Protective Effect of Probiotics on Cardiac Damage in Experimental Sepsis Model Induced by Lipopolysaccharide in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Grouping the Rats and Experimental Applications
- Sham Group (SG): this group was administered 0.5 mL of normal saline (0.9% NaCl) daily via oral gavage for 14 days;
- Probiotic Group (PG): this group received 0.5 mL of probiotics (25 mg/kg, 109 CFU/day) daily via oral gavage for 14 days;
- LPS Group (LPSG): this group served as the sepsis model, receiving lipopolysaccharide (LPS) administration;
- Probiotic + LPS Group (PLPSG): this group was treated with probiotics as described for PG and received LPS on day 14.
2.2. Anesthesia Procedure
2.3. Acquisition, Preservation, and Preparation of Serum and Tissue Specimens for Analysis
2.4. TNF-α, IL1β, and IL6 Analysis in Serum and Tissue Samples
2.5. CRP, CK-MB, and cTn-I Analysis in Serum Samples
2.6. MDA, GSH, SOD, TOS, and TAS Analysis in Tissue Sample
2.7. Calculation of Oxidative Stress Index
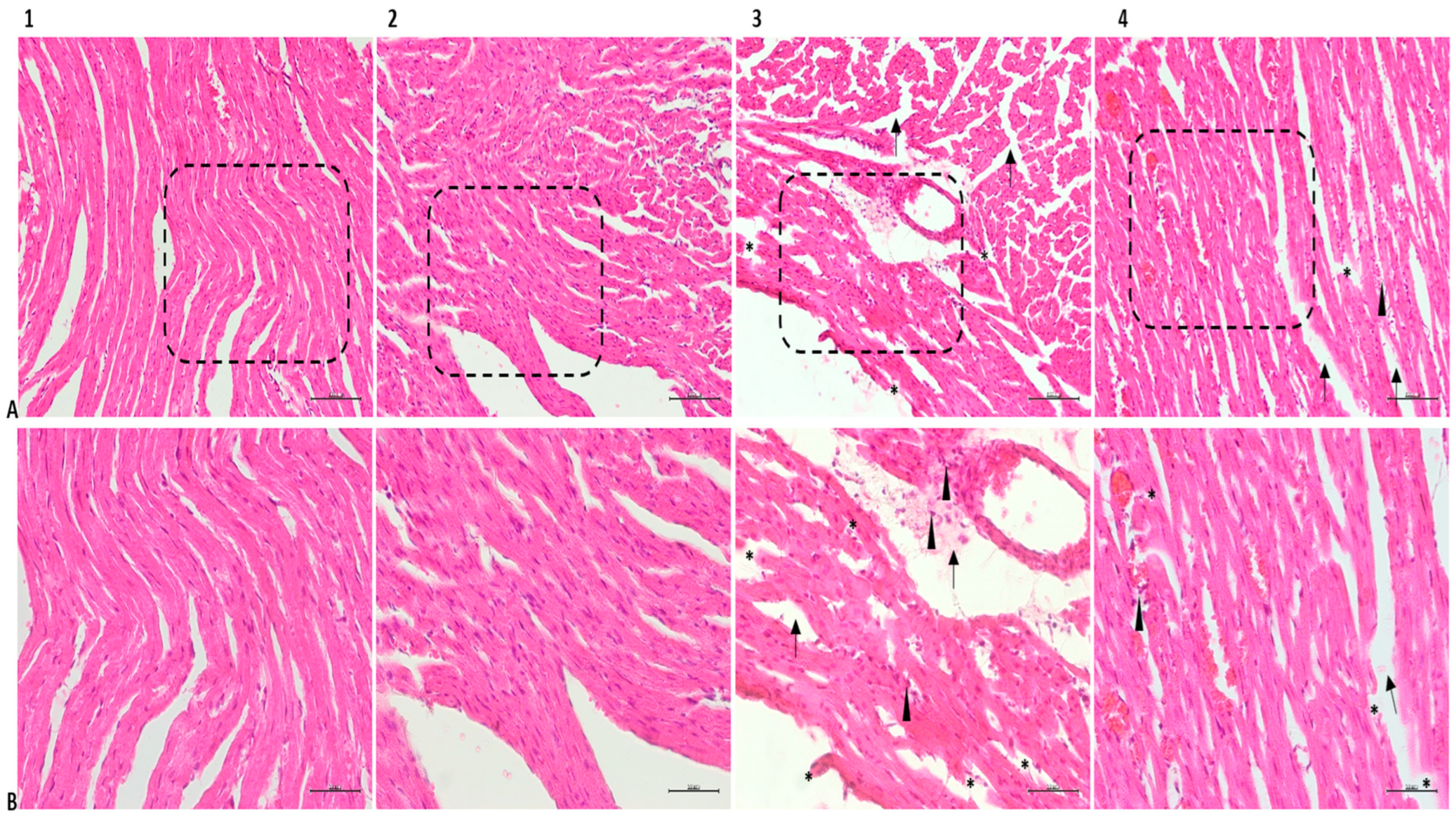
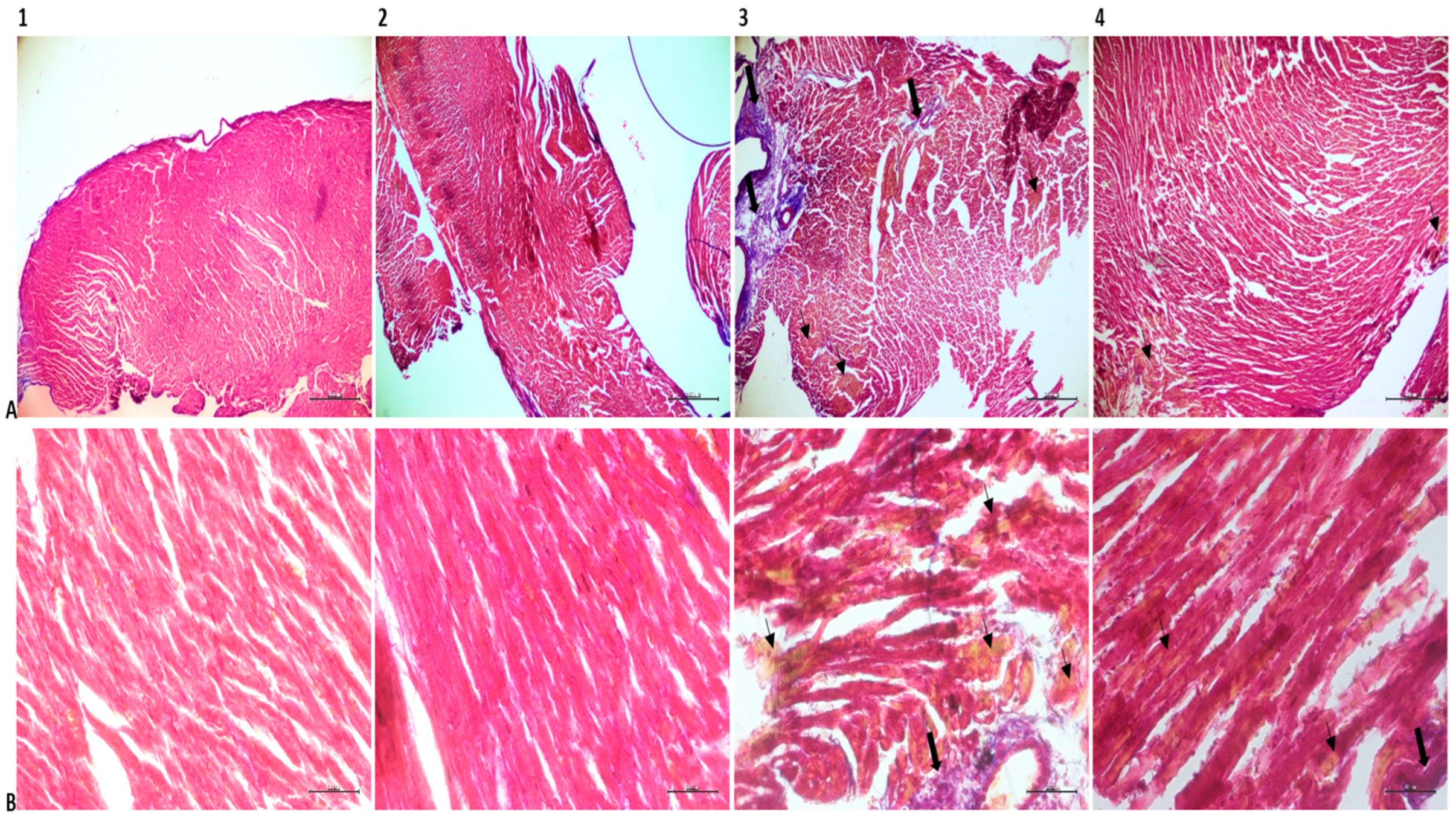
2.8. Histopathological Analysis
2.9. Statistical Analysis
3. Results
3.1. Biochemical Findings in Tissue and Serum
3.2. Findings in Serum CK-MB, CRP, and Troponin I
3.3. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishita Saha, I.; Bag, N.; Roy, S.; Ullah, Z.; Bardhan, S.; Karmakar, R.; Das, S.; Guo, B. The state-of-the-art therapeutic paradigms against sepsis. Smart. Mater. Med. 2024, 5, 425–446. [Google Scholar] [CrossRef]
