92 amyloidosis pts | adenosine | yes | Reduced MPR comparable to 3 vessel disease pts | - | Subendocardial/diffuse perfusion impairment |
| Chacko L, 2024 [71] | 93 amyloidosis pts (42 and 51 ATTR)/97 controls (74 3VD) | adenosine | yes | CMR stress perfusion mapping and histology demonstrate severe inducible myocardial ischemia in CA | - | Subendocardial/diffuse perfusion impairment |
| Katznelson E, 2024 [72] | 92 AL amyloidosis pts in rest CMR | - | yes | MBF and MWE decrease as cardiac amyloid burden (ECV) increase | - | Subendocardial/diffuse perfusion impairment |
| Tang L, 2025 [73] | 126 AL amyloidosis pts | adenosine | yes | ECV and MPR show high prognostic value (higher ECV and lower MPR—worse prognosis) | - | Subendocardial/diffuse perfusion impairment |
| Arrhythmogenic cardiomyopathy | ||||||
| Tung R, 2015 [74] | 103 pts in a PET/CMR study | - | no | Near 50% of unexplained cardiomyopathy and VA demonstrate inflammation, fibrotic remodeling and microvascular dysfunction | Perfusion defects present in
| Heterogeneous matching fibrotic and inflamed areas |
| Fabry disease | ||||||
| Knott KD, 2019 [75] | 44 Fabry pts/27 healthy controls | adenosine | yes |
| - | Predominantly subendocardial perfusion impairment |
4. Hypertrophic Cardiomyopathy
4.1. Non-CMR Studies on Myocardial Perfusion in HCM
4.2. CMR Studies on Myocardial Perfusion in HCM
5. Cardiac Amyloidosis
5.1. Non-CMR Studies on Myocardial Perfusion in CA
5.2. CMR Studies on Myocardial Perfusion in CA
6. Arrhythmogenic Cardiomyopathy
7. Fabry Disease
8. Limitations of CMR
9. Future Directions for Myocardial Perfusion in CMR
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIF | Arterial input function |
| ACM | Arrhythmogenic cardiomyopathy |
| AL | Light-chain amyloidosis |
| ATTR | Transthyretin amyloidosis |
| AVB | Atrioventricular block |
| CA | Cardiac amyloidosis |
| CAD | Coronary artery disease |
| CFR | Coronary flow reserve |
| CMD | Coronary microvascular dysfunction |
| CMR | Cardiovascular magnetic resonance |
| CTP | Computed tomography perfusion |
| DCM | Dilated cardiomyopathy |
| DPD | 3,3-Diphosphono-1,2-propanodicarboxylic acid (bone-avid SPECT tracer) |
| ECV | Extracellular volume |
| FD | Fabry disease |
| FGE | Fast gradient-echo |
| HCM | Hypertrophic cardiomyopathy |
| HF | heart failure |
| ICD | Implantable cardioverter–defibrillator |
| iGRASP | Iterative golden-angle radial sparse parallel magnetic resonance imaging |
| k-t PCA | Principal component analysis |
| k-t SENSE | SENSitivity Endocing |
| LAD | Left anterior descending |
| LGE | Late gadolinium enhancement |
| LRMC | Low-rank motion compensation |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| MBF | Myocardial blood flow |
| MCE | Myocardial contrast echocardiography |
| MPI | Myocardial perfusion reserve |
| MPR | Myocardial perfusion reserve |
| MPRI | Myocardial perfusion reserve index |
| MRS | Magnetic resonance spectroscopy |
| MyoTT | Myocardial transit-time |
| PET | Positron emission tomography |
| PYP | Pyrophosphate (Tc-99m-PYP SPECT tracer) |
| RV | Right ventricle |
| SCD | Sudden cardiac death |
| SPECT | Single-photon emission computed tomography |
| SSFP | Steady-state free precession |
| Tc-99m | Technetium-99m |
| SMS | Simultaneous multi-slice imaging |
| VUS | Variance of uncertain significance |
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies: Developed by the Task Force on the Management of Cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Salerno, M.; Kwong, R.Y.; Singh, A.; Heydari, B.; Kramer, C.M. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging. J. Am. Coll. Cardiol. 2021, 78, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Wilke, N.M.; Jerosch-Herold, M.; Zenovich, A.; Stillman, A.E. Magnetic Resonance First-Pass Myocardial Perfusion Imaging: Clinical Validation and Future Applications. J. Magn. Reson. Imaging 1999, 10, 676–685. [Google Scholar] [CrossRef]
- Jerosch-Herold, M.; Seethamraju, R.T.; Swingen, C.M.; Wilke, N.M.; Stillman, A.E. Analysis of Myocardial Perfusion MRI. J. Magn. Reson. Imaging 2004, 19, 758–770. [Google Scholar] [CrossRef]
- Arai, A.E. Magnetic Resonance First-Pass Myocardial Perfusion Imaging. Top. Magn. Reson. Imaging 2000, 11, 383–398. [Google Scholar] [CrossRef]
- Jogiya, R.; Schuster, A.; Zaman, A.; Motwani, M.; Kouwenhoven, M.; Nagel, E.; Kozerke, S.; Plein, S. Three-Dimensional Balanced Steady State Free Precession Myocardial Perfusion Cardiovascular Magnetic Resonance at 3T Using Dual-Source Parallel RF Transmission: Initial Experience. J. Cardiovasc. Magn. Reson. 2014, 16, 90. [Google Scholar] [CrossRef]
- Bertschinger, K.M.; Nanz, D.; Buechi, M.; Luescher, T.F.; Marincek, B.; von Schulthess, G.K.; Schwitter, J. Magnetic Resonance Myocardial First-Pass Perfusion Imaging: Parameter Optimization for Signal Response and Cardiac Coverage. J. Magn. Reson. Imaging 2001, 14, 556–562. [Google Scholar] [CrossRef]
- Villa, A.D.M.; Corsinovi, L.; Ntalas, I.; Milidonis, X.; Scannell, C.; Di Giovine, G.; Child, N.; Ferreira, C.; Nazir, M.S.; Karady, J.; et al. Importance of Operator Training and Rest Perfusion on the Diagnostic Accuracy of Stress Perfusion Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2018, 20, 74. [Google Scholar] [CrossRef]
- von Knobelsdorff-Brenkenhoff, F.; Schulz-Menger, J. Cardiovascular Magnetic Resonance in the Guidelines of the European Society of Cardiology: A Comprehensive Summary and Update. J. Cardiovasc. Magn. Reson. 2023, 25, 42. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction: Validation Against Invasive Coronary Physiology. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef]
