Impact of Para-Aortic Lymphadenectomy on Clinically FIGO Stage IIIC1 High-Grade Endometrial Cancer: A Retrospective Cohort Study from Two Tertiary Centers in Korea and Taiwan
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Study Population
3.2. Analyses of Factors Affecting Survival in Different Subgroups
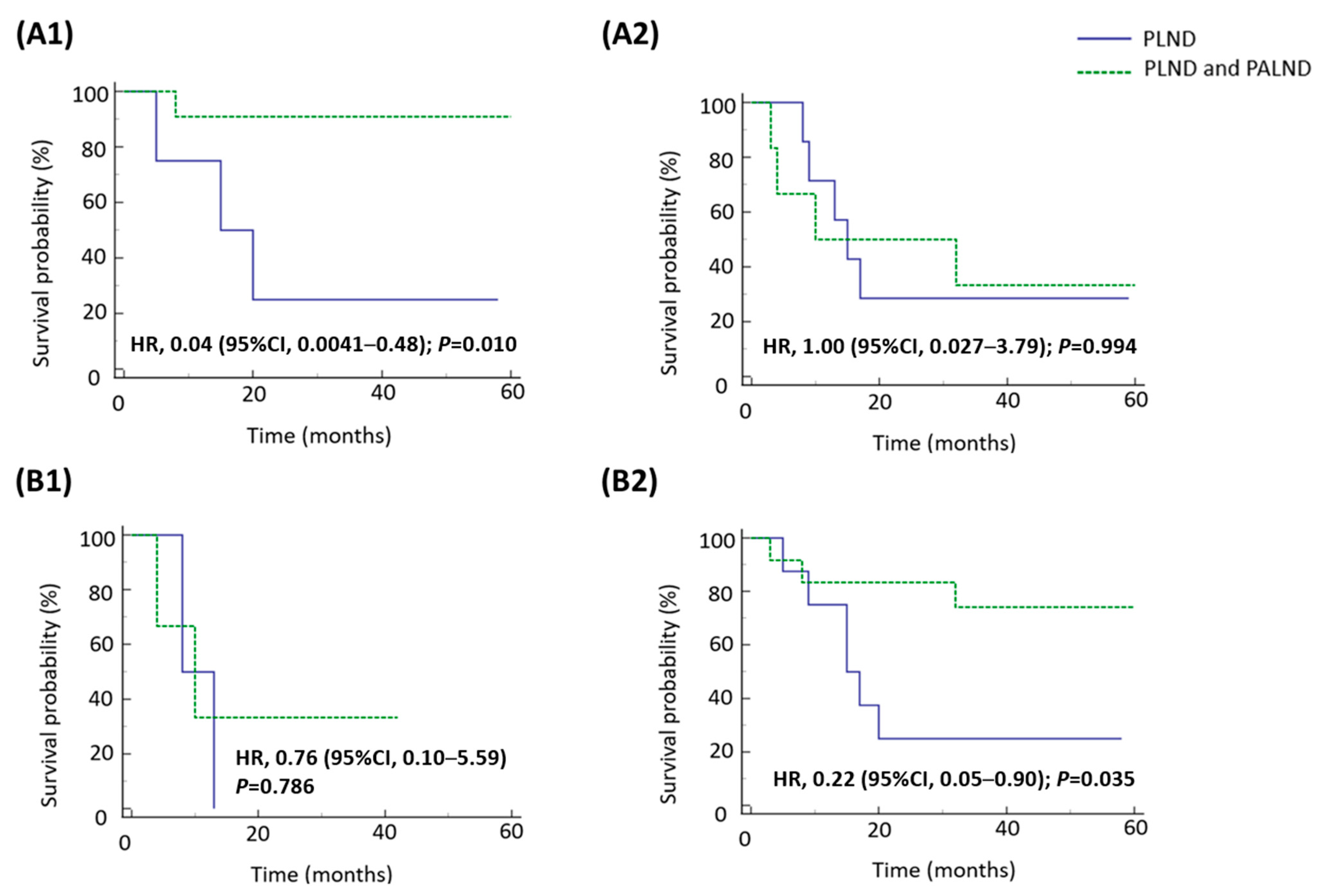
3.3. Survival Impact of Para-Aortic Lymphadenectomy on Patients with Pathologically Confirmed Nodal Involvement and Positive LVSI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FIGO | International Federation of Gynecology and Obstetrics |
| RFS | Recurrence-free survival |
| OS | Overall survival |
| HR | Hazard ratio |
| CI | Confidence interval |
| LVSI | Lymph-vascular space invasion |
| SMC | Samsung Medical Center |
| NTUH | National Taiwan University Hospital |
| MRI | Magnetic resonance imaging |
| CT | Computed tomography |
| BMI | Body mass index |
| CA-125 | Cancer antigen 125 |
| PLND | Pelvic lymphadenectomy |
| PALND | Para-aortic lymphadenectomy |
References
- Siegel, R.L.; Giaquinto, A.N. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Buras, A.L.; Mallen, A.; Wenham, R.; Montejo, M. Stage IIIC endometrial cancer review: Current controversies in adjuvant therapy. Gynecol. Oncol. Rep. 2021, 36, 100754. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Uterine Neoplasms (Version 1 2024); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Seagle, B.-L.L.; Kocherginsky, M.; Shahabi, S. Association of Pelvic and Para-Aortic Lymphadenectomy with Survival in Stage I Endometrioid Endometrial Cancer: Matched Cohort Analyses from the National Cancer Database. JCO Clin. Cancer Inform. 2017, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seagle, B.-L.L.; Gilchrist-Scott, D.; Graves, S.; Strohl, A.E.; Nieves-Neira, W.; Shahabi, S. Association of Lymph Node Count and Overall Survival in Node-Negative Endometrial Cancers. JCO Clin Cancer Inform. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Todo, Y.; Kato, H.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sakuragi, N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): A retrospective cohort analysis. Lancet 2010, 375, 1165–1172. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Chang, C.-S.; Chang, K.; Kim, H.-S.; Chen, J.; Cheng, W.-F.; Chen, Y.-L.; Lee, Y.-Y. Does para-aortic lymphadenectomy improve survival in pathologically diagnosed early-stage grade 3 endometrioid and non-endometrioid endometrial cancers? A retrospective cohort study in Korea and Taiwan. Gynecol. Oncol. 2022, 167, 65–72. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. Int. J. Gynaecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef]