- Majno, G. The ancient riddle of sigma eta psi iota sigma (sepsis). J. Infect. Dis. 1991, 163, 937–945. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing sepsis as a global health priority—A WHO resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Naghavi, M. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-pathophysiology and therapeutic concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Reinhart, K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar]
- Seymour, C.W.; Rea, T.D.; Kahn, J.M.; Walkey, A.J.; Yealy, D.M.; Angus, D.C. Severe sepsis in pre-hospital emergency care: Analysis of incidence, care, and outcome. Am. J. Respir. Crit. Care Med. 2012, 186, 1264–1271. [Google Scholar]
- Liu, D.; Huang, S.Y.; Sun, J.H.; Zhang, H.C.; Cai, Q.L.; Gao, C.; Zeng, L. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022, 9, 56. [Google Scholar]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine supplementation, physical exercise and oxidative stress markers: A review of the mechanisms and effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Evolution of the knowledge of free radicals and other oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef]
- van der Slikke, E.C.; Beumeler, L.F.E.; Holmqvist, M.; Linder, A.; Mankowski, R.T.; Bouma, H.R. Understanding post-sepsis syndrome: How can clinicians help? Infect. Drug Resist. 2023, 16, 6493–6511. [Google Scholar] [CrossRef]
- Aisa-Alvarez, A.; Soto, M.E.; Guarner-Lans, V.; Camarena-Alejo, G.; Franco-Granillo, J.; Martínez-Rodríguez, E.A.; Pérez-Torres, I. Usefulness of antioxidants as adjuvant therapy for septic shock: A randomized clinical trial. Medicina 2020, 56, 619. [Google Scholar] [CrossRef]
