Comparison of Rectal and Gastrointestinal Core Temperatures During Heat Tolerance Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Design
2.2. Procedure
2.2.1. Gastrointestinal Core Temperature Pills
2.2.2. Baseline Assessments
2.2.3. Heat Tolerance Test
2.2.4. Post-Exercise Assessments
2.3. Statistical Analyses
3. Results
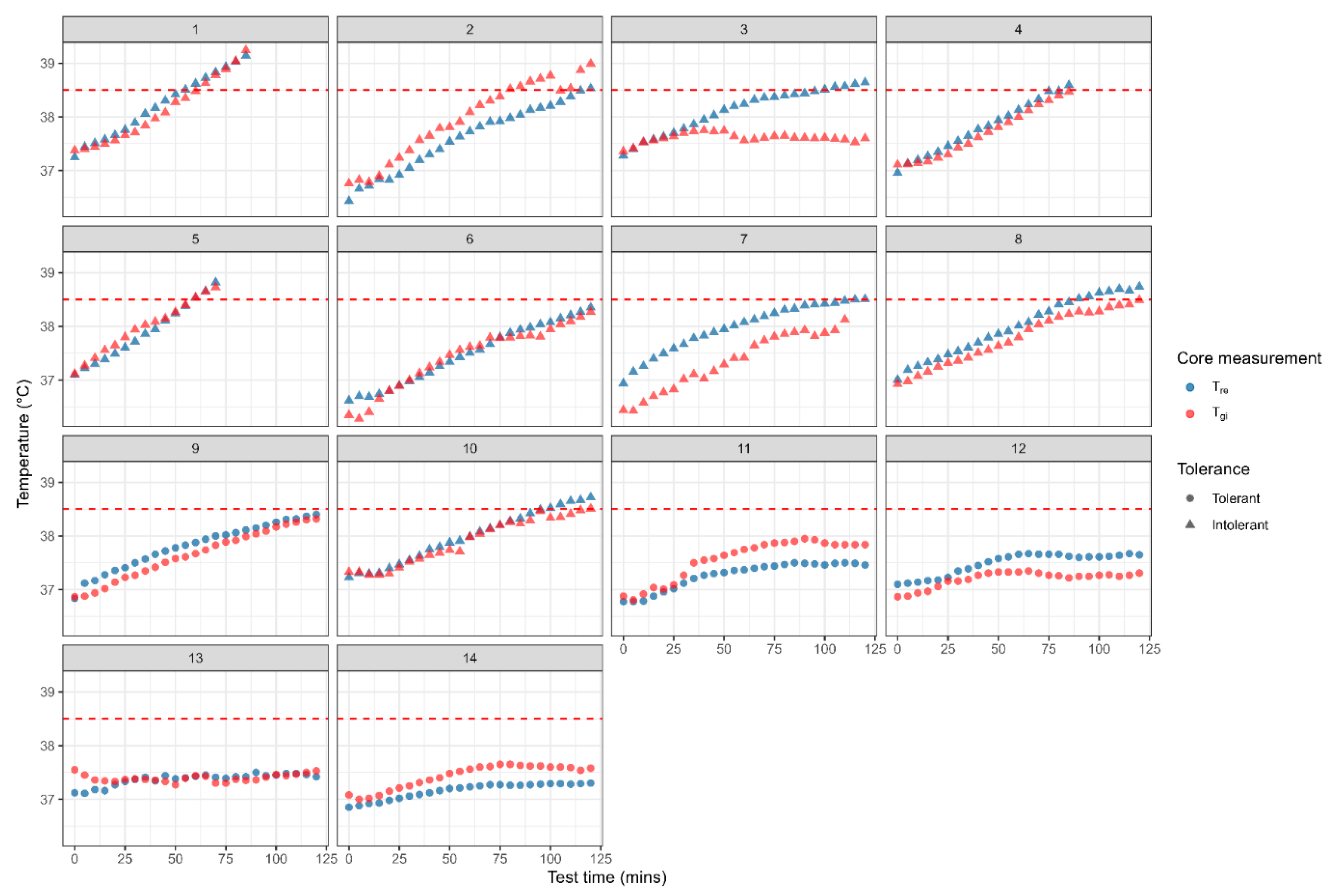
3.1. Heat Tolerance Test Outcomes Based on Rectal and Gastrointestinal Core Temperatures
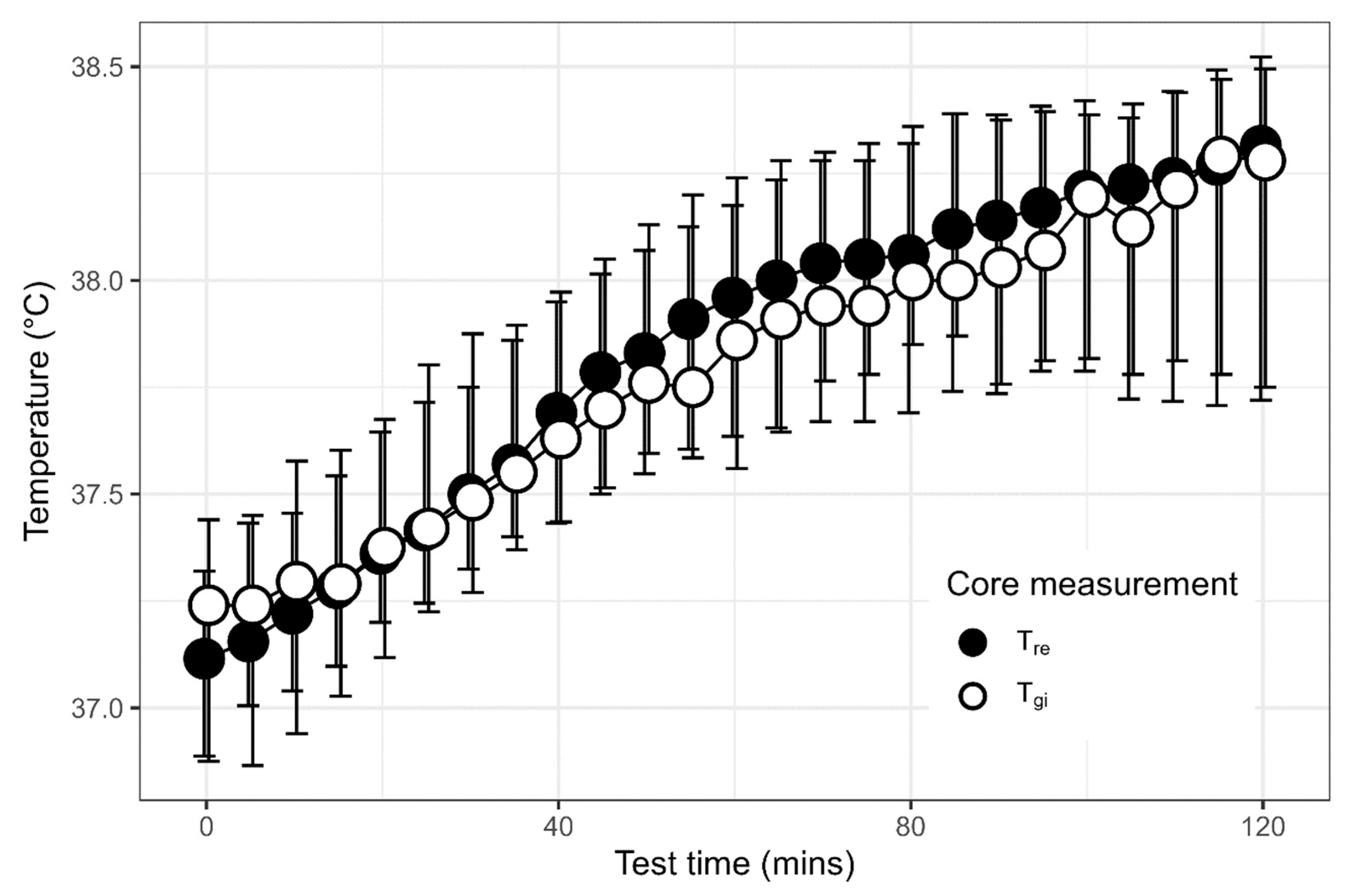
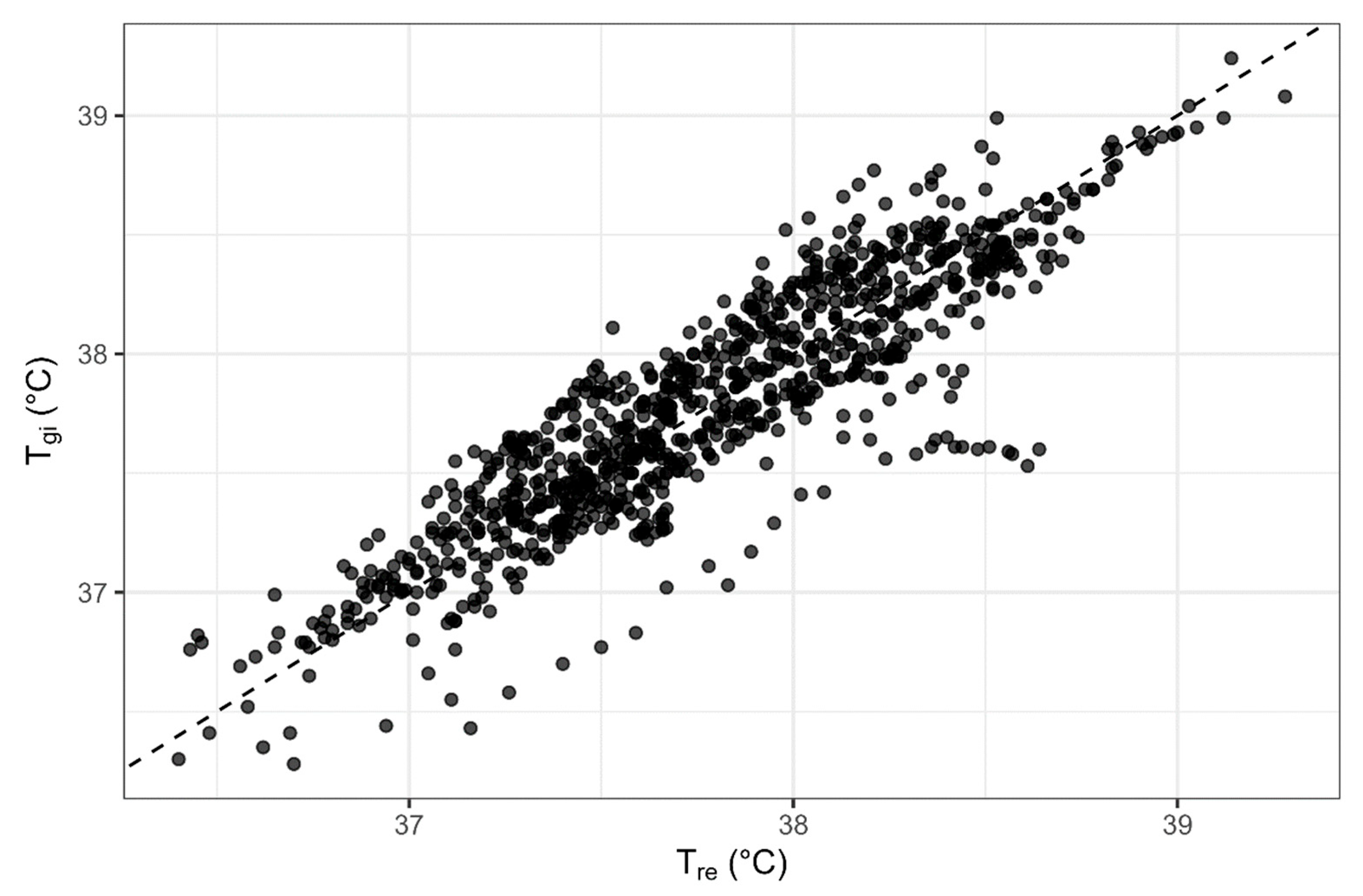
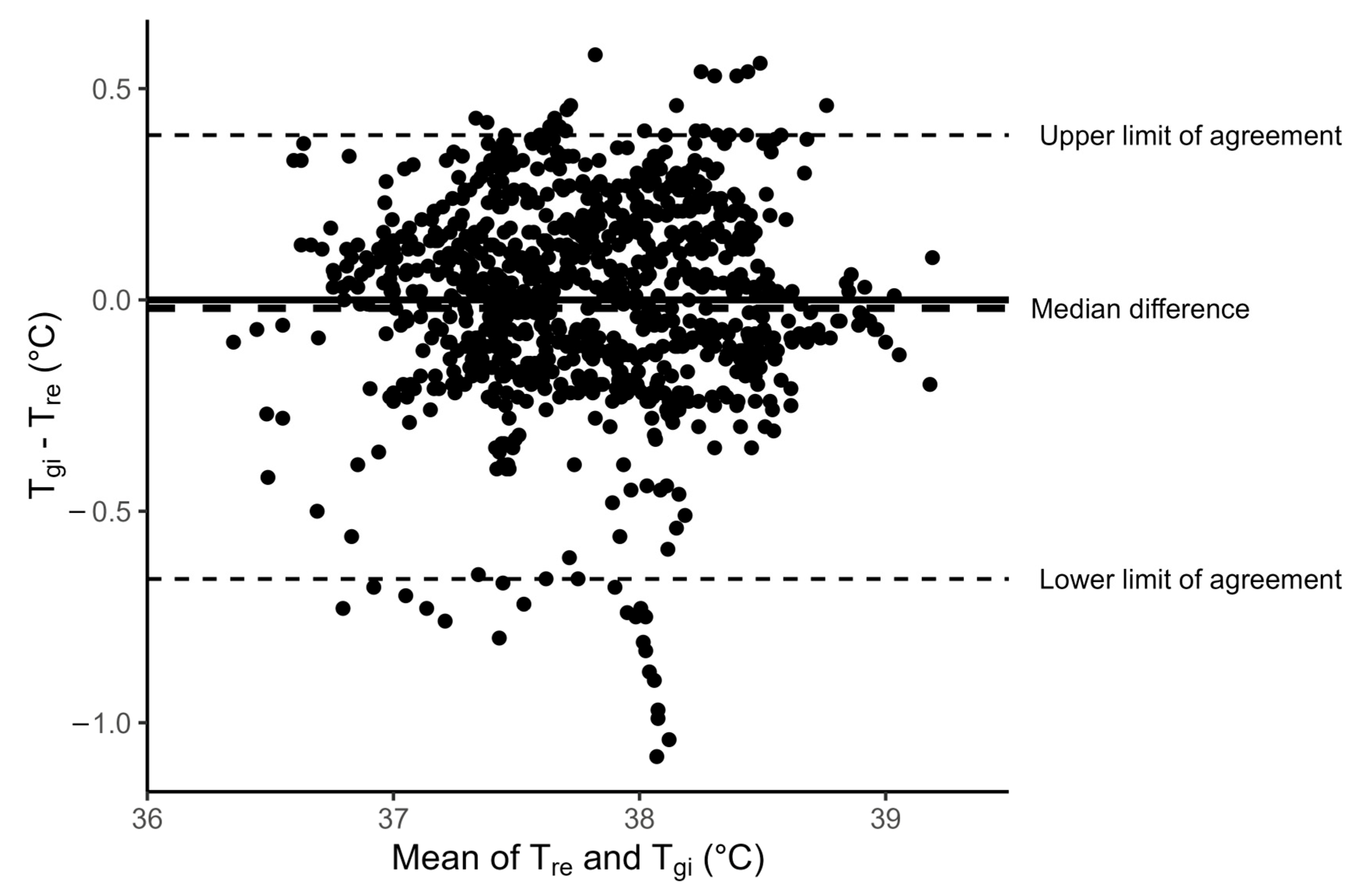
3.2. Comparison of Rectal and Gastrointestinal Temperatures
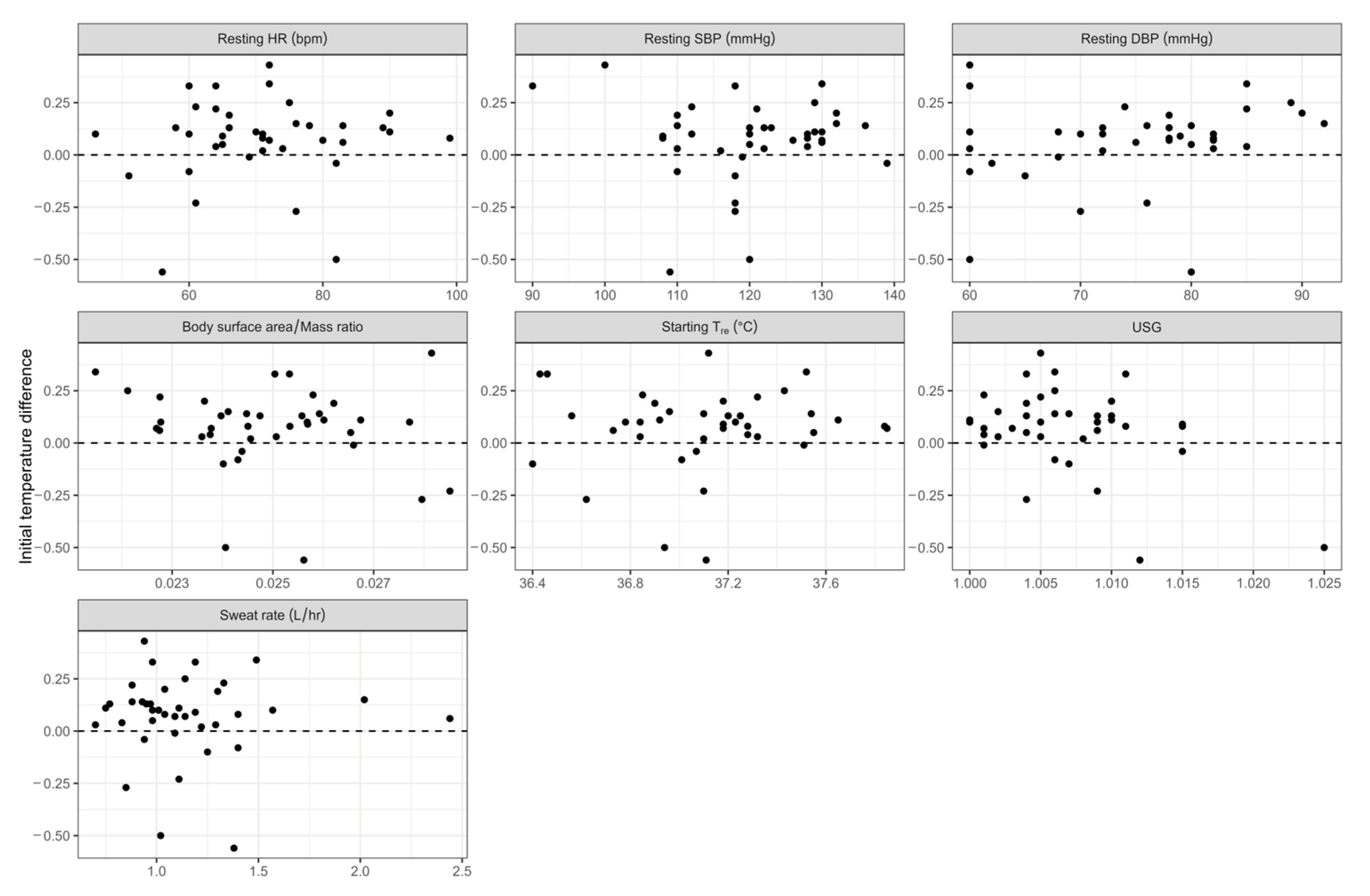
3.3. Influence of Covariates on Difference Between Rectal and Gastrointesinal Temperatures
3.4. Comparison of Covariates Between Heat-Tolerant and Heat-Intolerant Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
| ADF | Australian Defence Force |
| BP | Blood pressure |
| BSA | Body surface area |
| DBP | Diastolic blood pressure |
| EHS | Exertional heat stroke |
| HR | Heart rate |
| HTT | Heat tolerance test |
| IDF | Israeli Defence Force |
| IQR | Interquartile range |
| LoA | Limits of agreement |
| SBP | Systolic blood pressure |
| SR | Sweat rate |
| Tgi | Gastrointestinal core temperature |
| Tre | Rectal core temperature |
| USG | Urine specific gravity |
| VO2max | Maximal oxygen consumption |
References
