Successful Treatment of Abdominal Wall Advanced Endometriosis-Associated Clear Cell Carcinoma with AKT Pathway Inhibitor: Case Report
Abstract
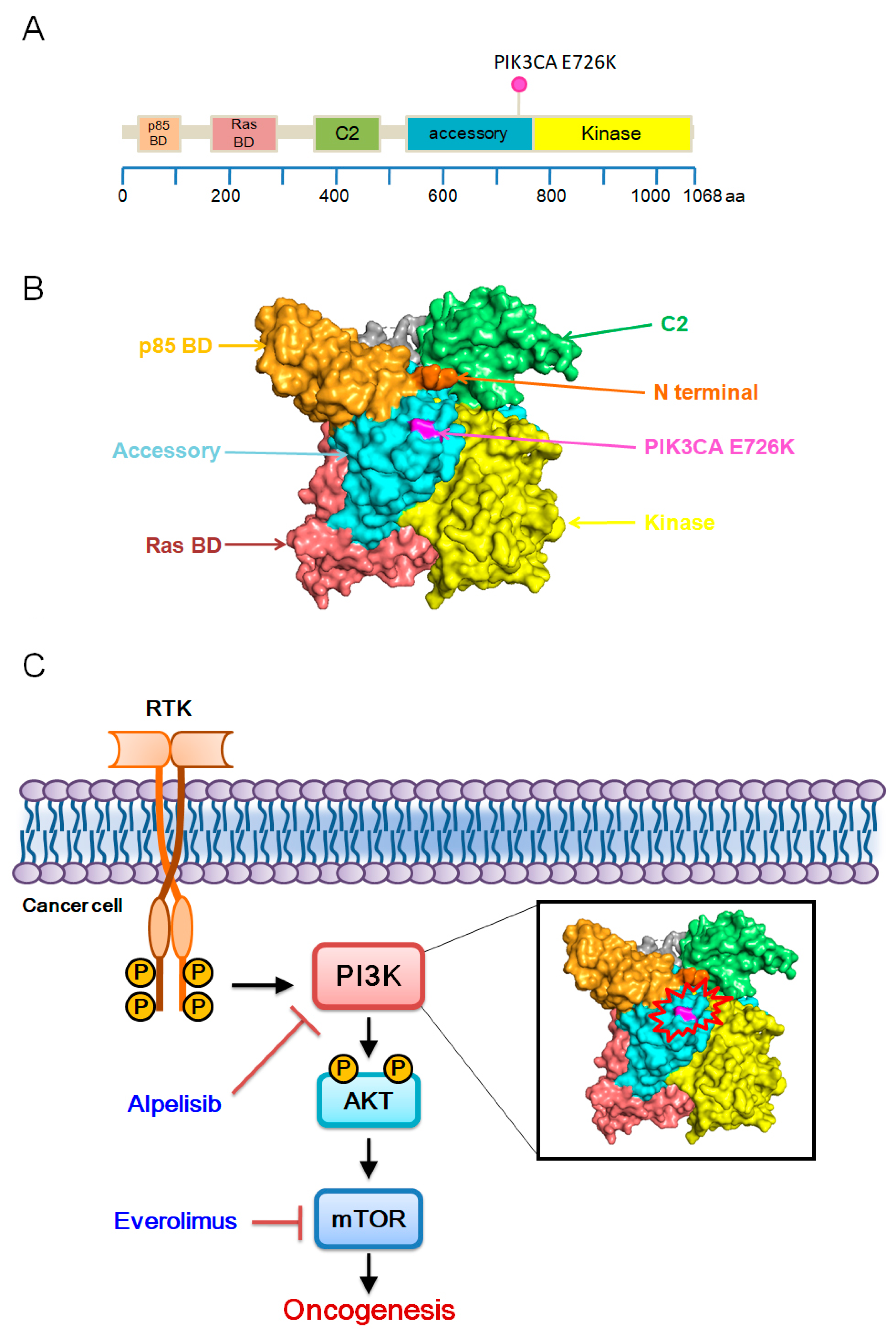
1. Introduction
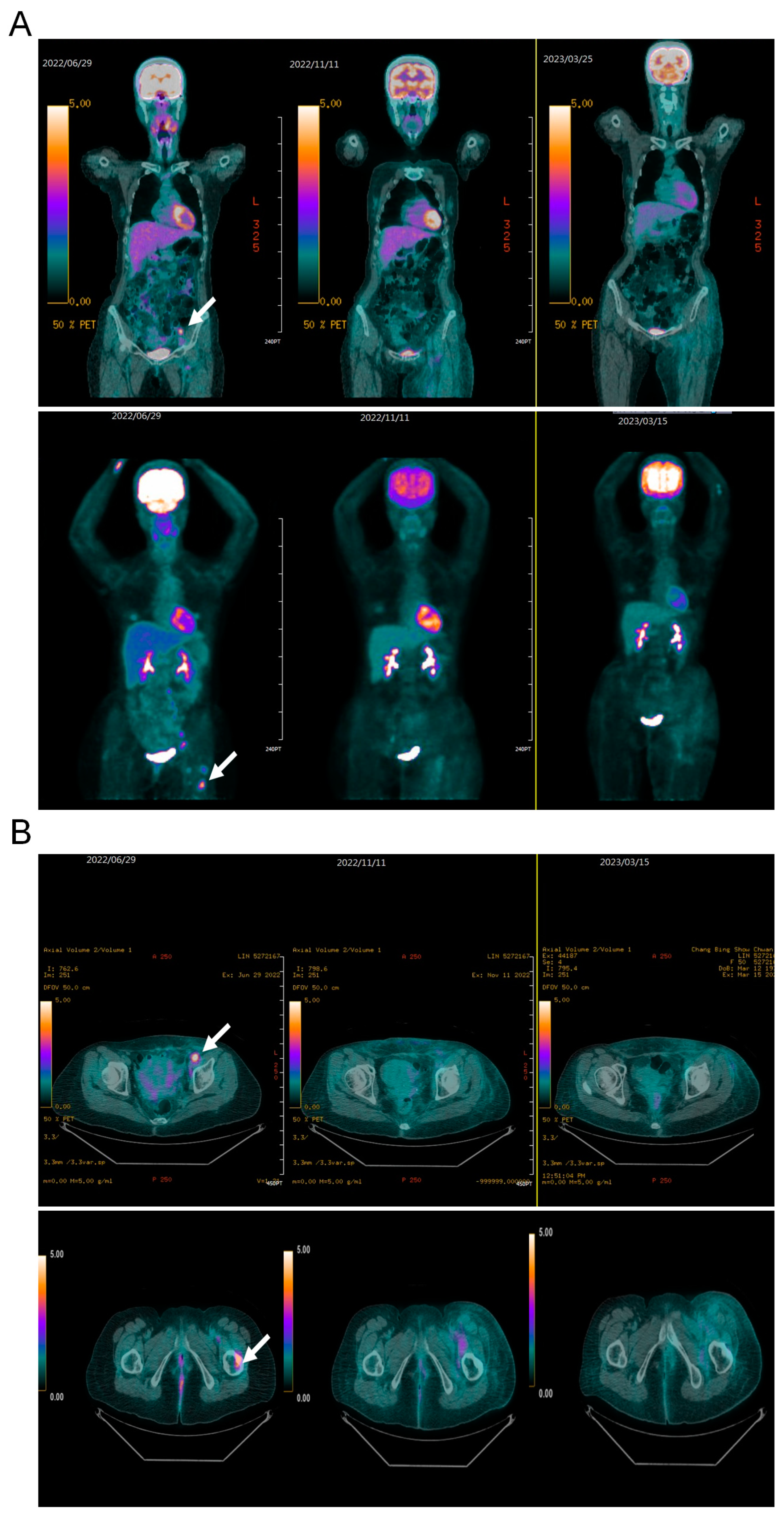
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, C.; Petignat, P.; Belisle, A.; Drouin, P. Primary abdominal wall clear cell carcinoma: Case report and review of literature. Anticancer. Res. 2009, 29, 1591–1593. [Google Scholar] [PubMed]
- Ferrandina, G.; Palluzzi, E.; Fanfani, F.; Gentileschi, S.; Valentini, A.L.; Mattoli, M.V.; Pennacchia, I.; Scambia, G.; Zannoni, G. Endometriosis-associated clear cell carcinoma arising in caesarean section scar: A case report and review of the literature. World J. Surg. Oncol. 2016, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wei, H.; Huang, J.; Shen, H.; Wang, X.; Hu, C. Clear Cell Adenocarcinoma Arising from Endometriosis in Abdominal Wall Cesarean Section Scar: A Case Report and Literature Review. Int. J. Womens Health 2023, 15, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Suehnholz, S.P.; Nissan, M.H.; Zhang, H.; Kundra, R.; Nandakumar, S.; Lu, C.; Carrero, S.; Dhaneshwar, A.; Fernandez, N.; Xu, B.W.; et al. Quantifying the Expanding Landscape of Clinical Actionability for Patients with Cancer. Cancer Discov. 