Role of Inflammatory Markers as a Risk Factor for Community-Acquired Pneumonia Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- -
- Age ≥ 18 years;
- -
- Diagnosed with community-acquired pneumonia;
- -
- Provided written informed consent.
- -
- Age under 18;
- -
- Inability to provide informed consent;
- -
- Patients with autoimmune diseases;
- -
- Patients with chronic lung diseases such as cystic fibrosis or COPD;
- -
- Contraindications to venipuncture.
2.2. Patients and Study Design
2.3. Blood Sampling and Determination of Serum Concentration of Inflammatory Markers
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Serum Inflammatory Marker Levels
3.3. Integration of Clinical and Laboratory Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAP | Community-acquired pneumonia |
| CBC | Complete blood count |
| CRP | C reactive protein |
| G-CSF | Granulocyte colony-stimulating factor |
| IL | Interleukin |
| IFN | Interferon |
| PCT | Procalcitonin |
| PSI | Pneumonia severity index |
References
- Ferreira-Coimbra, J.; Sarda, C.; Rello, J. Burden of Community-Acquired Pneumonia and Unmet Clinical Needs. Adv. Ther. 2020, 37, 1302–1318. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019. 9 December 2020. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 17 May 2025).
- World Health Organization. Global Health Observatory (GHO) Data: Causes of Death. Available online: https://www.who.int/data/gho (accessed on 17 May 2025).
- Partouche, H.; Lepoutre, A.; Vaure, C.B.D.; Poisson, T.; Toubiana, L.; Gilberg, S. Incidence of all-cause adult community-acquired pneumonia in primary care settings in France. Med. Mal. Infect. 2018, 48, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, A.; Westin, J.; Lindh, M.; Andersson, L.M.; Kristinsson, K.G.; Löve, A.; Baldursson, O.; Gottfredsson, M. Incidence, aetiology, and outcomes of community-acquired pneumonia: A population-based study. Open Forum Infect. Dis. 2018, 5, ofy010. [Google Scholar] [CrossRef]
- Lopardo, G.D.; Fridman, D.; Raimondo, E.; Albornoz, H.; Lopardo, A.; Bagnulo, H.; Goleniuk, D.; Sanabria, M.; Stamboulian, D. Incidence rate of community-acquired pneumonia in adults: A population-based prospective active surveillance study in three cities in South America. BMJ Open 2018, 8, e019439. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Zhanel, G.G. Streptococcus pneumoniae: Epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr. Opin. Pulm. Med. 2010, 16, 217–225. [Google Scholar] [CrossRef]
- Converso, T.R.; Goulart, C.; Rodriguez, D.; Guerra, M.E.S.; Darrieux, M.; Leite, L.C.C. Immune response induced in mice by a hybrid rPotD-PdT pneumococcal protein. PLoS ONE 2022, 17, e0273017. [Google Scholar] [CrossRef]