- Periard, J.D.; Eijsvogels, T.M.H.; Daanen, H.A.M. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef] [PubMed]
- World Meteorological Organization. State of the Global Climate. 2023. Available online: https://wmo.int/publication-series/state-of-global-climate-2023 (accessed on 2 December 2024).
- Lalli, K.; Charkoudian, N.; Moreh, Y.; Degroot, D.W. The role of motivation to excel in the etiology of exertional heat stroke. J. Spec. Oper. Med. 2024, 24, 28–33. [Google Scholar] [CrossRef]
- Alele, F.O.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O.; Crowe, M.J. Epidemiology of exertional heat illness in the military: A systematic review of observational studies. Int. J. Environ. Res. Public Health 2020, 17, 7037. [Google Scholar] [CrossRef]
- Bouchama, A.; Knochel, J.P. Heat stroke. N. Engl. J. Med. 2002, 346, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J. Don’t gamble: Complex considerations for return to play following exertional heat stroke. J. Sport Rehabil. 2007, 16, 161–162. [Google Scholar] [CrossRef]
- O’Connor, F.G.; Williams, A.D.; Blivin, S.; Heled, Y.; Deuster, P.; Flinn, S.D. Guidelines for return to duty (play) after heat illness: A military perspective. J. Sport Rehabil. 2007, 16, 227–237. [Google Scholar] [CrossRef]
- Koo, C.J.; Hintz, C.; Butler, C.R. Return to duty following exertional heat stroke: A review. Mil. Med. 2024, 189, e1312–e1317. [Google Scholar] [CrossRef]