- Pons-Lladó, G.; Kellman, P. State-of-the-Art of Myocardial Perfusion by CMR: A Practical View. Rev. Cardiovasc. Med. 2022, 23, 325. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, R.; van Assen, M.; Vliegenthart, R.; de Bock, G.H.; van der Harst, P.; Oudkerk, M. Diagnostic Performance of Semi-Quantitative and Quantitative Stress CMR Perfusion Analysis: A Meta-Analysis. J. Cardiovasc. Magn. Reson. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Gerber, B.L.; Raman, S.V.; Nayak, K.; Epstein, F.H.; Ferreira, P.; Axel, L.; Kraitchman, D.L. Myocardial First-Pass Perfusion Cardiovascular Magnetic Resonance: History, Theory, and Current State of the Art. J. Cardiovasc. Magn. Reson. 2008, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-Y.; Groves, D.W.; Aletras, A.H.; Kellman, P.; Arai, A.E. A Quantitative Pixel-Wise Measurement of Myocardial Blood Flow by Contrast-Enhanced First-Pass CMR Perfusion Imaging: Microsphere Validation in Dogs and Feasibility Study in Humans. JACC Cardiovasc. Imaging 2012, 5, 154–166. [Google Scholar] [CrossRef]
- Sharrack, N.; Chiribiri, A.; Schwitter, J.; Plein, S. How to Do Quantitative Myocardial Perfusion Cardiovascular Magnetic Resonance. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, 315–318. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized Cardiovascular Magnetic Resonance Imaging (CMR) Protocols: 2020 Update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Pingitore, A.; Lombardi, M.; Scattini, B.; De Marchi, D.; Aquaro, G.D.; Positano, V.; Picano, E. Head to Head Comparison between Perfusion and Function during Accelerated High-Dose Dipyridamole Magnetic Resonance Stress for the Detection of Coronary Artery Disease. Am. J. Cardiol. 2008, 101, 8–14. [Google Scholar] [CrossRef]
- Lee, D.C.; Johnson, N.P. Quantification of Absolute Myocardial Blood Flow by Magnetic Resonance Perfusion Imaging. JACC Cardiovasc. Imaging 2009, 2, 761–770. [Google Scholar] [CrossRef]
- Charoenpanichkit, C.; Hundley, W.G. The 20 Year Evolution of Dobutamine Stress Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 59. [Google Scholar] [CrossRef]
- Nolan, J.P.; Maconochie, I.; Soar, J.; Olasveengen, T.M.; Greif, R.; Wyckoff, M.H.; Singletary, E.M.; Aickin, R.; Berg, K.M.; Mancini, M.E.; et al. Executive Summary: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020, 142, S2–S27. [Google Scholar] [CrossRef]
- Gigli, M.; Stolfo, D.; Merlo, M.; Sinagra, G.; Taylor, M.R.G.; Mestroni, L. Pathophysiology of Dilated Cardiomyopathy: From Mechanisms to Precision Medicine. Nat. Rev. Cardiol. 2024, 22, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Kramer, C.M. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Mckenna, P.; et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Vanderheyden, M.; Bartunek, J.; Verstreken, S.; Mortier, L.; Goethals, M.; de Bruyne, B. Non-Invasive Assessment of Coronary Flow Reserve in Idiopathic Dilated Cardiomyopathy: Hemodynamic Correlations. Eur. J. Echocardiogr. 2005, 6, 47–53. [Google Scholar] [CrossRef]
- Rigo, F.; Gherardi, S.; Galderisi, M.; Sicari, R.; Picano, E. The Independent Prognostic Value of Contractile and Coronary Flow Reserve Determined by Dipyridamole Stress Echocardiography in Patients with Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2007, 99, 1154–1158. [Google Scholar] [CrossRef]
- Ciampi, Q.; Cortigiani, L.; Pratali, L.; Rigo, F.; Villari, B.; Picano, E.; Sicari, R. Left Bundle Branch Block Negatively Affects Coronary Flow Velocity Reserve and Myocardial Contractile Reserve in Nonischemic Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2016, 29, 112–118. [Google Scholar] [CrossRef]
- Mathew, R.C.; Bourque, J.M.; Salerno, M.; Kramer, C.M. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc. Imaging 2020, 13, 1577–1590. [Google Scholar] [CrossRef]
- Lima, M.F.; Mathias, W.; Sbano, J.C.N.; de la Cruz, V.Y.; Abduch, M.C.; Lima, M.S.M.; Bocchi, E.A.; Hajjar, L.A.; Ramires, J.A.F.; Kalil Filho, R.; et al. Prognostic Value of Coronary and Microvascular Flow Reserve in Patients with Nonischemic Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2013, 26, 278–287. [Google Scholar] [CrossRef]
- Ma, J.; Guan, L.; Yang, L.; Mahemuti, A.; Mu, Y. Relationship Between Myocardial Perfusion and Myocardial Function in Dilated Cardiomyopathy by Shown Ultrasonography. Int. Heart J. 2021, 62, 792–800. [Google Scholar] [CrossRef]
- Miyata-Fukuoka, Y.; Kawai, H.; Iseki, O.; Yamanaka, Y.; Ueda, Y.; Yokoyama, M.; Hirata, K.-I. Myocardial Blood Volume Reserve by Intravenous Contrast Echocardiography Predicts Improvement in Left Ventricular Function in Patients with Nonischemic Dilated Cardiomyopathy. J. Echocardiogr. 2016, 14, 163–170. [Google Scholar] [CrossRef]
- Nunes, R.; Martins, E.; Dias, P. Comparison between Myocardial Perfusion SPECT and Cardiac Magnetic Resonance Findings in Non-Ischemic Dilated Cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2024, 25, jeae142.061. [Google Scholar] [CrossRef]
- Sobajima, M.; Nozawa, T.; Suzuki, T.; Ohori, T.; Shida, T.; Matsuki, A.; Inoue, H. Impact of Myocardial Perfusion Abnormality on Prognosis in Patients with Non-Ischemic Dilated Cardiomyopathy. J. Cardiol. 2010, 56, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Danias, P.G.; Papaioannou, G.I.; Ahlberg, A.W.; O’Sullivan, D.M.; Mann, A.; Boden, W.E.; Heller, G.V. Usefulness of Electrocardiographic-Gated Stress Technetium-99m Sestamibi Single-Photon Emission Computed Tomography to Differentiate Ischemic from Nonischemic Cardiomyopathy. Am. J. Cardiol. 2004, 94, 14–19. [Google Scholar] [CrossRef]
- Matsuo, S.; Nakae, I.; Tsutamoto, T.; Okamoto, N.; Horie, M. A Novel Clinical Indicator Using Tc-99m Sestamibi for Evaluating Cardiac Mitochondrial Function in Patients with Cardiomyopathies. J. Nucl. Cardiol. 2007, 14, 215–220. [Google Scholar] [CrossRef]