- Paterson, G.K.; Orihuela, C.J. Pneumococci: Immunology of the innate host response. Respirology 2010, 15, 1057–1063. [Google Scholar] [CrossRef]
- Kumar, S. Mycoplasma pneumoniae: A significant but underrated pathogen in paediatric community-acquired lower respiratory tract infections. Indian J. Med. Res. 2018, 147, 23–31. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, S.; Zhu, C.; Zhou, R.; Leung, P.H.M. Mycoplasma pneumoniae infections: Pathogenesis and vaccine development. Pathogens 2021, 10, 119. [Google Scholar] [CrossRef]
- Naghib, M.; Hatam-Jahromi, M.; Niktab, M.; Ahmadi, R.; Kariminik, A. Mycoplasma pneumoniae and toll-like receptors: A mutual avenue. Allergol. Immunopathol. (Madr) 2018, 46, 508–513. [Google Scholar] [CrossRef]
- Tsai, T.A.; Tsai, C.K.; Kuo, K.C.; Yu, H.R. Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J. Microbiol. Immunol. Infect. 2021, 54, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Meng, F.; Gao, M.; Cheng, G.; Wang, X. Cytokine signatures associate with disease severity in children with Mycoplasma pneumoniae pneumonia. Sci. Rep. 2019, 9, 17853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, S.; Zhou, Y.; Yang, D.; Pan, T.; Chen, Z.; Wang, Q. TIPE2 negatively regulates mycoplasma pneumonia-triggered immune response via MAPK signaling pathway. Sci. Rep. 2017, 7, 13319. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Lu, Z.; Jiang, W.; Dai, G.; Huang, L.; Wang, M.; Zhu, C.; Wang, Y.; Hao, C.; et al. The CARDS toxin of Mycoplasma pneumoniae induces a positive feedback loop of type 1 immune response. Front. Immunol. 2022, 13, 1054788. [Google Scholar] [CrossRef]
- Zhang, Z.; Dou, H.; Tu, P.; Shi, D.; Wei, R.; Wan, R.; Jia, C.; Ning, L.; Wang, D.; Li, J.; et al. Serum cytokine profiling reveals different immune response patterns during general and severe Mycoplasma pneumoniae pneumonia. Front. Immunol. 2022, 13, 1088725. [Google Scholar] [CrossRef]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- Helble, J.D.; Starnbach, M.N. T cell responses to Chlamydia. Pathog. Dis. 2021, 79, ftab014. [Google Scholar] [CrossRef]
- Wang, X.; Rockey, D.D.; Dolan, B.P. Chlamydia lipooligosaccharide has varied direct and indirect roles in evading both innate and adaptive host immune responses. Infect. Immun. 2020, 88, e00198-20. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, B.; Greub, G. Chlamydiales and the innate immune response: Friend or foe? FEMS Immunol. Med. Microbiol. 2011, 61, 231–244. [Google Scholar] [CrossRef]
- Mygind, P.; Christiansen, G.; Persson, K.; Birkelund, S. Analysis of the Humoral Immune Response to Chlamydia Outer Membrane Protein 2. Clin. Diagn. Lab. Immunol. 1998, 5, 313–318. [Google Scholar] [CrossRef]