- Epstein, Y. Heat intolerance: Predisposing factor or residual injury? Med. Sci. Sports Exerc. 1990, 22, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.S.; Heled, Y.; Still, L.; Laor, A.; Shapiro, Y. Assessment of heat tolerance for post exertional heat stroke individuals. Med. Sci. Monit. 2004, 10, Cr252–Cr257. [Google Scholar]
- Moran, D.S.; Erlich, T.; Epstein, Y. The heat tolerance test: An efficient screening tool for evaluating susceptibility to heat. J. Sport Rehabil. 2007, 16, 215–221. [Google Scholar] [CrossRef]
- Druyan, A.; Ketko, I.; Yanovich, R.; Epstein, Y.; Heled, Y. Refining the distinction between heat tolerant and intolerant individuals during a heat tolerance test. J. Therm. Biol. 2013, 38, 539–542. [Google Scholar] [CrossRef]
- Gagnon, D.; Lemire, B.B.; Jay, O.; Kenny, G.P. Aural canal, esophageal, and rectal temperatures during exertional heat stress and the subsequent recovery period. J. Athl. Train. 2010, 45, 157–163. [Google Scholar] [CrossRef]
- Moran, D.S.; Mendal, L. Core temperature measurement: Methods and current insights. Sports Med. 2002, 32, 879–885. [Google Scholar] [CrossRef]
- Teunissen, L.P.J. Measurement and Manipulation of Body Temperature in Rest and Exercise. Ph.D. Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 2012. [Google Scholar]