- Aleman, R.S.; Yadav, A. Systematic review of probiotics and their potential for developing functional nondairy foods. Appl. Microbiol. 2024, 4, 47–69. [Google Scholar] [CrossRef]
- Momin, E.S.; Khan, A.A.; Kashyap, T.; Pervaiz, M.A.; Akram, A.; Mannan, V.; Sanusi, M.; Elshaikh, A.O. The Effects of probiotics on cholesterol levels in patients with metabolic syndrome: A systematic review. Cureus 2023, 15, e37567. [Google Scholar] [CrossRef]
- Szajewska, H.; Hojsak, I. Health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12 in children. Postgrad. Med. 2020, 132, 441–451. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Feng, X.; Feng, X.; Liu, C.; Guan, X.; Liu, S.; Long, Z.; Miao, Z.; He, F.; et al. Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 promote infected wound healing via regulation of the wound microenvironment. Microb. Biotechnol. 2024, 17, e70031. [Google Scholar] [CrossRef]
- Castro-Herrera, V.M.; Rasmussen, C.; Wellejus, A.; Miles, E.A.; Calder, P.C. In vitro effects of live and heat-inactivated Bifidobacterium animalis subsp. lactis, BB-12 and Lactobacillus rhamnosus GG on caco-2 cells. Nutrients 2020, 12, 1719. [Google Scholar] [CrossRef]
- Hamid, M.; Zahid, S. Ameliorative effects of probiotics in AlCl3-induced mouse model of Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2023, 107, 5803–5812. [Google Scholar] [CrossRef]