- Seligman, R.; Ramos-Lima, L.F.; Amaral Oliveira, V.; Sanvicente, C.; Pacheco, E.F.; Dalla Rosa, K. Biomarkers in community-acquired pneumonia: A state-of-the-art review. Clinics 2012, 67, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Siljan, W.W.; Holter, J.C.; Michelsen, A.E.; Nymo, S.H.; Lauritzen, T.; Oppen, K.; Husebye, E.; Ueland, T.; Mollnes, T.E.; Aukrust, P.; et al. Inflammatory biomarkers are associated with aetiology and predict outcomes in community-acquired pneumonia: Results of a 5-year follow-up cohort study. ERJ Open Res. 2019, 5, 00014-2019. [Google Scholar] [PubMed]
- Antunes, G.; Evans, S.A.; Lordan, J.L.; Frew, A.J. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur. Respir. J. 2002, 20, 990–995. [Google Scholar] [CrossRef]
- Rosolowski, M.; Oberle, V.; Ahnert, P.; Creutz, P.; Witzenrath, M.; Kiehntopf, M.; Loeffler, M.; Suttorp, N.; Scholz, M. Dynamics of cytokines, immune cell counts, and disease severity in patients with community-acquired pneumonia—Unravelling potential causal relationships. Cytokine 2020, 136, 155263. [Google Scholar] [CrossRef]
- Tille, P.M. Diagnostic Microbiology, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 878–891. [Google Scholar]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2009, 45, 27–37. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA) 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Cao, Y.; Han, Z.; Zhang, Y.; Luu, L.D.W.; Chen, L.; Yan, P.; Chen, W.; Wang, J.; Liang, Y.; et al. A pan-immune panorama of bacterial pneumonia revealed by a large-scale single-cell transcriptome atlas. Signal Transduct. Target. Ther. 2025, 10, 5. [Google Scholar] [CrossRef]
- Ding, L.; Jiang, Y. Biomarkers associated with the diagnosis and prognosis of Mycoplasma pneumoniae pneumonia in children: A review. Front. Cell. Infect. Microbiol. 2025, 15, 1552144. [Google Scholar] [CrossRef]
- Karakioulaki, M.; Stolz, D. Biomarkers in Pneumonia-Beyond Procalcitonin. Int. J. Mol. Sci. 2019, 20, 2004. [Google Scholar] [CrossRef]
- Carbonell, R.; Moreno, G.; Martín-Loeches, I.; Bodí, M.; Rodríguez, A. The Role of Biomarkers in Influenza and COVID-19 Community-Acquired Pneumonia in Adults. Antibiotics 2023, 12, 161. [Google Scholar] [CrossRef]
- Lin, J.; Chen, J.; Yuan, S.; Tang, M.; Yang, G.; Zhang, H.; Li, W.; Zhao, H.; Zhang, J.; Zhang, L.; et al. Local and systemic cytokine profiles in children with pneumonia-associated lung consolidation. Front. Immunol. 2025, 16, 1546730. [Google Scholar] [CrossRef] [PubMed]