- Takehana, K.; Maeba, H.; Ueyama, T.; Iwasaka, T. Direct Correlation between Regional Systolic Function and Regional Washout Rate of 99mTc-Sestamibi in Patients with Idiopathic Dilated Cardiomyopathy. Nucl. Med. Commun. 2011, 32, 1174–1178. [Google Scholar] [CrossRef]
- Caobelli, F.; Bengel, F.M. Ischaemic vs Non-Ischaemic Dilated Cardiomyopathy: The Value of Nuclear Cardiology Techniques. J. Nucl. Cardiol. 2015, 22, 971–974. [Google Scholar] [CrossRef]
- Bateman, T.M. Advantages and Disadvantages of PET and SPECT in a Busy Clinical Practice. J. Nucl. Cardiol. 2012, 19 (Suppl. S1), S3–S11. [Google Scholar] [CrossRef]
- Bravo, P.E.; Di Carli, M.F.; Dorbala, S. Role of PET to Evaluate Coronary Microvascular Dysfunction in Non-Ischemic Cardiomyopathies. Heart Fail. Rev. 2017, 22, 455–464. [Google Scholar] [CrossRef]
- Murthy, V.L.; Bateman, T.M.; Beanlands, R.S.; Berman, D.S.; Borges-Neto, S.; Chareonthaitawee, P.; Cerqueira, M.D.; deKemp, R.A.; DePuey, E.G.; Dilsizian, V.; et al. Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J. Nucl. Cardiol. 2018, 25, 269–297. [Google Scholar] [CrossRef]
- Robinson, A.A.; Bourque, J.M. Emerging Techniques for Cardiovascular PET. Cardiovasc. Innov. Appl. 2019, 4, 13–24. [Google Scholar] [CrossRef]
- van den Heuvel, A.F.; van Veldhuisen, D.J.; van der Wall, E.E.; Blanksma, P.K.; Siebelink, H.M.; Vaalburg, W.M.; van Gilst, W.H.; Crijns, H.J. Regional Myocardial Blood Flow Reserve Impairment and Metabolic Changes Suggesting Myocardial Ischemia in Patients with Idiopathic Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2000, 35, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Michelassi, C.; Trivieri, M.G.; Sambuceti, G.; Giorgetti, A.; Pratali, L.; Gallopin, M.; Salvadori, P.; Sorace, O.; Carpeggiani, C.; et al. Prognostic Role of Myocardial Blood Flow Impairment in Idiopathic Left Ventricular Dysfunction. Circulation 2002, 105, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Majmudar, M.D.; Murthy, V.L.; Shah, R.V.; Kolli, S.; Mousavi, N.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Sitek, A.; et al. Quantification of Coronary Flow Reserve in Patients with Ischaemic and Non-Ischaemic Cardiomyopathy and Its Association with Clinical Outcomes. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 900–909. [Google Scholar] [CrossRef]
- Middour, T.G.; Rosenthal, T.M.; Abi-Samra, F.M.; Bernard, M.L.; Khatib, S.; Polin, G.M.; Rogers, P.A.; Bober, R.M.; Morin, D.P. Positron Emission Tomography Absolute Stress Myocardial Blood Flow for Risk Stratification in Nonischemic Cardiomyopathy. J. Cardiovasc. Electrophysiol. 2020, 31, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Range, F.T.; Paul, M.; Schäfers, K.P.; Acil, T.; Kies, P.; Hermann, S.; Schober, O.; Breithardt, G.; Wichter, T.; Schäfers, M.A. Myocardial Perfusion in Nonischemic Dilated Cardiomyopathy with and without Atrial Fibrillation. J. Nucl. Med. 2009, 50, 390–396. [Google Scholar] [CrossRef]
- Bell, S.P.; Adkisson, D.W.; Ooi, H.; Sawyer, D.B.; Lawson, M.A.; Kronenberg, M.W. Impairment of Subendocardial Perfusion Reserve and Oxidative Metabolism in Nonischemic Dilated Cardiomyopathy. J. Card. Fail. 2013, 19, 802–810. [Google Scholar] [CrossRef]
- Slivnick, J.A.; Zareba, K.M.; Truong, V.T.; Liu, E.; Barnes, A.; Mazur, W.; Binkley, P. Impairment in Quantitative Microvascular Function in Non-Ischemic Cardiomyopathy as Demonstrated Using Cardiovascular Magnetic Resonance. PLoS ONE 2022, 17, e0264454. [Google Scholar] [CrossRef]
- Javed, W.; Goh, Z.; Shabi, M.; Sharrack, N.; Gorecka, M.; Levelt, E.; Xue, H.; Dall’Armellina, E.; Kellman, P.; Greenwood, J.P.; et al. 19 Myocardial Perfusion Reserve by Quantitative Perfusion Cardiovascular Magnetic Resonance in Dilated Cardiomyopathy: Association with Major Adverse Cardiovascular Events. Heart 2023, 109, A16–A17. [Google Scholar] [CrossRef]
- Dass, S.; Holloway, C.J.; Cochlin, L.E.; Rider, O.J.; Mahmod, M.; Robson, M.; Sever, E.; Clarke, K.; Watkins, H.; Ashrafian, H.; et al. No Evidence of Myocardial Oxygen Deprivation in Nonischemic Heart Failure. Circ. Heart Fail. 2015, 8, 1088–1093. [Google Scholar] [CrossRef]
- Gulati, A.; Ismail, T.F.; Ali, A.; Hsu, L.-Y.; Gonçalves, C.; Ismail, N.A.; Krishnathasan, K.; Davendralingam, N.; Ferreira, P.; Halliday, B.P.; et al. Microvascular Dysfunction in Dilated Cardiomyopathy: A Quantitative Stress Perfusion Cardiovascular Magnetic Resonance Study. JACC Cardiovasc. Imaging 2019, 12, 1699–1708. [Google Scholar] [CrossRef]
- Rahman, H.; Scannell, C.M.; Demir, O.M.; Ryan, M.; McConkey, H.; Ellis, H.; Masci, P.G.; Perera, D.; Chiribiri, A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Cadour, F.; Quemeneur, M.; Biere, L.; Donal, E.; Bentatou, Z.; Eicher, J.-C.; Roubille, F.; Lalande, A.; Giorgi, R.; Rapacchi, S.; et al. Prognostic Value of Cardiovascular Magnetic Resonance T1 Mapping and Extracellular Volume Fraction in Nonischemic Dilated Cardiomyopathy. J. Cardiovasc. Magn. Reson. 2023, 25, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mėlinytė-Ankudavičė, K.; Marcinkevičienė, K.; Galnaitienė, G.; Bučius, P.; Lapinskas, T.; Ereminienė, E.; Šakalytė, G.; Jurkevičius, R. Potential Prognostic Impact of Left-Ventricular Global Longitudinal Strain in Analysis of Whole-Heart Myocardial Mechanics in Nonischemic Dilated Cardiomyopathy. Int. J. Cardiovasc. Imaging 2024, 40, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Dentamaro, I.; La Mura, L.; Alifragki, A.; Marketou, M.; Cavarretta, E.; Papadakis, M.; Androulakis, E. Current Insights and Novel Cardiovascular Magnetic Resonance-Based Techniques in the Prognosis of Non-Ischemic Dilated Cardiomyopathy. J. Clin. Med. 2024, 13, 1017. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, J.; Abuzaid, A.; Brian, A.-E.; Ordovas, K. Cardiovascular Magnetic Resonance Imaging Tissue Characterization in Non-Ischemic Cardiomyopathies. Curr. Treat. Options Cardio Med. 2020, 22, 16. [Google Scholar] [CrossRef]