- Yaegashi, N.; Ito, K.; Niikura, H. Lymphadenectomy for endometrial cancer: Is paraaortic lymphadenectomy necessary? Int. J. Clin. Oncol. 2007, 12, 176–180. [Google Scholar] [CrossRef]
- Frederick, P.J.; Straughn, J.M., Jr. The role of comprehensive surgical staging in patients with endometrial cancer. Cancer Control. 2009, 16, 23–29. [Google Scholar] [CrossRef]
- AlHilli, M.M.; Mariani, A. The role of para-aortic lymphadenectomy in endometrial cancer. Int. J. Clin. Oncol. 2013, 18, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; El-Balat, A.; du Bois, A.; Sehouli, J.; Harter, P.; Muallem, M.Z.; Krätschell, R.W.; Traut, A.; Heitz, F. Systematic pelvic and paraaortic lymphadenectomy in early high-risk or advanced endometrial cancer. Arch. Gynecol. Obstet. 2015, 292, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.R.; So, K.; Kim, T.J.; Lim, K.; Lee, K.H.; Kim, M.-K. Role of systematic lymphadenectomy in patients with intermediate to high-risk early stage endometrial cancer. J. Gynecol. Oncol. 2023, 34, e23. [Google Scholar] [CrossRef]
- Panici, P.B.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef]
- ASTEC Study Group; Kitchener, H.; Swart, A.M.C.; Qian, Q.; Amos, C.; Parmar, M.K.B. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar]
- Guntupalli, S.R.; Zighelboim, I.; Kizer, N.T.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Goodfellow, P.J.; Mutch, D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012, 124, 31–35. [Google Scholar] [CrossRef]
- Hahn, H.; Lee, I.; Kim, T.; Lee, K.; Shim, J.; Kim, J.; Lim, K. Lymphovascular space invasion is highly associated with lymph node metastasis and recurrence in endometrial cancer. Aust. N. Z. J. Obstet. Gynaecol. 2013, 53, 293–297. [Google Scholar] [CrossRef]
- Stålberg, K.; Bjurberg, M.; Borgfeldt, C.; Carlson, J.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Hjerpe, E.; Holmberg, E.; Kjølhede, P.; et al. Lymphovascular space invasion as a predictive factor for lymph node metastases and survival in endometrioid endometrial cancer - a Swedish Gynecologic Cancer Group (SweGCG) study. Acta Oncol. 2019, 58, 1628–1633. [Google Scholar] [CrossRef]
- Kang, S.; Kang, W.D.; Chung, H.H.; Jeong, D.H.; Seo, S.-S.; Lee, J.-M.; Lee, J.-K.; Kim, J.W.; Kim, S.-M.; Park, S.-Y.; et al. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: A Korean gynecologic oncology group study. J. Clin. Oncol. 2012, 30, 1329–1334. [Google Scholar] [CrossRef]
- Kang, S.; Nam, J.; Bae, D.; Kim, M.; Chen, X.; No, J.; Lee, J.; Kim, J.; Watari, H.; Kim, S.M.; et al. Preoperative assessment of lymph node metastasis in endometrial cancer: A Korean Gynecologic Oncology Group study. Cancer 2017, 123, 263–272. [Google Scholar] [CrossRef]