- Castro-Herrera, V.M.; Fisk, H.L.; Wootton, M.; Lown, M.; Owen-Jones, E.; Lau, M.; Lowe, R.; Hood, K.; Gillespie, D.; Hobbs, F.D.R.; et al. Combination of the probiotics Lacticaseibacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis, BB-12 has limited effect on biomarkers of immunity and inflammation in older people resident in care homes: Results from the probiotics to reduce infections iN CarE home reSidentS Randomized, Controlled Trial. Front. Immunol. 2021, 12, 643321. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Ahtesh, F.B.; Stojanovska, L.; Apostolopoulos, V. Anti-hypertensive peptides released from milk proteins by probiotics. Maturitas 2018, 115, 103–109. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The role of probiotics in cancer prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Yang, S.; Qiao, J.; Zhang, M.; Kwok, L.Y.; Matijašić, B.B.; Zhang, H.; Zhang, W. Prevention and treatment of antibiotics-associated adverse effects through the use of probiotics: A review. J. Adv. Res. 2024, S2090–1232, 00230–00233. [Google Scholar] [CrossRef]
- Font, M.D.; Thyagarajan, B.; Khanna, A.K. Sepsis and septic shock—Basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. N. Am. 2020, 104, 573–585. [Google Scholar] [CrossRef]
- Poveda-Jaramillo, R. Heart Dysfunction in Sepsis. J. Cardiothorac. Vasc. Anesth. 2021, 35, 298–309. [Google Scholar] [CrossRef]
- Fang, C.H.; Ravindra, V.; Akhter, S.; Adibuzzaman, M.; Griffin, P.; Subramaniam, S.; Grama, A. Identifying and analyzing sepsis states: A retrospective study on patients with sepsis in ICUs. PLoS Digit. Health 2022, 1, e0000130. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: An update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Firdausa, A.Y.; Ahimsa, S.S.; Ahmada, R.A.; Sukmawati, N.F.; Ernawati, D.S.; Parmadiati, A.E.; Soebadi, B.; Radithia, D.; Winias, S.; Mahdani, F.Y.; et al. Malondialdehyde level and tissue apoptosis count as an early-detection marker of oral potentially malignant disorders. Eur. J. Dent. 2023, 17, 155–160. [Google Scholar] [CrossRef]
- Essadek, S.; Bouchab, H.; El Kebbaj, R.; Gondcaille, C.; El Kamouni, S.; Savary, S.; Vamecq, J.; Essamadi, A.; Cherkaoui-Malki, M.; Nasser, B.; et al. Effects of a short-term lipopolysaccharides challenge on mouse brain and liver peroxisomal antioxidant and β-oxidative functions: Protective action of argan oil. Pharmaceuticals 2022, 15, 465. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Mohammed, N.A.; Sleem, A.A. The effects of trimetazidine on lipopolysaccharide-induced oxidative stress in mice. EXCLI J. 2011, 10, 162–172. [Google Scholar]
- Li, Y.; Chen, Y.; Shao, B.; Liu, J.; Hu, R.; Zhao, F.; Cui, X.; Zhao, X.; Wang, Y. Evaluation of creatine kinase (CK)-MB to total CK ratio as a diagnostic biomarker for primary tumors and metastasis screening. Pract. Lab. Med. 2023, 37, e00336. [Google Scholar] [CrossRef]
- Hadi, R.N.; Al-Amran, F.G.; Kadhum, A.J. The cardioprotective potential of melatonin after heterotopic cardiac transplantation in male rats. PhOL 2016, 1, 175–184. [Google Scholar]
- Hyun, S.H.; Kim, Y.M.; Park, S.J. The effects of preceding exercise on myocardial damage in rats. J. Phys. Ther. Sci. 2017, 29, 508–510. [Google Scholar]