- Petersen, S.E.; Jerosch-Herold, M.; Hudsmith, L.E.; Robson, M.D.; Francis, J.M.; Doll, H.A.; Selvanayagam, J.B.; Neubauer, S.; Watkins, H. Evidence for Microvascular Dysfunction in Hypertrophic Cardiomyopathy: New Insights from Multiparametric Magnetic Resonance Imaging. Circulation 2007, 115, 2418–2425. [Google Scholar] [CrossRef]
- Ismail, T.F.; Hsu, L.-Y.; Greve, A.M.; Gonçalves, C.; Jabbour, A.; Gulati, A.; Hewins, B.; Mistry, N.; Wage, R.; Roughton, M.; et al. Coronary Microvascular Ischemia in Hypertrophic Cardiomyopathy—A Pixel-Wise Quantitative Cardiovascular Magnetic Resonance Perfusion Study. J. Cardiovasc. Magn. Reson. 2014, 16, 49. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Z.; Sun, J.; Wen, L.; Zhang, G.; Zhang, S.; Guo, Y. The Regional Myocardial Microvascular Dysfunction Differences in Hypertrophic Cardiomyopathy Patients with or without Left Ventricular Outflow Tract Obstruction: Assessment with First-Pass Perfusion Imaging Using 3.0-T Cardiac Magnetic Resonance. Eur. J. Radiol. 2014, 83, 665–672. [Google Scholar] [CrossRef]
- Chiribiri, A.; Leuzzi, S.; Conte, M.R.; Bongioanni, S.; Bratis, K.; Olivotti, L.; De Rosa, C.; Lardone, E.; Di Donna, P.; Villa, A.D.M.; et al. Rest Perfusion Abnormalities in Hypertrophic Cardiomyopathy: Correlation with Myocardial Fibrosis and Risk Factors for Sudden Cardiac Death. Clin. Radiol. 2015, 70, 495–501. [Google Scholar] [CrossRef]
- Villa, A.D.M.; Sammut, E.; Zarinabad, N.; Carr-White, G.; Lee, J.; Bettencourt, N.; Razavi, R.; Nagel, E.; Chiribiri, A. Microvascular Ischemia in Hypertrophic Cardiomyopathy: New Insights from High-Resolution Combined Quantification of Perfusion and Late Gadolinium Enhancement. J. Cardiovasc. Magn. Reson. 2016, 18, 4. [Google Scholar] [CrossRef]
- Tezuka, D.; Kosuge, H.; Terashima, M.; Koyama, N.; Kishida, T.; Tada, Y.; Suzuki, J.-I.; Sasano, T.; Ashikaga, T.; Hirao, K.; et al. Myocardial Perfusion Reserve Quantified by Cardiac Magnetic Resonance Imaging Is Associated with Late Gadolinium Enhancement in Hypertrophic Cardiomyopathy. Heart Vessel. 2018, 33, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Lee, S.-C.; Chang, S.-A.; Jang, S.-Y.; Kim, S.M.; Park, S.-J.; Choi, J.-O.; Park, S.W.; Jeon, E.-S.; Choe, Y.H. Prevalence and Clinical Significance of Cardiovascular Magnetic Resonance Adenosine Stress-Induced Myocardial Perfusion Defect in Hypertrophic Cardiomyopathy. J. Cardiovasc. Magn. Reson. 2020, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, C.; Knott, K.D.; Augusto, J.B.; Seraphim, A.; Rosmini, S.; Ricci, F.; Boubertakh, R.; Xue, H.; Hughes, R.; Captur, G.; et al. Inline Perfusion Mapping Provides Insights into the Disease Mechanism in Hypertrophic Cardiomyopathy. Heart 2020, 106, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Tunnicliffe, E.M.; Chan, K.; Ariga, R.; Hundertmark, M.; Ohuma, E.O.; Sivalokanathan, S.; Tan, Y.J.G.; Mahmod, M.; Hess, A.T.; et al. Association between Sarcomeric Variants in Hypertrophic Cardiomyopathy and Myocardial Oxygenation: Insights from a Novel Oxygen-Sensitive Cardiovascular Magnetic Resonance Approach. Circulation 2021, 144, 1656–1658. [Google Scholar] [CrossRef]
- Hughes, R.K.; Camaioni, C.; Augusto, J.B.; Knott, K.; Quinn, E.; Captur, G.; Seraphim, A.; Joy, G.; Syrris, P.; Elliott, P.M.; et al. Myocardial Perfusion Defects in Hypertrophic Cardiomyopathy Mutation Carriers. J. Am. Heart Assoc. 2021, 10, e020227. [Google Scholar] [CrossRef]
- Das, A.; Kelly, C.; Teh, I.; Nguyen, C.; Brown, L.A.E.; Chowdhary, A.; Jex, N.; Thirunavukarasu, S.; Sharrack, N.; Gorecka, M.; et al. Phenotyping Hypertrophic Cardiomyopathy Using Cardiac Diffusion Magnetic Resonance Imaging: The Relationship between Microvascular Dysfunction and Microstructural Changes. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, 352–362. [Google Scholar] [CrossRef]
- Garcia Brás, P.; Rosa, S.A.; Cardoso, I.; Branco, L.M.; Galrinho, A.; Gonçalves, A.V.; Thomas, B.; Viegas, J.M.; Fiarresga, A.; Branco, G.; et al. Microvascular Dysfunction Is Associated with Impaired Myocardial Work in Obstructive and Nonobstructive Hypertrophic Cardiomyopathy: A Multimodality Study. J. Am. Heart Assoc. 2023, 12, e028857. [Google Scholar] [CrossRef]
- Li, R.; Yang, Z.; Wen, L.; Liu, X.; Xu, H.; Zhang, Q.; Guo, Y. Regional Myocardial Microvascular Dysfunction in Cardiac Amyloid Light-Chain Amyloidosis: Assessment with 3T Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2016, 18, 16. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Brown, J.; Little, C.; Knight, D.; Steriotis, A.; Patel, N.; Rakhit, R.; Hawkins, P.; Gillmore, J.; et al. 19 Myocardial Perfusion Mapping in Cardiac Amyloidosis- Unearthing the Spectrum from Infiltration to Ischaemia. Heart 2019, 105, A17. [Google Scholar] [CrossRef]
- Ioannou, A.; Chacko, L.; Kotecha, T.; Patel, R.K.; Razvi, Y.; Porcari, A.; Venneri, L.; Martinez-Naharro, A.; Knight, D.; Brown, J.; et al. Myocardial Ischaemia in Cardiac Amyloidosis: A Change of Perspective. Eur. Heart J. 2022, 43, ehac544.1761. [Google Scholar] [CrossRef]
- Chacko, L.; Kotecha, T.; Ioannou, A.; Patel, N.; Martinez-Naharro, A.; Razvi, Y.; Patel, R.; Massa, P.; Venneri, L.; Brown, J.; et al. Myocardial Perfusion in Cardiac Amyloidosis. Eur. J. Heart Fail. 2024, 26, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, E.; Jerosch-Herold, M.; Cuddy, S.A.M.; Clerc, O.F.; Benz, D.C.; Taylor, A.; Rao, S.; Kijewski, M.F.; Liao, R.; Landau, H.; et al. Mechanisms of Left Ventricular Systolic Dysfunction in Light Chain Amyloidosis: A Multiparametric Cardiac MRI Study. Front. Cardiovasc. Med. 2024, 11, 1371810. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhao, W.; Li, K.; Tian, L.; Zhou, X.; Guo, H.; Zeng, M. Assessing Microvascular Dysfunction and Predicting Long-Term Prognosis in Patients with Cardiac Amyloidosis by Cardiovascular Magnetic Resonance Quantitative Stress Perfusion. J. Cardiovasc. Magn. Reson. 2025, 27, 101134. [Google Scholar] [CrossRef]