- Imai, K.; Kato, H.; Katayama, K.; Nakanishi, K.; Kawano, A.; Iura, A.; Konnai, K.; Onose, R.; Hirahara, F.; Miyagi, E. A preoperative risk-scoring system to predict lymph node metastasis in endometrial cancer and stratify patients for lymphadenectomy. Gynecol. Oncol. 2016, 142, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Okamoto, K.; Hayashi, M.; Minobe, S.; Nomura, E.; Hareyama, H.; Takeda, M.; Ebina, Y.; Watari, H.; Sakuragi, N. A validation study of a scoring system to estimate the risk of lymph node metastasis for patients with endometrial cancer for tailoring the indication of lymphadenectomy. Gynecol. Oncol. 2007, 104, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Watari, H.; Todo, Y.; Kato, T.; Konno, Y.; Hosaka, M.; Sakuragi, N. Lymphadenectomy can be omitted for low-risk endometrial cancer based on preoperative assessments. J. Gynecol. Oncol. 2014, 25, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Sakuragi, N.; Nishida, R.; Yamada, T.; Ebina, Y.; Yamamoto, R.; Fujimoto, S. Combined use of magnetic resonance imaging, CA 125 assay, histologic type, and histologic grade in the prediction of lymph node metastasis in endometrial carcinoma. Am. J. Obstet. Gynecol. 2003, 188, 1265–1272. [Google Scholar] [CrossRef]
- Aznar, A.L.; Bebia, V.; Gomez-Hidalgo, N.R.; López-Gil, C.; Miguez, M.; Colas, E.; Pérez-Benavente, A.; Gil-Moreno, A.; Cabrera, S. Molecular profile in endometrial carcinoma: Can we predict the lymph node status? A systematic review and meta-analysis. Clin. Transl. Oncol. 2024, 26, 1768–1778. [Google Scholar] [CrossRef]
- Jamieson, A.; Thompson, E.F.; Huvila, J.; Leung, S.; Lum, A.; Morin, C.; Ennour-Idrissi, K.; Sebastianelli, A.; Renaud, M.-C.; Gregoire, J.; et al. Endometrial carcinoma molecular subtype correlates with the presence of lymph node metastases. Gynecol. Oncol. 2022, 165, 376–384. [Google Scholar] [CrossRef]

| PLND (n = 18) | PLND and PALND (n = 38) | Total (n = 56) | p Value a | |
|---|---|---|---|---|
| Age (median, range) | 64.0 (33.0–78.0) | 58.0 (31.0–82.0) | 59.5 (31.0–82.0) | 0.642 |
| <60 years | 8 (44.4%) | 28 (73.7%) | 36 (64.3%) | |
| ≥60 years | 10 (55.6%) | 10 (26.3%) | 20 (35.7%) | |
| BMI (median, range) | 23.0 (20.4–29.7) | 25.7 (16.3–34.6) | 26.6 (16.3–34.6) | 0.313 |
| Pre-operative CA-125 (U/mL) (median, range) | 176.2 (4.8–449.4) | 41.3 (4.8–944.3) | 145.5 (4.8–944.3) | 0.563 |
| Surgical method | 0.296 | |||
| Laparotomy | 13 (72.2%) | 32 (84.2%) | 45 (80.4%) | |
| Laparoscopy b | 5 (27.8%) | 6 (15.8%) | 11 (19.6%) | |
| Histology | 0.257 c | |||
| Grade 3 endometrioid | 7 (38.9%) | 21 (55.3%) | 28 (50.0%) | |
| Non-endometrioid | 11 (61.1%) | 17 (44.7%) | 28 (50.0%) | |
| Serous | 4 (22.2%) | 3 (7.9%) | 7 (12.5%) | |
| Clear cell | 1 (5.6%) | 2 (5.3%) | 3 (5.4%) | |
| Mixed type | 2 (11.1%) | 5 (13.1%) | 7 (12.5%) | |
| Others d | 4 (22.2%) | 7 (18.4%) | 11 (19.6%) | |
| Pathologic FIGO stage e | 0.278 f | |||