- Gökçek, İ. Cardioprotective effect of oleuropein in a cisplatin-induced cardiotoxicity model in rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2024, 397, 3403–3410. [Google Scholar] [CrossRef]
- Ali, S.; Zehra, A.; Khalid, M.U.; Hassan, M.; Shah, S.I.A. Role of C-reactive protein in disease progression, diagnosis and management. Discoveries 2023, 11, e179. [Google Scholar] [CrossRef]
- Shahzad, S.; Mateen, S.; Naeem, S.S.; Akhtar, K.; Rizvi, W.; Moin, S. Syringic acid protects from isoproterenol induced cardiotoxicity in rats. Eur. J. Pharmacol. 2019, 849, 135–145. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Özak, H.E. Investigation of TAS TOS SCUBE-1 and Ichemic Modified Albumin Level in Experimental Acute Pancratitis in Rats. Specialization Thesis in Medicine, Sağlık Bilimleri Üniversitesi, Antalya, Turkey, 2022. [Google Scholar]
- Zhou, Y.; Liu, X.; Gao, W.; Luo, X.; Lv, J.; Wang, Y.; Liu, D. The role of intestinal flora on tumor immunotherapy: Recent progress and treatment implications. Heliyon 2023, 10, e23919. [Google Scholar] [CrossRef]
- Wickens, K.; Black, P.N.; Stanley, T.V.; Mitchell, E.; Fitzharris, P.; Tannock, G.W.; Probiotic Study Group. A differential effect of 2 probiotics in the prevention of eczema and atopy: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2008, 122, 788–794. [Google Scholar]
- Vajro, P.; Mandato, C.; Licenziati, M.R.; Franzese, A.; Vitale, D.F.; Lenta, S.; Meli, R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 740–743. [Google Scholar] [CrossRef]