- Slănină, A.M.; Coman, A.E.; Petroaie, A.D.; Barbacariu, C.L.; Novac, O.; Popa, E.; Manole, M.; Bacușcă, A.I.; Cosmescu, A. Mycoplasma pneumoniae and Chlamydia pneumoniae community-acquired pneumonia in young adults from a family medicine practice. GERMS 2025, 15, 64–70. [Google Scholar] [CrossRef]
- Dukhinova, M.; Kokinos, E.; Kuchur, P.; Komissarov, A.; Shtro, A. Macrophage-derived cytokines in pneumonia: Linking cellular immunology and genetics. Cytokine Growth Factor. Rev. 2021, 59, 46–61. [Google Scholar] [CrossRef]
- Menendez, R.; Sahuquillo-Arce, J.M.; Reyes, S.; Martínez, R.; Polverino, E.; Cillóniz, C.; Córdoba, J.G.; Montull, B.; Torres, A. Cytokine activation patterns and biomarkers are influenced by microorganisms in community-acquired pneumonia. Chest 2012, 141, 1537–1545. [Google Scholar] [CrossRef]
- Rendon, A.; Rendon-Ramirez, E.J.; Rosas-Taraco, A.G. Relevant Cytokines in the Management of Community-Acquired Pneumonia. Curr. Infect. Dis. Rep. 2016, 18, 10. [Google Scholar] [CrossRef]
- Endeman, H.; Meijvis, S.C.A.; Rijkers, G.T.; Velzen-Blad, H.; Moorsel, C.H.M.; Grutters, J.C.; Biesma, D. Systemic cytokine response in patients with community-acquired pneumonia. Eur. Respir. J. 2011, 37, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Botran, R.; Uriarte, S.M.; Arnold, F.W.; Rodriguez-Hernandez, L.; Rane, M.J.; Peyrani, P.; Wiemken, T.; Kelley, R.; Uppatla, S.; Cavallazzi, R.; et al. Contrasting inflammatory responses in severe and non-severe community-acquired pneumonia. Inflammation 2014, 37, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Botran, R.; Wiemken, T.L.; Kelley, R.R.; Peyrani, P.; Bordon, J.; Cavallazzi, R.; Ramirez, J.A. Analysis of the Local and Systemic Cytokine Response Profiles in Patients with Community-Acquired Pneumonia: Relationship with Outcomes and Disease Severity. Univ. Louisville J. Respir. Infect. 2017, 1, 13–20. [Google Scholar] [CrossRef]
- Paats, M.S.; Bergen, I.M.; Hanselaar, W.E.; van Zoelen, E.C.G.; Hoogsteden, H.C.; Hendriks, R.W.; Van der Eerden, M.M. Local and systemic cytokine profiles in non-severe and severe community-acquired pneumonia. Eur. Respir. J. 2013, 41, 1378–1385. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Valles, X.; Menendez, R.; Sibila, O.; Montull, B.; Cilloniz, C.; Artigas, A.; Torres, A. Predicting treatment failure in patients with community-acquired pneumonia: A case-control study. Respir. Res. 2014, 15, 75. [Google Scholar] [CrossRef]
- Martınez, R.; Menendez, R.; Reyes, S.; Polverino, E.; Cilloniz, C.; Martınez, A.; Esquinas, C.; Filella, X.; Ramírez, P.; Torres, A. Factors associated with inflammatory cytokine patterns in community-acquired pneumonia. Eur. Respir. J. 2011, 37, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Tripon, R.E.; Neagoe, I.B.; Budisan, L.; Pop, T.L.; Cristea, V.; Stanca, L.M.; Lupse, M.S. Systemic interleukin levels in community-acquired pneumonia and their association with adverse outcomes. Rev. Română Med. Lab. 2019, 27, 189–198. [Google Scholar] [CrossRef]