- Tung, R.; Bauer, B.; Schelbert, H.; Lynch, J.P.; Auerbach, M.; Gupta, P.; Schiepers, C.; Chan, S.; Ferris, J.; Barrio, M.; et al. Incidence of Abnormal Positron Emission Tomography in Patients with Unexplained Cardiomyopathy and Ventricular Arrhythmias: The Potential Role of Occult Inflammation in Arrhythmogenesis. Heart Rhythm. 2015, 12, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Augusto, J.B.; Nordin, S.; Kozor, R.; Camaioni, C.; Xue, H.; Hughes, R.K.; Manisty, C.; Brown, L.A.E.; Kellman, P.; et al. Quantitative Myocardial Perfusion in Fabry Disease. Circ. Cardiovasc. Imaging 2019, 12, e008872. [Google Scholar] [CrossRef]
- Maron, B.J. Clinical Course and Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2018, 379, 655–668. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
- Treibel, T.A.; Kozor, R.; Menacho, K.; Castelletti, S.; Bulluck, H.; Rosmini, S.; Nordin, S.; Maestrini, V.; Fontana, M.; Moon, J.C. Left Ventricular Hypertrophy Revisited: Cell and Matrix Expansion Have Disease-Specific Relationships. Circulation 2017, 136, 2519–2521. [Google Scholar] [CrossRef]
- Del Franco, A.; Ruggieri, R.; Pieroni, M.; Ciabatti, M.; Zocchi, C.; Biagioni, G.; Tavanti, V.; Del Pace, S.; Leone, O.; Favale, S.; et al. Atlas of Regional Left Ventricular Scar in Nonischemic Cardiomyopathies: Substrates and Etiologies. JACC Adv. 2024, 3, 101214. [Google Scholar] [CrossRef]
- Knaapen, P.; Germans, T.; Camici, P.G.; Rimoldi, O.E.; ten Cate, F.J.; ten Berg, J.M.; Dijkmans, P.A.; Boellaard, R.; van Dockum, W.G.; Götte, M.J.W.; et al. Determinants of Coronary Microvascular Dysfunction in Symptomatic Hypertrophic Cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H986–H993. [Google Scholar] [CrossRef]
- Raphael, C.E.; Cooper, R.; Parker, K.H.; Collinson, J.; Vassiliou, V.; Pennell, D.J.; de Silva, R.; Hsu, L.Y.; Greve, A.M.; Nijjer, S.; et al. Mechanisms of Myocardial Ischemia in Hypertrophic Cardiomyopathy: Insights From Wave Intensity Analysis and Magnetic Resonance. J. Am. Coll. Cardiol. 2016, 68, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Roldan, P.; Ravi, S.; Hodovan, J.; Belcik, J.T.; Heitner, S.B.; Masri, A.; Lindner, J.R. Myocardial Contrast Echocardiography Assessment of Perfusion Abnormalities in Hypertrophic Cardiomyopathy. Cardiovasc. Ultrasound 2022, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.I.I.; Knaapen, P.; Geleijnse, M.L.; Dijkmans, P.A.; Anwar, A.M.; Nemes, A.; Michels, M.; Vletter, W.B.; Lammertsma, A.A.; ten Cate, F.J. Assessment of Intravascular and Extravascular Mechanisms of Myocardial Perfusion Abnormalities in Obstructive Hypertrophic Cardiomyopathy by Myocardial Contrast Echocardiography. Heart 2007, 93, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Balázs, E.; Soliman, O.I.I.; Sepp, R.; Csanády, M.; Forster, T. Long-Term Prognostic Value of Coronary Flow Velocity Reserve in Patients with Hypertrophic Cardiomyopathy: 9-Year Follow-up Results from SZEGED Study. Heart Vessel. 2009, 24, 352–356. [Google Scholar] [CrossRef]
- Yamada, M.; Elliott, P.M.; Kaski, J.C.; Prasad, K.; Gane, J.N.; Lowe, C.M.; Doi, Y.; McKenna, W.J. Dipyridamole Stress Thallium-201 Perfusion Abnormalities in Patients with Hypertrophic Cardiomyopathy. Relationship to Clinical Presentation and Outcome. Eur. Heart J. 1998, 19, 500–507. [Google Scholar] [CrossRef]
- Sorajja, P.; Chareonthaitawee, P.; Ommen, S.R.; Miller, T.D.; Hodge, D.O.; Gibbons, R.J. Prognostic Utility of Single-Photon Emission Computed Tomography in Adult Patients with Hypertrophic Cardiomyopathy. Am. Heart J. 2006, 151, 426–435. [Google Scholar] [CrossRef]
- Ziolkowska, L.; Boruc, A.; Sobielarska-Lysiak, D.; Grzyb, A.; Petryka-Mazurkiewicz, J.; Mazurkiewicz, Ł.; Brzezinska-Rajszys, G. Prognostic Significance of Myocardial Ischemia Detected by Single-Photon Emission Computed Tomography in Children with Hypertrophic Cardiomyopathy. Pediatr. Cardiol. 2021, 42, 960–968. [Google Scholar] [CrossRef]
- Kaimoto, S.; Kawasaki, T.; Kuribayashi, T.; Yamano, M.; Miki, S.; Kamitani, T.; Matsubara, H. Myocardial Perfusion Abnormality in the Area of Ventricular Septum-Free Wall Junction and Cardiovascular Events in Nonobstructive Hypertrophic Cardiomyopathy. Int. J. Cardiovasc. Imaging 2012, 28, 1829–1839. [Google Scholar] [CrossRef]
- Schindler, T.H.; Fearon, W.F.; Pelletier-Galarneau, M.; Ambrosio, G.; Sechtem, U.; Ruddy, T.D.; Patel, K.K.; Bhatt, D.L.; Bateman, T.M.; Gewirtz, H.; et al. PET for Detection and Reporting Coronary Microvascular Dysfunction: A JACC: Cardiovascular Imaging Expert Panel Statement. JACC. Cardiovasc. Imaging 2023, 16, 536–548. [Google Scholar] [CrossRef]
- Timmer, S.A.J.; Knaapen, P. Coronary Microvascular Function, Myocardial Metabolism, and Energetics in Hypertrophic Cardiomyopathy: Insights from Positron Emission Tomography. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 95–101. [Google Scholar] [CrossRef]