2024, 14, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Stephens, L.; Hawkins, P. PI3K signalling: The path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 2012, 13, 195–203. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef]
- Murakami, R.; Matsumura, N.; Brown, J.B.; Higasa, K.; Tsutsumi, T.; Kamada, M.; Abou-Taleb, H.; Hosoe, Y.; Kitamura, S.; Yamaguchi, K.; et al. Exome Sequencing Landscape Analysis in Ovarian Clear Cell Carcinoma Shed Light on Key Chromosomal Regions and Mutation Gene Networks. Am. J. Pathol. 2017, 187, 2246–2258. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Bashashati, A.; Wang, Y.K.; Senz, J.; Ha, G.; Yang, W.; Aniba, M.R.; Prentice, L.M.; Farahani, H.; Li Chang, H.; et al. Multifocal endometriotic lesions associated with cancer are clonal and carry a high mutation burden. J. Pathol. 2015, 236, 201–209. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Andre, F.; Jiang, Z.; Shao, Z.; Mano, M.S.; Neciosup, S.P.; Tseng, L.M.; Zhang, Q.; Shen, K.; Liu, D.; et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015, 16, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, X.; Zhen, L.; Chen, W.; Mu, L.; Zhang, Y.; Song, A. Everolimus inhibits breast cancer cell growth through PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2018, 17, 7163–7169. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.L.; Morice, P.; Enomoto, T.; Takehara, K.; Denys, H.; Lorusso, D.; Coleman, R.; et al. Clear cell carcinoma of the endometrium. Gynecol. Oncol. 2022, 164, 658–666. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.-T.; Wu, C.-H.; Chang, C.-S.; Lai, D.-W.; Liu, T.-C. Successful Treatment of Abdominal Wall Advanced Endometriosis-Associated Clear Cell Carcinoma with AKT Pathway Inhibitor: Case Report. Medicina 2024, 60, 1946. https://doi.org/10.3390/medicina60121946
Ko Y-T, Wu C-H, Chang C-S, Lai D-W, Liu T-C. Successful Treatment of Abdominal Wall Advanced Endometriosis-Associated Clear Cell Carcinoma with AKT Pathway Inhibitor: Case Report. Medicina. 2024; 60(12):1946. https://doi.org/10.3390/medicina60121946
Chicago/Turabian StyleKo, Ya-Ting, Ching-Hsuan Wu, Cheng-Shyong Chang, De-Wei Lai, and Ta-Chih Liu. 2024. "Successful Treatment of Abdominal Wall Advanced Endometriosis-Associated Clear Cell Carcinoma with AKT Pathway Inhibitor: Case Report" Medicina 60, no. 12: 1946. https://doi.org/10.3390/medicina60121946
APA StyleKo, Y.-T., Wu, C.-H., Chang, C.-S., Lai, D.-W., & Liu, T.-C. (2024). Successful Treatment of Abdominal Wall Advanced Endometriosis-Associated Clear Cell Carcinoma with AKT Pathway Inhibitor: Case Report. Medicina, 60(12), 1946. https://doi.org/10.3390/medicina60121946