- Nascimento-Carvalho, E.C.; Vasconcellos, A.G.; Clarêncio, J.; Andrade, D.; Barral, A.; Barral-Netto, M. Evolution of cytokines/chemokines in cases with community-acquired pneumonia and distinct etiologies. Pediatr. Pulmonol. 2020, 55, 169–176. [Google Scholar] [CrossRef] [PubMed]
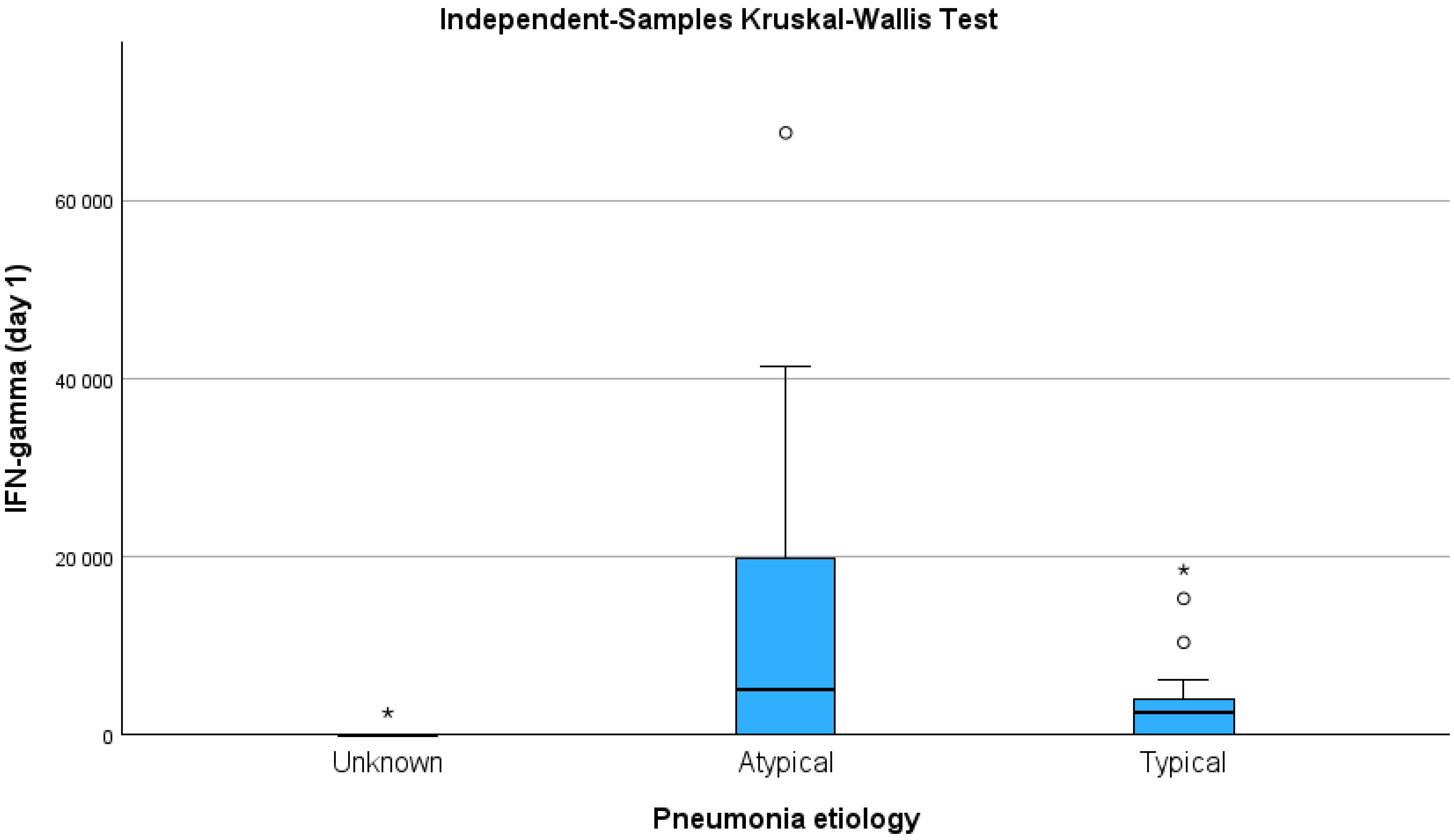
| Characteristic | CAP of Unknown Aetiology, n = 5 n (%) | Typical Bacterial Pathogen, n = 22 n (%) | Atypical Pathogen, n = 14 n (%) | p Value |
|---|---|---|---|---|
| Age (M (IQR)) | 57 (38) | 71.5 (27) * | 41(41) * | 0.030 |
| >65 years | 2 (40) | 14 (63.6) * | 3 (21.4) ** | 0.040 |
| ≤65 years | 3 (60) | 8 (36.4) * | 11 (78.6) ** | |
| Smokers, n (%) | ||||
| Current or past (n = 13) | 2 (15.4) *,** | 10 (76.9) * | 1 (7.7) ** | 0.040 |
| Never (n = 28) | 3 (10.7) *,** | 12 (42.9) * | 12 (46.4) ** | |
| Place of treatment, n (%) | ||||
| Ambulatory | 2 (40) | 9 (40.9) | 5 (35.7) | 1.000 |
| Hospital | 3 (60) | 13 (59.1) | 9 (64.3) | |
| Duration of treatment days Mean (SD) | 5.7 (1.1) | 8.3 (2.7) | 8.6 (2.2) | 0.193 |
| PSI score, n (%) | ||||
| Risk class I | 2 (40) | 4 (18.2) | 4 (28.6) | 0.551 |
| Risk class II−III | 3 (60) | 17 (77.3) | 8 (57.1) | |
| Risk class IV−V | 0 (0) | 1 (4.5) | 2 (14.3) | |
| Signs and symptoms, n (%) | ||||
| Fever | 3 (75) | 15 (68.2) | 11 (84.6) | 0.663 |
| Cough | 4 (100) | 19 (86.4) | 13 (100) | 0.484 |
| Sore throat | 3 (75) | 12 (54.5) | 9 (69.2) | 0.628 |
| Dyspnoea | 3 (75) | 14 (63.6) | 8 (61.5) | 1.000 |
| Headache | 0 (0) | 4 (18.2) | 3 (23.1) | 0.852 |
| Sputum | 4 (100) | 19 (86.4) | 10 (76.9) | 0.678 |
| Laboratory parameters | ||||
| WBC count (×109/L) | 11 (6.61) * | 6.9 (3.16) * | 7.82 (7.09) | 0.026 |
| Platelet (×109/L) | 223 (207.5) | 271.5 (85.5) | 256.5 (100.3) | 0.451 |
| Neutrophil count (×109/L) | 6.76 (6.39) | 5.55 (4.06) | 6.52 (5.28) | 0.370 |
| Lymphocyte count (×109/L) | 1.43 (0.89) | 1.14 (1.11) | 1.37 (0.93) | 0.688 |
| CRP (mg/L) | 11 (84) | 70.5 (136) | 93.5 (144) | 0.107 |
| Inflammatory Markers | Day 1 M (IQR) pg/mL | Day 7 M (IQR) pg/mL | p Value |
|---|---|---|---|
| IL-8 | 24,225.0 (28,861.0) | 18,849 (22,285.0) | 0.382 |
| IL-6 | 7241.0 (12,114.0) | 2195 (9362.8) | 0.021 |
| IFN-γ | 2445.0 (5877.9) | 0.83 (2444.3) | 0.004 |
| G-CSF | 14,129.5 (41,837.0) | 7.0 (3890.8) | <0.001 |
| Inflammatory Markers | Inpatient Treatment | Outpatient Treatment | p Value | |
|---|---|---|---|---|