- Knaapen, P.; van Dockum, W.G.; Götte, M.J.W.; Broeze, K.A.; Kuijer, J.P.A.; Zwanenburg, J.J.M.; Marcus, J.T.; Kok, W.E.M.; van Rossum, A.C.; Lammertsma, A.A.; et al. Regional Heterogeneity of Resting Perfusion in Hypertrophic Cardiomyopathy Is Related to Delayed Contrast Enhancement but Not to Systolic Function: A PET and MRI Study. J. Nucl. Cardiol. 2006, 13, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-Y.; Yalçin, H.; Yalçin, F.; Zhao, M.; Sivalokanathan, S.; Valenta, I.; Tahari, A.; Pomper, M.G.; Abraham, T.P.; Schindler, T.H.; et al. Stress Myocardial Blood Flow Heterogeneity Is a Positron Emission Tomography Biomarker of Ventricular Arrhythmias in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 121, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Timmer, S.A.J.; Germans, T.; Götte, M.J.W.; Rüssel, I.K.; Lubberink, M.; Ten Berg, J.M.; Ten Cate, F.J.; Lammertsma, A.A.; Knaapen, P.; van Rossum, A.C. Relation of Coronary Microvascular Dysfunction in Hypertrophic Cardiomyopathy to Contractile Dysfunction Independent from Myocardial Injury. Am. J. Cardiol. 2011, 107, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, H.; Valenta, I.; Yalçin, F.; Corona-Villalobos, C.; Vasquez, N.; Ra, J.; Kucukler, N.; Tahari, A.; Pozios, I.; Zhou, Y.; et al. Effect of Diffuse Subendocardial Hypoperfusion on Left Ventricular Cavity Size by 13N-Ammonia Perfusion PET in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2016, 118, 1908–1915. [Google Scholar] [CrossRef]
- Sciagrà, R.; Calabretta, R.; Cipollini, F.; Passeri, A.; Castello, A.; Cecchi, F.; Olivotto, I.; Pupi, A. Myocardial Blood Flow and Left Ventricular Functional Reserve in Hypertrophic Cardiomyopathy: A 13NH3 Gated PET Study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 866–875. [Google Scholar] [CrossRef]
- Castagnoli, H.; Ferrantini, C.; Coppini, R.; Passeri, A.; Baldini, K.; Berti, V.; Cecchi, F.; Olivotto, I.; Sciagrà, R. Role of Quantitative Myocardial Positron Emission Tomography for Risk Stratification in Patients with Hypertrophic Cardiomyopathy: A 2016 Reappraisal. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2413–2422. [Google Scholar] [CrossRef]
- Chatzantonis, G.; Bietenbeck, M.; Florian, A.; Meier, C.; Stalling, P.; Korthals, D.; Reinecke, H.; Yilmaz, A. Diagnostic Value of the Novel CMR Parameter “Myocardial Transit-Time” (MyoTT) for the Assessment of Microvascular Changes in Cardiac Amyloidosis and Hypertrophic Cardiomyopathy. Clin. Res. Cardiol. 2021, 110, 136–145. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients with Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Bravo, P.E.; Zimmerman, S.L.; Luo, H.-C.; Pozios, I.; Rajaram, M.; Pinheiro, A.; Steenbergen, C.; Kamel, I.R.; Wahl, R.L.; Bluemke, D.A.; et al. Relationship of Delayed Enhancement by Magnetic Resonance to Myocardial Perfusion by Positron Emission Tomography in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2013, 6, 210–217. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L.; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef]
- Dorbala, S.; Cuddy, S.; Falk, R.H. How to Image Cardiac Amyloidosis: A Practical Approach. JACC Cardiovasc. Imaging 2020, 13, 1368–1383. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and Treatment of Cardiac Amyloidosis. A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2021, 23, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Bauersachs, J.; Bengel, F.; Büchel, R.; Kindermann, I.; Klingel, K.; Knebel, F.; Meder, B.; Morbach, C.; Nagel, E.; et al. Diagnosis and Treatment of Cardiac Amyloidosis: Position Statement of the German Cardiac Society (DGK). Clin. Res. Cardiol. 2021, 110, 479–506. [Google Scholar] [CrossRef]
- Abdelmoneim, S.S.; Bernier, M.; Bellavia, D.; Syed, I.S.; Mankad, S.V.; Chandrasekaran, K.; Pellikka, P.A.; Mulvagh, S.L. Myocardial Contrast Echocardiography in Biopsy-Proven Primary Cardiac Amyloidosis. Eur. J. Echocardiogr. 2008, 9, 338–341. [Google Scholar] [CrossRef][Green Version]
- Nam, M.C.Y.; Nel, K.; Senior, R.; Greaves, K. Abnormal Myocardial Blood Flow Reserve Observed in Cardiac Amyloidosis. J. Cardiovasc. Ultrasound 2016, 24, 64–67. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Eiskjær, H.; Mølgaard, H.; Larsen, A.H.; Soerensen, J.; Andersen, N.F.; Tolbod, L.P.; Harms, H.J.; Poulsen, S.H. Abnormal Coronary Flow Velocity Reserve and Decreased Myocardial Contractile Reserve Are Main Factors in Relation to Physical Exercise Capacity in Cardiac Amyloidosis. J. Am. Soc. Echocardiogr. 2018, 31, 71–78. [Google Scholar] [CrossRef]
- Soh, R.Y.H.; Soo, W.M.; Wong, R.C.C. A Unique Pitfall of Myocardial Perfusion Imaging in Transthyretin Amyloid Cardiomyopathy. J. Clin. Images Med. Case Rep. 2022, 3, 2017. [Google Scholar] [CrossRef]
- Suenaga, H.; Fukushima, K.; Ishii, S.; Hasegawa, O.; Muto, Y.; Yamakuni, R.; Sugawara, S.; Sekino, H.; Sato, A.; Oikawa, M.; et al. Global and Regional Reduction of Myocardial Perfusion in Patients with Transthyretin Type of Cardiac Amyloidosis: A Dual SPECT Study Using 99mTc Pyrophosphate and 201Thallium. Ann. Nucl. Cardiol. 2024, 10, 16–22. [Google Scholar] [CrossRef]
- Deux, J.-F.; Nouri, R.; Tacher, V.; Zaroui, A.; Derbel, H.; Sifaoui, I.; Chevance, V.; Ridouani, F.; Galat, A.; Kharoubi, M.; et al. Diagnostic Value of Extracellular Volume Quantification and Myocardial Perfusion Analysis at CT in Cardiac Amyloidosis. Radiology 2021, 300, 326–335. [Google Scholar] [CrossRef]
- Dorbala, S.; Vangala, D.; Bruyere, J.; Quarta, C.; Kruger, J.; Padera, R.; Foster, C.; Hanley, M.; Di Carli, M.F.; Falk, R. Coronary Microvascular Dysfunction Is Related to Abnormalities in Myocardial Structure and Function in Cardiac Amyloidosis. JACC Heart Fail. 2014, 2, 358–367. [Google Scholar] [CrossRef]
- Harms, H.J.; Clemmensen, T.; Rosengren, S.; Tolbod, L.; Pilebro, B.; Wikström, G.; Granstam, S.-O.; Kero, T.; Di Carli, M.; Poulsen, S.H.; et al. Association of Right Ventricular Myocardial Blood Flow with Pulmonary Pressures and Outcome in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2023, 16, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Zumbo, G.; Knight, D.S.; Kotecha, T.; Francis, R.; Hutt, D.F.; Rezk, T.; et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2017, 70, 466–477. [Google Scholar] [CrossRef]