- O’Brien, C.; Hoyt, R.W.; Buller, M.J.; Castellani, J.W.; Young, A.J. Telemetry pill measurement of core temperature in humans during active heating and cooling. Med. Sci. Sports Exerc. 1998, 30, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Lim, C.L. The ingestible telemetric body core temperature sensor: A review of validity and exercise applications. Br. J. Sports Med. 2007, 41, 126–133. [Google Scholar] [CrossRef]
- Casa, D.J.; Becker, S.M.; Ganio, M.S.; Brown, C.M.; Yeargin, S.W.; Roti, M.W.; Siegler, J.; Blowers, J.A.; Glaviano, N.R.; Huggins, R.A.; et al. Validity of devices that assess body temperature during outdoor exercise in the heat. J. Athl. Train. 2007, 42, 333–342. [Google Scholar] [PubMed]
- Gant, N.; Atkinson, G.; Williams, C. The validity and reliability of intestinal temperature during intermittent running. Med. Sci. Sports Exerc. 2006, 38, 1926–1931. [Google Scholar] [CrossRef]
- Ganio, M.S.; Brown, C.M.; Casa, D.J.; Becker, S.M.; Yeargin, S.W.; McDermott, B.P.; Boots, L.M.; Boyd, P.W.; Armstrong, L.E.; Maresh, C.M. Validity and reliability of devices that assess body temperature during indoor exercise in the heat. J. Athl. Train. 2009, 44, 124–135. [Google Scholar] [CrossRef]
- Mundel, T.; Carter, J.M.; Wilkinson, D.M.; Jones, D.A. A comparison of rectal, oesophageal and gastro-intestinal tract temperatures during moderate-intensity cycling in temperate and hot conditions. Clin. Physiol. Funct. Imaging 2016, 36, 11–16. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Adams, W.M.; Stearns, R.L.; Casa, D.J. Comparison of Gastrointestinal and Rectal Temperatures During Recovery After a Warm-Weather Road Race. J. Athl. Train. 2016, 51, 382–388. [Google Scholar] [CrossRef]