- Ludwig, I.S.; Broere, F.; Manurung, S.; Lambers, T.T.; Van der Zee, R.; Van Eden, W. Lactobacillus rhamnosus GG-derived soluble mediators modulate adaptive immune cells. Front. Immunol. 2018, 9, 1546. [Google Scholar]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
| Biomarkers | Rat Groups | Pairwise Comparison p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG (1) | LPSG (2) | PLPSG (3) | PG (4) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| IN TISSUE | ||||||||||
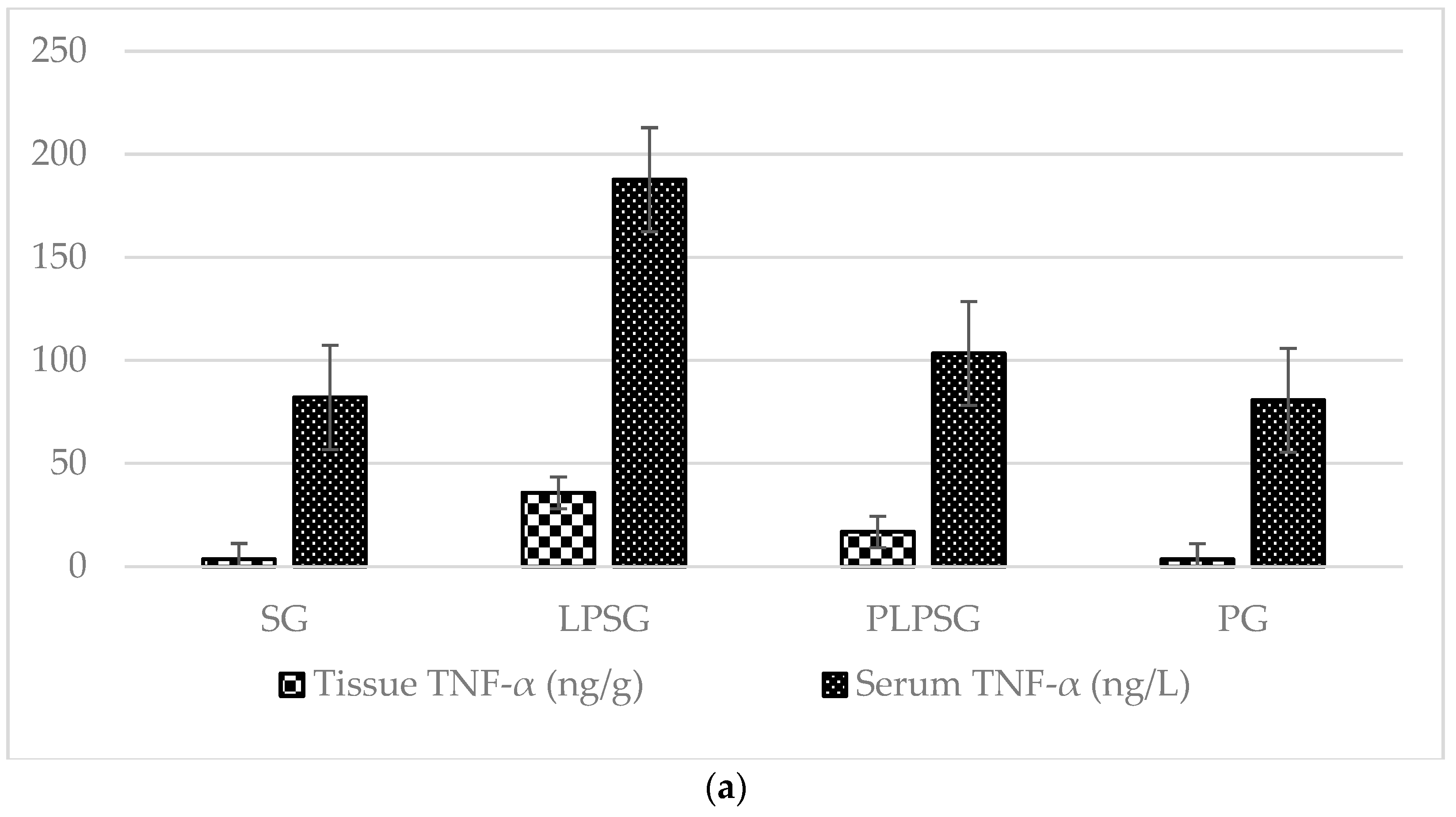
| TNF-α (ng/g) | 3.60 ± 1.18 | 35.75 ± 8.21 | 16.85 ± 9.04 | 3.49 ± 2.02 | <0.001 | <0.01 | 1.000 | <0.001 | <0.001 | <0.01 |
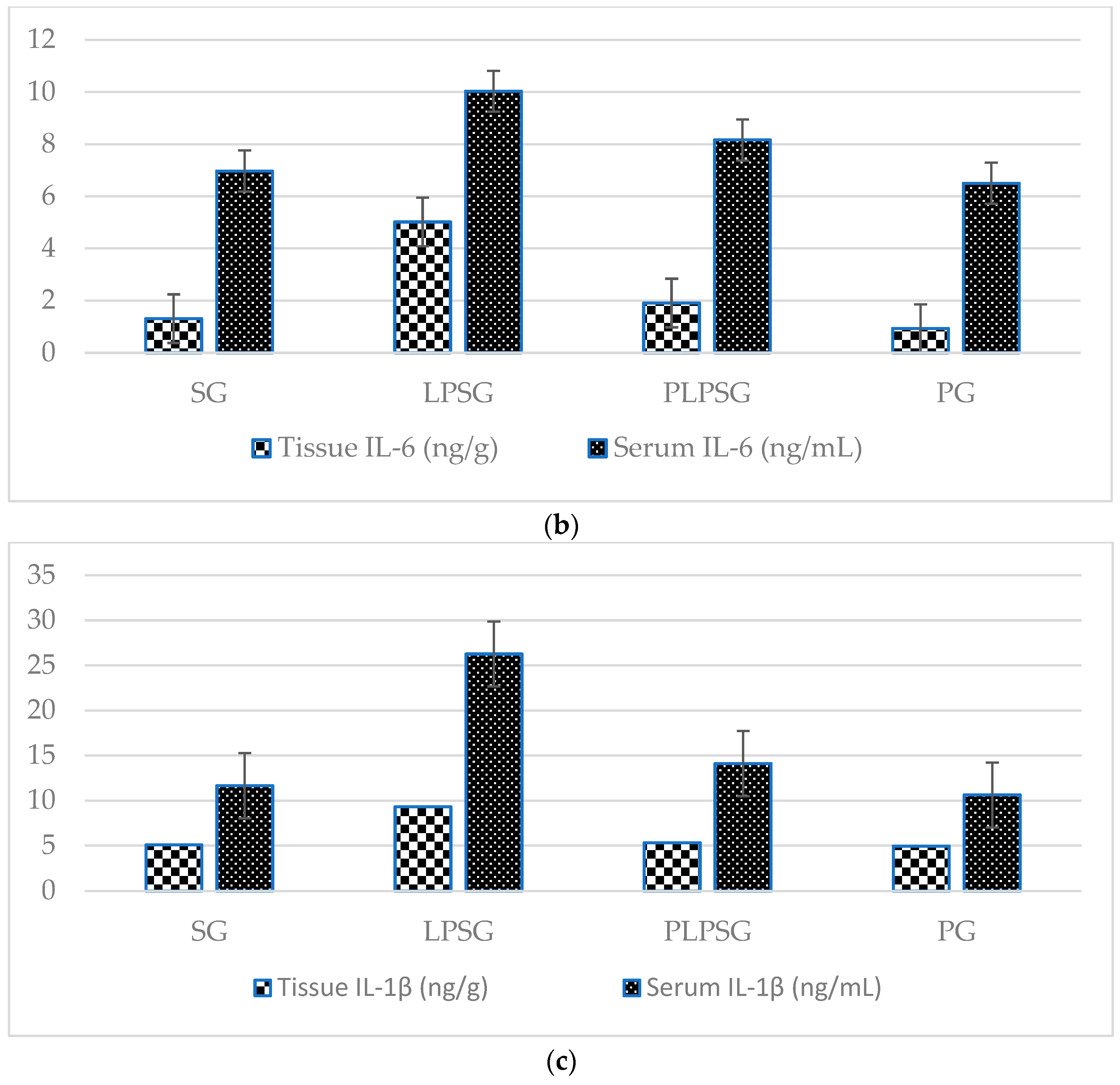
| IL-6 (ng/g) | 1.31 ± 1.39 | 5.02 ± 2.90 | 1.91 ± 0.30 | 0.93 ± 0.78 | <0.05 | 0.922 | 0.978 | <0.05 | <0.05 | <0.01 |
| IL-1β (ng/mg) | 5.08 ± 0.43 | 9.31 ± 4.83 | 5.32 ± 0.25 | 4.94 ± 0.29 | <0.05 | 0.998 | 1.000 | <0.05 | <0.05 | 0.993 |
| IN SERUM | ||||||||||
| TNF-α (ng/L) | 82.06 ± 6.80 | 187.68 ± 86.55 | 103.40 ± 16.24 | 80.69 ± 15.29 | <0.01 | 0.842 | 1.000 | <0.05 | <0.01 | 0.816 |
| IL-6 (ng/L) | 6.97 ± 1.46 | 10.03 ± 1.61 | 8.16 ± 1.10 | 6.50 ± 1.17 | <0.01 | 0.445 | 0.931 | 0.111 | <0.01 | 0.181 |
| IL-1β (ng/mL) | 11.66 ± 0.77 | 26.27 ± 6.68 | 14.11 ± 3.02 | 10.62 ± 1.32 | <0.001 | 0.675 | 0.963 | <0.001 | <0.001 | 0.395 |
| Biomarkers | Rat Groups | Pairwise Comparison p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG (1) | LPSG (2) | PLPSG (3) | PG (4) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
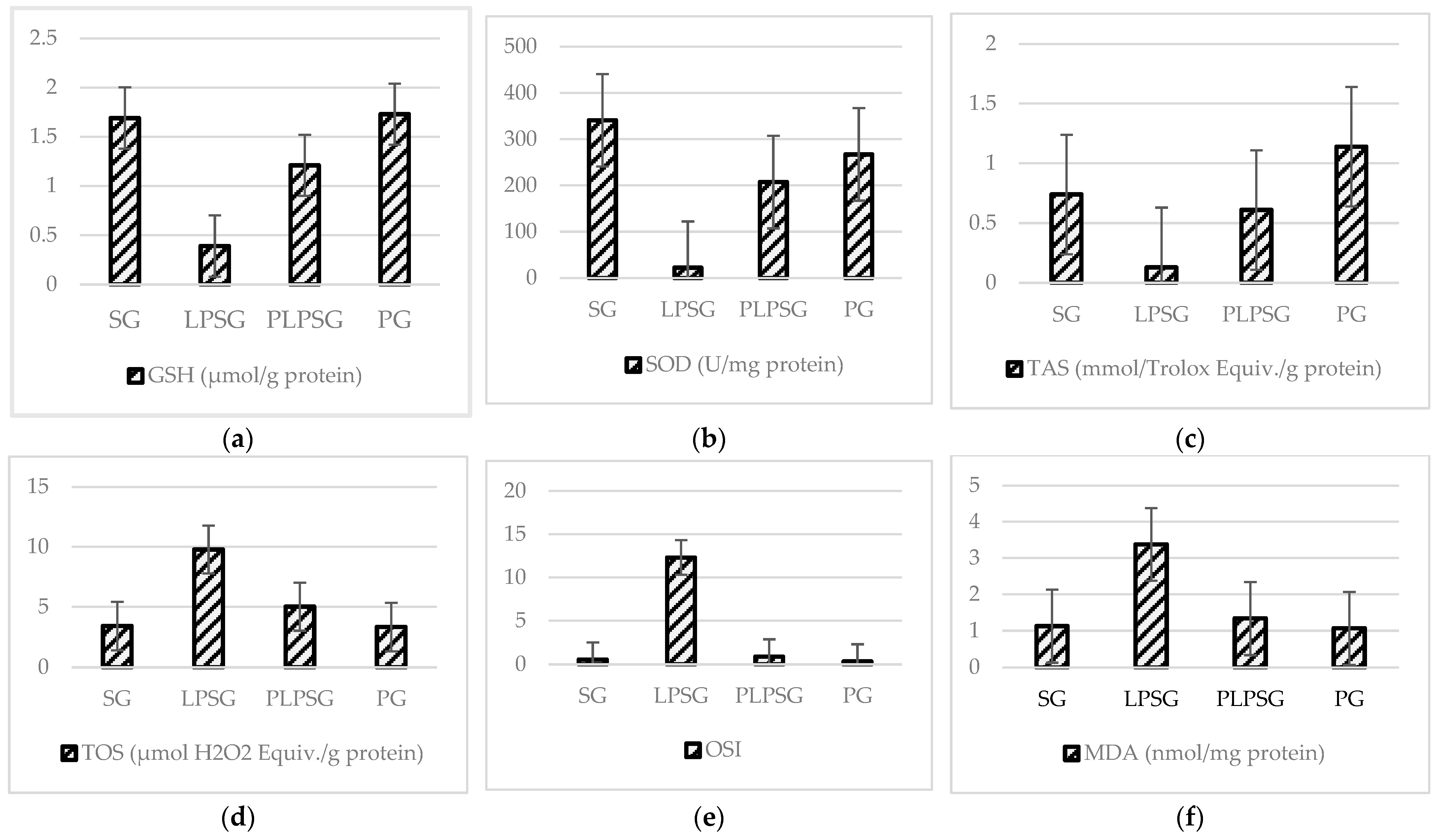