| I | 1 (5.6%) | 9 (23.7%) | 10 (17.8%) | |
| II | 3 (16.6%) | 5 (13.2%) | 8 (14.3%) | |
| IIIA | 1 (5.6%) | 1 (2.6%) | 2 (3.6%) | |
| IIIB | 0 (0.0%) | 1 (2.6%) | 1 (1.8%) | |
| IIIC1 | 13 (72.2%) | 13 (34.2%) | 26 (46.4%) | |
| IIIC2 | 0 (0.0%) | 8 (21.1%) | 8 (14.3%) | |
| IVA | 0 (0.0%) | 1 (2.6%) | 1 (1.8%) | |
| Myometrial invasion | 0.179 | |||
| <1/2 | 3 (16.7%) | 13 (34.2%) | 16 (28.6%) | |
| ≥1/2 | 15 (83.3%) | 25 (65.8%) | 40 (71.4%) | |
| LVSI | 0.741 | |||
| Present | 12 (66.7%) | 27 (71.1%) | 39 (69.6%) | |
| Absent | 6 (33.3%) | 11 (28.9%) | 17 (30.4%) | |
| Peritoneal washing cytology | 0.426 | |||
| Positive | 2 (11.1%) | 3 (7.9%) | 5 (8.9%) | |
| Negative | 15 (83.3%) | 28 (73.7%) | 43 (76.8%) | |
| Unknown | 1 (5.6%) | 7 (18.4%) | 8 (14.3%) | |
| Adjuvant treatment | 0.349 | |||
| None | 1 (5.6%) | 2 (5.2%) | 3 (5.4%) | |
| Chemotherapy | 4 (22.2%) | 9 (23.7%) | 13 (23.2%) | |
| Radiotherapy g | 2 (11.1%) | 12 (31.6%) | 14 (25.0%) | |
| Combination treatment | 11 (61.1%) | 15 (39.5%) | 26 (46.4%) |
| RFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariable | Multivariable | Univariable | Multivariable | ||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | ||||||||
| <60 years | 1.00 | 1.00 | ||||||
| ≥60 years | 1.74 (0.64–4.76) | 0.278 | 0.71 (0.15–3.33) | 0.665 | ||||
| FIGO stage | ||||||||
| I-II | 1.00 | 1.00 | 1.00 | |||||
| III-IVA | 3.65 (1.34–9.95) | 0.012 | 8.65 (1.15–65.32) | 0.037 | 3.38 (1.79–14.48) | 0.040 | ||
| Histology | ||||||||
| Grade 3 endometrioid | 1.00 | 1.00 | ||||||
| Non-endometrioid | 2.21 (0.85–5.77) | 0.105 | 2.58 (0.58–11.37) | 0.211 | ||||
| Surgical method | ||||||||
| Laparotomy | 1.00 | 1.00 | ||||||
| Laparoscopy | 0.57 (0.18–1.82) | 0.341 | 0.75 (0.11–4.99) | 0.763 | ||||
| Extent of lymphadenectomy | ||||||||
| PLND | 1.00 | 1.00 | ||||||
| PLND and PALND | 0.45 (0.16–1.27) | 0.129 | 2.10 (0.40–10.89) | 0.379 | ||||
| LVSI | ||||||||
| Absent | 1.00 | 1.00 | ||||||
| Present | 3.59 (1.31–9.84) | 0.013 | 1.97 (0.37–10.49) | 0.426 | ||||
| RFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariable | Multivariable | Univariable | Multivariable | ||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | ||||||||
| <60 years | 1.00 | 1.00 | ||||||
| ≥60 years | 1.82 (0.58–5.74) | 0.308 | 0.92 (0.18–4.66) | 0.92 | ||||
| Histology | ||||||||
| Grade 3 endometrioid | 1.00 | 1.00 | 1.00 | |||||
| Non-endometrioid | 3.98 (1.30–12.17) | 0.016 | 3.34 (1.11–10.05) | 0.032 | 4.03 (0.88–18.53) | 0.073 | ||
| Surgical method | ||||||||
| Laparotomy | 1.00 | 1.00 | ||||||
| Laparoscopy | 0.65 (0.18–2.33) | 0.505 | 0.89 (0.11–7.12) | 0.915 | ||||
| Extent of lymphadenectomy | ||||||||
| PLND | 1.00 | 1.00 | ||||||
| PLND and PALND | 0.36 (0.12–1.09) | 0.07 | 2.50 (0.50–12.38) | 0.263 | ||||