- Surgeon General Australian Defence Force. HD 286: Health Management for the Prevention and Treatment of Heat Casualties; Australian Defence Force, Ed.; Surgeon General Australian Defence Force: Canberra, Australia, 2008. [Google Scholar]
- Alele, F.O.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O.; Crowe, M.J. Individual anthropometric, aerobic capacity and demographic characteristics as predictors of heat intolerance in military populations. Medicina 2021, 57, 173. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.M.; Salgado, R.M.; Bradbury, K.E.; Charkoudian, N.; Kenefick, R.W.; Cheuvront, S.N. Heat acclimation improves heat tolerance test specificity in a criteria-dependent manner. Med. Sci. Sports Exerc. 2021, 53, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef]
- Mitchell, K.M.; Cheuvront, S.N.; King, M.A.; Mayer, T.A.; Leon, L.R.; Kenefick, R.W. Use of the heat tolerance test to assess recovery from exertional heat stroke. Temperature 2019, 6, 106–119. [Google Scholar] [CrossRef]
- Epstein, Y.; Yanovich, R.; Heled, Y. Heat tolerance test or race simulation test for return to activity after heat stroke. Med. Sci. Sports Exerc. 2016, 48, 1428. [Google Scholar] [CrossRef]
- Butler, C.; Dierickx, E.; Bruneau, M.; Stearns, R.; Casa, D.J. Current clinical concepts: Heat tolerance testing. J. Athl. Train. 2023, 58, 84–90. [Google Scholar] [CrossRef]
- Johnson, E.C.; Kolkhorst, F.W.; Richburg, A.; Schmitz, A.; Martinez, J.; Armstrong, L.E. Specific exercise heat stress protocol for a triathlete’s return from exertional heat stroke. Curr. Sports Med. Rep. 2013, 12, 106–109. [Google Scholar] [CrossRef]
- Ketko, I.; Eliyahu, U.; Epstein, Y.; Heled, Y. The thermal-circulatory ratio (TCR): An index to evaluate the tolerance to heat. Temperature 2014, 1, 101–106. [Google Scholar] [CrossRef]
- Shapiro, Y.; Magazanik, A.; Udassin, R.; Ben-Baruch, G.; Shvartz, E.; Shoenfeld, Y. Heat intolerance in former heatstroke patients. Ann. Intern. Med. 1979, 90, 913–916. [Google Scholar] [CrossRef]
- Wilkinson, D.M.; Carter, J.M.; Richmond, V.L.; Blacker, S.D.; Rayson, M.P. The effect of cool water ingestion on gastrointestinal pill temperature. Med. Sci. Sports Exerc. 2008, 40, 523–528. [Google Scholar] [CrossRef]
- Easton, C.; Fudge, B.W.; Pitsiladis, Y.P. Rectal, telemetry pill and tympanic membrane thermometry during exercise heat stress. J. Therm. Biol. 2007, 32, 78–86. [Google Scholar] [CrossRef]
- Lee, S.M.C.; Williams, W.J.; Schneider, S.M. Core Temperature Measurement During Submaximal Exercise: Esophageal, Rectal, and Intestinal Temperatures; NASA: Houston, DC, USA, 2000.