- Corrado, D.; Zorzi, A.; Cipriani, A.; Bauce, B.; Bariani, R.; Beffagna, G.; De Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; et al. Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e021987. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Anastasakis, A.; Basso, C.; Bauce, B.; Blomström-Lundqvist, C.; Bucciarelli-Ducci, C.; Cipriani, A.; De Asmundis, C.; Gandjbakhch, E.; Jiménez-Jáimez, J.; et al. Proposed Diagnostic Criteria for Arrhythmogenic Cardiomyopathy: European Task Force Consensus Report. Int. J. Cardiol. 2024, 395, 131447. [Google Scholar] [CrossRef]
- Bauer, B.S.; Li, A.; Bradfield, J.S. Arrhythmogenic Inflammatory Cardiomyopathy: A Review. Arrhythm. Electrophysiol. Rev. 2018, 7, 181–186. [Google Scholar] [CrossRef]
- Bassetto, G.; Angriman, F.; detto Gava, C.P.L.; Paldino, A.; Perotto, M.; Bordignon, L.; Gigli, M.; Ferro, M.D.; Massa, L.; Altinier, A.; et al. Hot Phases Cardiomyopathy: Pathophysiology, Diagnostic Challenges, and Emerging Therapies. Curr. Cardiol. Rep. 2025, 27, 11. [Google Scholar] [CrossRef]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef]
- Tomberli, B.; Cecchi, F.; Sciagrà, R.; Berti, V.; Lisi, F.; Torricelli, F.; Morrone, A.; Castelli, G.; Yacoub, M.H.; Olivotto, I. Coronary Microvascular Dysfunction Is an Early Feature of Cardiac Involvement in Patients with Anderson-Fabry Disease. Eur. J. Heart Fail. 2013, 15, 1363–1373. [Google Scholar] [CrossRef]
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Cooper, J.A.; Manisty, C.; Bhuva, A.N.; et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence-Based Approach Using Perfusion Mapping. Circulation 2020, 141, 1282–1291. [Google Scholar] [CrossRef]
- Barison, A.; Baritussio, A.; Cipriani, A.; De Lazzari, M.; Aquaro, G.D.; Guaricci, A.I.; Pica, S.; Pontone, G.; Todiere, G.; Indolfi, C.; et al. Cardiovascular Magnetic Resonance: What Clinicians Should Know about Safety and Contraindications. Int. J. Cardiol. 2021, 331, 322–328. [Google Scholar] [CrossRef]
- Barison, A.; Ricci, F.; Pavon, A.G.; Muscogiuri, G.; Bisaccia, G.; Camastra, G.; De Lazzari, M.; Lanzillo, C.; Raguso, M.; Monti, L.; et al. Cardiovascular Magnetic Resonance in Patients with Cardiac Electronic Devices: Evidence from a Multicenter Study. J. Clin. Med. 2023, 12, 6673. [Google Scholar] [CrossRef] [PubMed]
- Woolen, S.A.; Shankar, P.R.; Gagnier, J.J.; MacEachern, M.P.; Singer, L.; Davenport, M.S. Risk of Nephrogenic Systemic Fibrosis in Patients with Stage 4 or 5 Chronic Kidney Disease Receiving a Group II Gadolinium-Based Contrast Agent: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2020, 180, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, N.; Amico, M.A.; Guaricci, A.I.; Carella, M.C.; Maestrini, V.; Monosilio, S.; Pedrotti, P.; Ricci, F.; Monti, L.; Figliozzi, S.; et al. CMR Mapping: The 4th-Era Revolution in Cardiac Imaging. J. Clin. Med. 2024, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Karamitsos, T.D. Oxygenation-Sensitive Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2013, 15, 43. [Google Scholar] [CrossRef]
- Goykhman, P.; Mehta, P.K.; Agarwal, M.; Shufelt, C.; Slomka, P.J.; Yang, Y.; Xu, Y.; Shaw, L.J.; Berman, D.S.; Merz, N.B.; et al. Reproducibility of Myocardial Perfusion Reserve—Variations in Measurements from Post Processing Using Commercially Available Software. Cardiovasc. Diagn. Ther. 2012, 2, 268–277. [Google Scholar] [CrossRef]
- Kero, T.; Johansson, E.; Engström, M.; Eggers, K.M.; Johansson, L.; Ahlström, H.; Lubberink, M. Evaluation of Quantitative CMR Perfusion Imaging by Comparison with Simultaneous 15O-Water-PET. J. Nucl. Cardiol. 2021, 28, 1252–1266. [Google Scholar] [CrossRef]
- Brown, L.A.E.; Onciul, S.C.; Broadbent, D.A.; Johnson, K.; Fent, G.J.; Foley, J.R.J.; Garg, P.; Chew, P.G.; Knott, K.; Dall’Armellina, E.; et al. Fully Automated, Inline Quantification of Myocardial Blood Flow with Cardiovascular Magnetic Resonance: Repeatability of Measurements in Healthy Subjects. J. Cardiovasc. Magn. Reson. 2018, 20, 48. [Google Scholar] [CrossRef]
- Vitanis, V.; Manka, R.; Boesiger, P.; Pedersen, H.; Kozerke, S. High Resolution 3D Cardiac Perfusion Imaging Using Compartment Based K-t PCA. J. Cardiovasc. Magn. Reson. 2010, 12, P107. [Google Scholar] [CrossRef]
- Otazo, R.; Kim, D.; Axel, L.; Sodickson, D.K. Combination of Compressed Sensing and Parallel Imaging for Highly Accelerated First-Pass Cardiac Perfusion MRI. Magn. Reson. Med. 2010, 64, 767–776. [Google Scholar] [CrossRef]
- Likhite, D.; Adluru, G.; Hu, N.; McGann, C.; DiBella, E. Quantification of Myocardial Perfusion with Self-Gated Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2015, 17, 14. [Google Scholar] [CrossRef]
- Cruz, G.; Hua, A.; Munoz, C.; Ismail, T.F.; Chiribiri, A.; Botnar, R.M.; Prieto, C. Low-Rank Motion Correction for Accelerated Free-Breathing First-Pass Myocardial Perfusion Imaging. Magn. Reson. Med. 2023, 90, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Manka, R.; Wissmann, L.; Gebker, R.; Jogiya, R.; Motwani, M.; Frick, M.; Reinartz, S.; Schnackenburg, B.; Niemann, M.; Gotschy, A.; et al. Multicenter Evaluation of Dynamic Three-Dimensional Magnetic Resonance Myocardial Perfusion Imaging for the Detection of Coronary Artery Disease Defined by Fractional Flow Reserve. Circ. Cardiovasc. Imaging 2015, 8, e003061. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Grimm, R.; Block, K.T.; Chandarana, H.; Kim, S.; Xu, J.; Axel, L.; Sodickson, D.K.; Otazo, R. Golden-Angle Radial Sparse Parallel MRI: Combination of Compressed Sensing, Parallel Imaging, and Golden-Angle Radial Sampling for Fast and Flexible Dynamic Volumetric MRI. Magn. Reson. Med. 2014, 72, 707–717. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Frijia, F.; Positano, V.; Menichetti, L.; Santarelli, M.F.; Ardenkjaer-Larsen, J.H.; Wiesinger, F.; Lionetti, V.; Romano, S.L.; Bianchi, G.; et al. 3D CMR Mapping of Metabolism by Hyperpolarized 13C-Pyruvate in Ischemia-Reperfusion. JACC Cardiovasc. Imaging 2013, 6, 743–744. [Google Scholar] [CrossRef]