| IL-8 M (IQR) pg/mL | Day 1 | 34,763.5 (38,799.5) | 14,696.5 (24,524.5) | 0.001 |
| Day 7 | 22,166.5 (32,024.3) | 12,876.0 (13,766.8) | 0.017 | |
| IL-6 M (IQR) pg/mL | Day 1 | 8658.0 (12,114.0) | 2928.0 (10,757.8) | 0.234 |
| Day 7 | 3653.5 (10,066.8) | 727.6 (2744.1) | 0.029 | |
| IFN-γ M (IQR) pg/mL | Day 1 | 2445.0 (10,747.2) | 2445 (3868.3) | 0.420 |
| Day 7 | 0.8 (4325.3) | 0.83 (1410.3) | 0.141 | |
| G-CSF M (IQR) pg/mL | Day 1 | 27,964.0 (36,832.5) | 7.0 (20,470.0) | 0.013 |
| Day 7 | 7.0 (7124.0) | 7.0 (0.0) | 0.652 | |
| IL-8 | IL-6 | IFN-γ | G-CSF | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Age | –0.008 | 0.960 | 0.385 * | 0.015 | –0.288 | 0.072 | 0.023 | 0.888 |
| CRP | 0.348 * | 0.028 | 0.461 ** | 0.003 | 0.398 * | 0.011 | 0.396 * | 0.011 |
| WBC | 0.111 | 0.496 | 0.186 | 0.257 | −0.350 * | 0.027 | 0.175 | 0.280 |
| PLT | −0.197 | 0.224 | −0.065 | 0.692 | 0.033 | 0.842 | −0.080 | 0.626 |
| PSI score | 0.188 | 0.244 | 0.346 * | 0.031 | 0.114 | 0.483 | 0.167 | 0.303 |
| IL-8 | 1.000 | – | 0.110 | 0.507 | 0.174 | 0.283 | 0.189 | 0.244 |
| IL-6 | 0.110 | 0.507 | 1.000 | – | −0.110 | 0.506 | 0.305 | 0.059 |
| IFN-γ (1 day) | 0.174 | 0.283 | −0.110 | 0.506 | 1.000 | – | 0.259 | 0.107 |
| G-CSF | 0.189 | 0.244 | 0.305 | 0.059 | 0.259 | 0.107 | 1.000 | – |
| Lymphocytes | −0.135 | 0.414 | −0.112 | 0.503 | −0.291 | 0.072 | −0.328 * | 0.042 |
| Neutrophils | 0.021 | 0.897 | 0.214 | 0.191 | −0.185 | 0.253 | 0.328 * | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nutautiene, R.; Aleksa, I.; Janulaityte, I.; Skrodeniene, E.; Bieksiene, K.; Zaliaduonyte, D.; Batulevicius, D.; Vitkauskiene, A. Role of Inflammatory Markers as a Risk Factor for Community-Acquired Pneumonia Management. Medicina 2025, 61, 1078. https://doi.org/10.3390/medicina61061078
Nutautiene R, Aleksa I, Janulaityte I, Skrodeniene E, Bieksiene K, Zaliaduonyte D, Batulevicius D, Vitkauskiene A. Role of Inflammatory Markers as a Risk Factor for Community-Acquired Pneumonia Management. Medicina. 2025; 61(6):1078. https://doi.org/10.3390/medicina61061078
Chicago/Turabian StyleNutautiene, Ruta, Irmantas Aleksa, Ieva Janulaityte, Erika Skrodeniene, Kristina Bieksiene, Diana Zaliaduonyte, Darius Batulevicius, and Astra Vitkauskiene. 2025. "Role of Inflammatory Markers as a Risk Factor for Community-Acquired Pneumonia Management" Medicina 61, no. 6: 1078. https://doi.org/10.3390/medicina61061078
APA StyleNutautiene, R., Aleksa, I., Janulaityte, I., Skrodeniene, E., Bieksiene, K., Zaliaduonyte, D., Batulevicius, D., & Vitkauskiene, A. (2025). Role of Inflammatory Markers as a Risk Factor for Community-Acquired Pneumonia Management. Medicina, 61(6), 1078. https://doi.org/10.3390/medicina61061078