- Ruddock, A.D.; Tew, G.A.; Purvis, A.J. Reliability of intestinal temperature using an ingestible telemetry pill system during exercise in a hot environment. J. Strength Cond. Res. 2014, 28, 861–869. [Google Scholar] [CrossRef]
- Darwent, D.; Zhou, X.; van den Heuvel, C.; Sargent, C.; Roach, G.D. The validity of temperature-sensitive ingestible capsules for measuring core body temperature in laboratory protocols. Chronobiol. Int. 2011, 28, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Keren, G.; Epstein, Y.; Magazanik, A. Temporary heat intolerance in a heatstroke patient. Aviat. Space Environ. Med. 1981, 52, 116–117. [Google Scholar] [PubMed]
- McDermott, B.P.; Casa, D.J.; Yeargin, S.W.; Ganio, M.S.; Armstrong, L.E.; Maresh, C.M. Recovery and return to activity following exertional heat stroke: Considerations for the sports medicine staff. J. Sport Rehabil. 2007, 16, 163–181. [Google Scholar] [CrossRef]
- Roberts, W.O.; Armstrong, L.E.; Sawka, M.N.; Yeargin, S.W.; Heled, Y.; O’Connor, F.G. ACSM Expert consensus statement on exertional heat illness: Recognition, management, and return to activity. Curr. Sports Med. Rep. 2021, 20, 470–484. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Stearns, R.L.; Casa, D.J. Is heat intolerance state or trait? Sports Med. 2019, 49, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, C.G.; Saltin, B. Cardiovascular adaptations to physical training. Annu. Rev. Physiol. 1983, 45, 169–189. [Google Scholar] [CrossRef]
- Reimers, A.K.; Knapp, G.; Reimers, C.D. Effects of Exercise on the resting heart rate: A systematic review and meta-analysis of interventional studies. J. Clin. Med. 2018, 7, 503. [Google Scholar] [CrossRef]
- Shvartz, E.; Shapiro, Y.; Magazanik, A.; Meroz, A.; Birnfeld, H.; Mechtinger, A.; Shibolet, S. Heat acclimation, physical fitness, and responses to exercise in temperate and hot environments. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 43, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Havenith, G.; Luttikholt, V.G.; Vrijkotte, T.G. The relative influence of body characteristics on humid heat stress response. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Lisman, P.; Kazman, J.B.; O’Connor, F.G.; Heled, Y.; Deuster, P.A. Heat tolerance testing: Association between heat intolerance and anthropometric and fitness measurements. Mil. Med. 2014, 179, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Maresh, C.M. The induction and decay of heat acclimatisation in trained athletes. Sports Med. 1991, 12, 302–312. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Reilly, T.; Cable, N.T. Thermoregulation. In Kinanthropometry and Exercise Physiology Laboratory Manual: Tests, Procedures and Data, 2nd ed.; Eston, R., Reilly, T., Eds.; Routledge: London, UK, 2001; pp. 193–210. [Google Scholar]
- Taylor, K.M.; Giersch, G.E.W.; Caldwell, A.R.; Epstein, Y.; Charkoudian, N. Relation of body surface area-to-mass ratio to risk of exertional heat stroke in healthy men and women. J. Appl. Physiol. 2024, 136, 549–554. [Google Scholar] [CrossRef]
- Akavian, I.; Epstein, Y.; Rabotin, A.; Peretz, S.; Charkoudian, N.; Ketko, I. The significance of body surface area to mass ratio for thermal responses to a standardized exercise-heat stress test. Med. Sci. Sports Exerc. 2025, 57, 88–93. [Google Scholar] [CrossRef]
- Rabotin, A.; Epstein, Y.; Ketko, I. Heat intolerance, body size, and return to duty. Aerosp. Med. Hum. Perform. 2023, 94, 546–549. [Google Scholar] [CrossRef] [PubMed]
- NCD-RisC. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Cawley, J.; Maclean, J.C. Unfit for service: The implications of rising obesity for US military recruitment. Health Econ. 2012, 21, 1348–1366. [Google Scholar] [CrossRef]