- Flögel, U.; Ding, Z.; Hardung, H.; Jander, S.; Reichmann, G.; Jacoby, C.; Schubert, R.; Schrader, J. In Vivo Monitoring of Inflammation after Cardiac and Cerebral Ischemia by Fluorine Magnetic Resonance Imaging. Circulation 2008, 118, 140–148. [Google Scholar] [CrossRef]
- Giovannetti, G.; Flori, A.; Santarelli, M.F.; Positano, V.; Martini, N.; Francischello, R.; Schulte, R.F.; Ardenkjaer-Larsen, J.H.; Menichetti, L.; Aquaro, G.D.; et al. Radio Frequency Coils for Hyperpolarized 13C Magnetic Resonance Experiments with a 3T MR Clinical Scanner: Experience from a Cardiovascular Lab. Electronics 2021, 10, 366. [Google Scholar] [CrossRef]
- Meng, Y.; Mo, Z.; Hao, J.; Peng, Y.; Yan, H.; Mu, J.; Ma, D.; Zhang, X.; Li, Y. High-Resolution Intravascular Magnetic Resonance Imaging of the Coronary Artery Wall at 3.0 Tesla: Toward Evaluation of Atherosclerotic Plaque Vulnerability. Quant. Imaging Med. Surg. 2021, 11, 4522–4529. [Google Scholar] [CrossRef]
- Gosling, R.C.; Williams, G.; Al Baraikan, A.; Alabed, S.; Levelt, E.; Chowdhary, A.; Swoboda, P.P.; Halliday, I.; Hose, D.R.; Gunn, J.P.; et al. Quantifying Myocardial Blood Flow and Resistance Using 4D-Flow Cardiac Magnetic Resonance Imaging. Cardiol. Res. Pract. 2023, 2023, 3875924. [Google Scholar] [CrossRef]
- Scannell, C.M.; Chiribiri, A.; Leiner, T. Chapter 12—Artificial Intelligence: The next Frontier of Perfusion Imaging? In Advances in Magnetic Resonance Technology and Applications; Cheng, H.-L.M., Strijkers, G.J., Eds.; Quantitative Perfusion MRI; Academic Press: Cambridge, MA, USA, 2023; Volume 11, pp. 291–311. [Google Scholar] [CrossRef]
- Wang, J.; Salerno, M. Deep Learning-Based Rapid Image Reconstruction and Motion Correction for High-Resolution Cartesian First-Pass Myocardial Perfusion Imaging at 3T. Magn. Reson. Med. 2024, 92, 1104–1114. [Google Scholar] [CrossRef]
| TTE | CMR | SPECT/PET | CT/PC-CT | |
|---|---|---|---|---|
| Technical characteristics | ||||
| Availability | +++ | + | ++/− | ++/− |
| Spatial resolution (mm) | 0.5–1 | 1–2 | 4–8/5–15 | 0.5/0.1–0.2 |
| Temporal resolution (ms) | <10 | 20–50 | 100–300 | 80–135/50–100 |
| Radiation exposure | − | − | +++/++ | +++/++ |
| Cost | € | €€€ | €€/€€€ | €€/€€€ |
| Cardiac morphology and function | ||||
| Chamber volume | ++ | +++ | + | ++/+++ |
| Wall thickness | ++ | +++ | − | ++/+++ |
| Systolic function | ++ | +++ | + | +/++ |
| Diastolic function | +++ | ++ | + | +/++ |
| Myocardial tissue characterization | ||||
| Inflammation | − | +++ | +++ | − |
| Fibrosis | + | +++ | + | +/++ |
| Amyloidosis | + | +++ | +++ | +/++ |
| Metabolism | − | ++ (MRS) | +++ (FDG-PET) | − |
| Oxygenation | − | ++ (OS-CMR) | − | − |
| Myocardial perfusion and coronary artery disease | ||||
| Coronary anatomy | +/− (Coronary origin) | + (Coronary origin) | − | +++ |
| Coronary flow (noncontrast) | ++ (Doppler in LAD) | + (CS phase contrast) | − | − |
| Contrast for perfusion | Microbubbles | Gadolinium | 99mTc, 201Tl/ 82Rb, 13N-NH3, 15O-H2O, 18F-flurpiridaz | Iodine |
| Contrast safety limitations | Headache (rare) Paresthesia (rare) Nausea (rare) Allergy (very rare) | Nausea (rare) Allergy (very rare) Nefrogenic sclerosis (exceptional, only few patients with GFR < 30 mL/min exposed to old linear chelates) | Radioactivity | Nausea (rare) Allergy (rare) Thyreotoxicosis (rare) Renal failure (rare, only in patients with GFR <30 mL/min) |
| Qualitative perfusion | + | +++ | ++/+++ | ++ |
| Semiquantitative perfusion | + | ++ | ++/+++ | + |
| Quantitative perfusion | − | +++ | +/+++ | ++ |
| Microvascular assessment | + | ++ | ++ | + |
| Cycloergometer stress | +++ | − (experimental) | +++ | − (experimental) |
| Pharmacological stress | Vasodilator (dipyridamole, adenosine, regadenoson), dobutamine in selected cases | |||
| Feasibility in patients with | ||||
| Severe renal failure | +++ | + | +++ | − |
| Arrhythmias | +++ | + | ++ | +/++ |
| Haemodynamic instability | +++ | − | − | ++ |
| Pacemaker/defibrillator | +++ | + | +++ | ++/+++ |
| Claustrophobia | +++ | + | +++ | ++ |
| COPD | + | +++ | +++ | +++ |
| Obesity | + | ++ | ++ | ++/+++ |
| Pregnancy | +++ | ++ | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharka, I.; Panichella, G.; Grigoratos, C.; Muca, M.; De Gori, C.; Keilberg, P.; Novani, G.; Barra, V.; Hlavata, H.; Bianchi, M.; et al. Myocardial Perfusion Imaging with Cardiovascular Magnetic Resonance in Nonischemic Cardiomyopathies: An In-Depth Review of Techniques and Clinical Applications. Medicina 2025, 61, 875. https://doi.org/10.3390/medicina61050875
Sharka I, Panichella G, Grigoratos C, Muca M, De Gori C, Keilberg P, Novani G, Barra V, Hlavata H, Bianchi M, et al. Myocardial Perfusion Imaging with Cardiovascular Magnetic Resonance in Nonischemic Cardiomyopathies: An In-Depth Review of Techniques and Clinical Applications. Medicina. 2025; 61(5):875. https://doi.org/10.3390/medicina61050875
Chicago/Turabian StyleSharka, Ilir, Giorgia Panichella, Chrysanthos Grigoratos, Matilda Muca, Carmelo De Gori, Petra Keilberg, Giovanni Novani, Valerio Barra, Hana Hlavata, Matteo Bianchi, and et al. 2025. "Myocardial Perfusion Imaging with Cardiovascular Magnetic Resonance in Nonischemic Cardiomyopathies: An In-Depth Review of Techniques and Clinical Applications" Medicina 61, no. 5: 875. https://doi.org/10.3390/medicina61050875
APA StyleSharka, I., Panichella, G., Grigoratos, C., Muca, M., De Gori, C., Keilberg, P., Novani, G., Barra, V., Hlavata, H., Bianchi, M., Zai, D. S., Frijia, F., Clemente, A., Todiere, G., & Barison, A. (2025). Myocardial Perfusion Imaging with Cardiovascular Magnetic Resonance in Nonischemic Cardiomyopathies: An In-Depth Review of Techniques and Clinical Applications. Medicina, 61(5), 875. https://doi.org/10.3390/medicina61050875