| LVSI | ||||||||
| Absent | 1.00 | 1.00 | ||||||
| Present | 2.40 (0.67–8.60) | 0.179 | 1.65 (0.28–9.75) | 0.583 | ||||
| Peritoneal washing cytology | ||||||||
| Negative | 1.00 | 1.00 | ||||||
| Positive | 7.90 (1.36–45.87) | 0.021 | 59.88 (4.48–800.24) | 0.002 | ||||
| Adjuvant treatment | ||||||||
| Single treatment | 1.00 | 1.00 | ||||||
| Combination treatment | 0.24 (0.08–0.78) | 0.018 | 0.51 (0.11–2.37) | 0.387 | ||||
| RFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariable | Multivariable | Univariable | Multivariable | ||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | ||||||||
| <60 years | 1.00 | 1.00 | ||||||
| ≥60 years | 1.44 (0.44–4.73) | 0.548 | 0.55 (0.0.9–3.32) | 0.55 | ||||
| Histology | ||||||||
| Grade 3 endometrioid | 1.00 | 1.00 | 1.00 | |||||
| Non-endometrioid | 3.64 (1.18–11.26) | 0.025 | 3.44 (1.05–11.26) | 0.041 | 4.86 (0.97–24.37) | 0.054 | ||
| Surgical method | ||||||||
| Laparotomy | 1.00 | 1.00 | ||||||
| Laparoscopy | 1.24 (0.24–6.34) | 0.796 | 1.91 (0.14–26.37) | 0.629 | ||||
| Extent of lymphadenectomy | ||||||||
| PLND | 1.00 | 1.00 | ||||||
| PLND and PALND | 0.30 (0.09–0.96) | 0.042 | 2.38 (0.43–13.08) | 0.319 | ||||
| Peritoneal washing cytology | ||||||||
| Negative | 1.00 | 1.00 | ||||||
| Positive | 5.04 (0.97–26.10) | 0.054 | 25.38 (2.34–274.91) | 0.008 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-L.; Seo, J.-H.; Chang, K.; Kim, H.-S.; Chen, J.; Yang, T.-S.; Chen, Y.-L.; Lee, Y.-Y. Impact of Para-Aortic Lymphadenectomy on Clinically FIGO Stage IIIC1 High-Grade Endometrial Cancer: A Retrospective Cohort Study from Two Tertiary Centers in Korea and Taiwan. Medicina 2025, 61, 1079. https://doi.org/10.3390/medicina61061079
Lai Y-L, Seo J-H, Chang K, Kim H-S, Chen J, Yang T-S, Chen Y-L, Lee Y-Y. Impact of Para-Aortic Lymphadenectomy on Clinically FIGO Stage IIIC1 High-Grade Endometrial Cancer: A Retrospective Cohort Study from Two Tertiary Centers in Korea and Taiwan. Medicina. 2025; 61(6):1079. https://doi.org/10.3390/medicina61061079
Chicago/Turabian StyleLai, Yen-Ling, Jun-Hyeong Seo, Koping Chang, Hyun-Soo Kim, Jung Chen, Tyan-Shin Yang, Yu-Li Chen, and Yoo-Young Lee. 2025. "Impact of Para-Aortic Lymphadenectomy on Clinically FIGO Stage IIIC1 High-Grade Endometrial Cancer: A Retrospective Cohort Study from Two Tertiary Centers in Korea and Taiwan" Medicina 61, no. 6: 1079. https://doi.org/10.3390/medicina61061079
APA StyleLai, Y.-L., Seo, J.-H., Chang, K., Kim, H.-S., Chen, J., Yang, T.-S., Chen, Y.-L., & Lee, Y.-Y. (2025). Impact of Para-Aortic Lymphadenectomy on Clinically FIGO Stage IIIC1 High-Grade Endometrial Cancer: A Retrospective Cohort Study from Two Tertiary Centers in Korea and Taiwan. Medicina, 61(6), 1079. https://doi.org/10.3390/medicina61061079