- Fox, R.H.; Goldsmith, R.; Hampton, I.F.; Hunt, T.J. Heat acclimatization by controlled hyperthermia in hot-dry and hot-wet climates. J. Appl. Physiol. 1967, 22, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Henane, R.; Valatx, J.L. Thermoregulatory changes induced during heat acclimatization by controlled hyperthermia in man. J. Physiol. 1973, 230, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Cubel, C.; Fischer, M.; Stampe, D.; Klaris, M.B.; Bruun, T.R.; Lundby, C.; Nordsborg, N.B.; Nybo, L. Time-course for onset and decay of physiological adaptations in endurance trained athletes undertaking prolonged heat acclimation training. Temperature 2024, 11, 350–362. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Levels of Physical Inactivity in Adults: Off Track for 2030; (Electronic Version); World Health Organization: Geneva, Switzerland, 2024; ISBN 9789240096905. [Google Scholar]

| Characteristic | Cases (n = 26) * | Controls (n = 14) | p-Value |
|---|---|---|---|
| Age (years) | 26.0 (21.3–33.0) | 27.7 (18.9–34.4) | 0.340 |
| Height (cm) | 178.0 (173.4–181.1) | 178.5 (170.7–181.4) | 0.888 |
| Weight (kg) | 78.0 (72.4–90.3) | 81.5 (65.6–86.8) | 0.461 |
| BSA/mass ratio | 0.0250 (0.0237–0.0257) | 0.0248 (0.0242–0.0272) | 0.307 |
| Heat-intolerant (n) # | 12 (46.2%) | 9 (64.3%) | 0.333 |
| Heat Tolerance Outcome by Tgi (Failed HTT by Tgi) | |||
|---|---|---|---|
| Heat tolerance outcome by Tre (Failed HTT by Tre) | False (Tolerant) | True (Intolerant) | |
| False (Tolerant) | 20 | 2 | |
| True (Intolerant) | 6 | 12 | |
| Covariate | Regression Coefficient | 95% Confidence Intervals | p-Value |
|---|---|---|---|
| Resting HR (bpm) | 0.00121 | −0.00462 to 0.00704 | 0.676 |
| Resting SBP (mmHg) | −0.000399 | −0.00683 to 0.00604 | 0.901 |
| Resting DBP (mmHg) | 0.00289 | −0.00406 to 0.00984 | 0.405 |
| BSA/mass ratio | −19.1 | −58.2 to 20.0 | 0.329 |
| Commencing core temperature (Tre; °C) | 0.0356 | −0.147 to 0.218 | 0.695 |
| Baseline USG # | −7.337 | −21.4 to 6.69 | 0.296 |
| Sweat rate (L/hr) | −0.00583 | −0.204 to 0.193 | 0.953 |
| Characteristic | Heat-Tolerant (n = 19) | Heat-Intolerant (n = 21) | p-Value |
|---|---|---|---|
| Resting HR (bpm) | 66.0 (60.5–73.0) | 75.0 (71.0–83.0) | 0.0154 |
| Resting SBP (mmHg) | 118.0 (110.0–121.0) | 128.0 (118.0–130.0) | 0.0465 |
| Resting DBP (mmHg) | 76.0 (68.0–79.5) | 78.0 (70.0–82.0) | 0.473 |
| BSA/mass ratio | 0.0257 (0.0249–0.0266) | 0.0241 (0.0228–0.0250) | 0.00133 |
| Commencing core temperature (Tre; °C) | 37.10 (36.85–37.18) | 37.25 (36.96–37.52) | 0.0830 |
| Final core temperature (Tre; °C) | 37.99 (37.65–38.26) | 38.59 (38.52–38.78) | <0.001 |
| Baseline USG # | 1.007 (1.004–1.010) | 1.006 (1.003–1.008) | 0.384 |
| Sweat rate (L/hr) | 1.04 (0.96–1.22) | 1.09 (0.88–1.40) | 0.626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowe, M.J.; Meehan, M.T.; Jones, R.E. Comparison of Rectal and Gastrointestinal Core Temperatures During Heat Tolerance Testing. Medicina 2025, 61, 1111. https://doi.org/10.3390/medicina61061111
Crowe MJ, Meehan MT, Jones RE. Comparison of Rectal and Gastrointestinal Core Temperatures During Heat Tolerance Testing. Medicina. 2025; 61(6):1111. https://doi.org/10.3390/medicina61061111
Chicago/Turabian StyleCrowe, Melissa J., Michael T. Meehan, and Rhondda E. Jones. 2025. "Comparison of Rectal and Gastrointestinal Core Temperatures During Heat Tolerance Testing" Medicina 61, no. 6: 1111. https://doi.org/10.3390/medicina61061111
APA StyleCrowe, M. J., Meehan, M. T., & Jones, R. E. (2025). Comparison of Rectal and Gastrointestinal Core Temperatures During Heat Tolerance Testing. Medicina, 61(6), 1111. https://doi.org/10.3390/medicina61061111