| TAS (mmol Eq/g prot) | 0.74 ± 0.23 | 0.13 ± 0.09 | 0.61 ± 0.19 | 1.14 ± 0.36 | <0.01 | 0.808 | <0.05 | <0.05 | <0.001 | <0.01 |
| TOS (µmol Eq/g prot) | 3.42 ± 1.23 | 9.78 ± 3.88 | 5.02 ± 0.55 | 3.34 ± 0.90 | <0.001 | 0.563 | 1.000 | <0.01 | <0.001 | 0.522 |
| OSI | 0.53 ± 0.28 | 12.30 ± 13.43 | 0.89 ± 0.30 | 0.33 ± 0.16 | <0.05 | 1.000 | 1.000 | <0.05 | <0.05 | 0.999 |
| MDA (nmol/mg prot) | 1.13 ± 0.40 | 3.38 ± 1.33 | 1.34 ± 0.61 | 1.07 ± 0.37 | <0.001 | 0.967 | 0.999 | <0.001 | <0.001 | 0.928 |
| GSH (µmol/g prot) | 1.69 ± 0.22 | 0.39 ± 0.31 | 1.21 ± 0.37 | 1.73 ± 0.32 | <0.001 | 0.072 | 0.994 | <0.001 | <0.001 | <0.05 |
| SOD (U/mg prot) | 340.78 ± 69.84 | 22.35 ± 14.96 | 207.63 ± 173.46 | 267.24 ± 74.74 | <0.001 | 0.135 | 0.597 | <0.05 | <0.01 | 0.738 |
| Biomarkers | Rat Groups | Pairwise Comparison p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG (1) | LPSG (2) | PLPSG (3) | PG (4) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
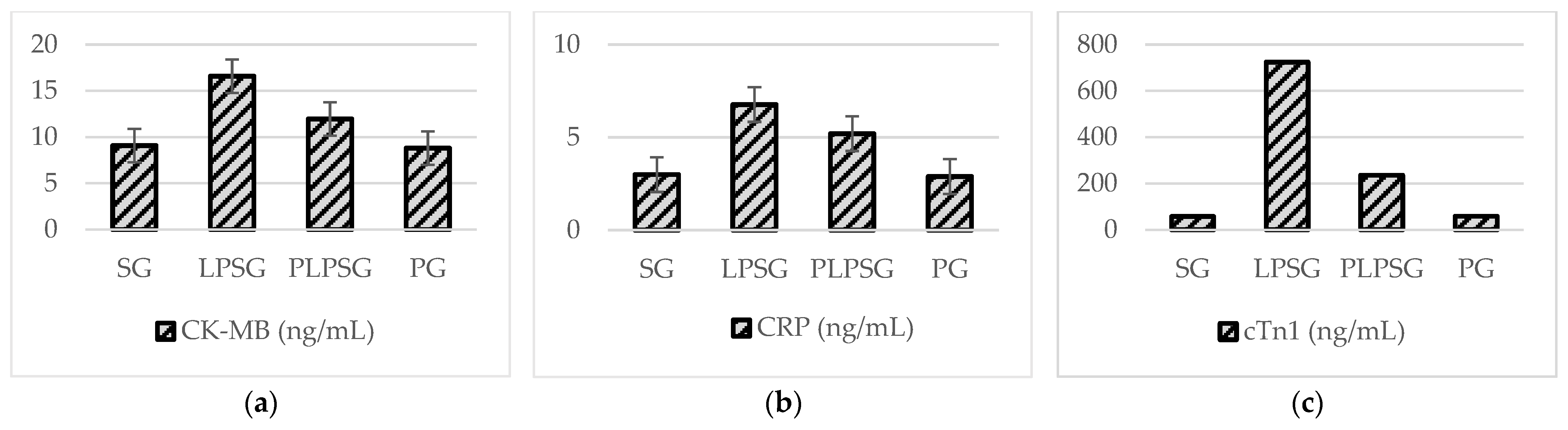
| CK-MB (ng/mL) | 9.08 ± 1.08 | 16.59 ± 3.08 | 11.95 ± 2.35 | 8.80 ± 1.86 | <0.001 | 0.147 | 0.996 | <0.01 | <0.001 | 0.098 |
| CRP (ng/mL) | 2.99 ± 0.43 | 6.77 ± 2.19 | 5.20 ± 2.03 | 2.89 ± 0.70 | <0.01 | 0.096 | 0.999 | 0.328 | <0.01 | 0.077 |
| cTn-I (ng/mL) | 57.21 ± 49.92 | 724.16 ± 277.23 | 235.63 ± 68.37 | 58.51 ± 22.07 | <0.001 | 0.179 | 1.000 | <0.001 | <0.001 | 0.184 |
| Histopathological Status | Groups | Pairwise Comparison p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG (1) | LPSG (2) | PLPSG (3) | PG (4) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
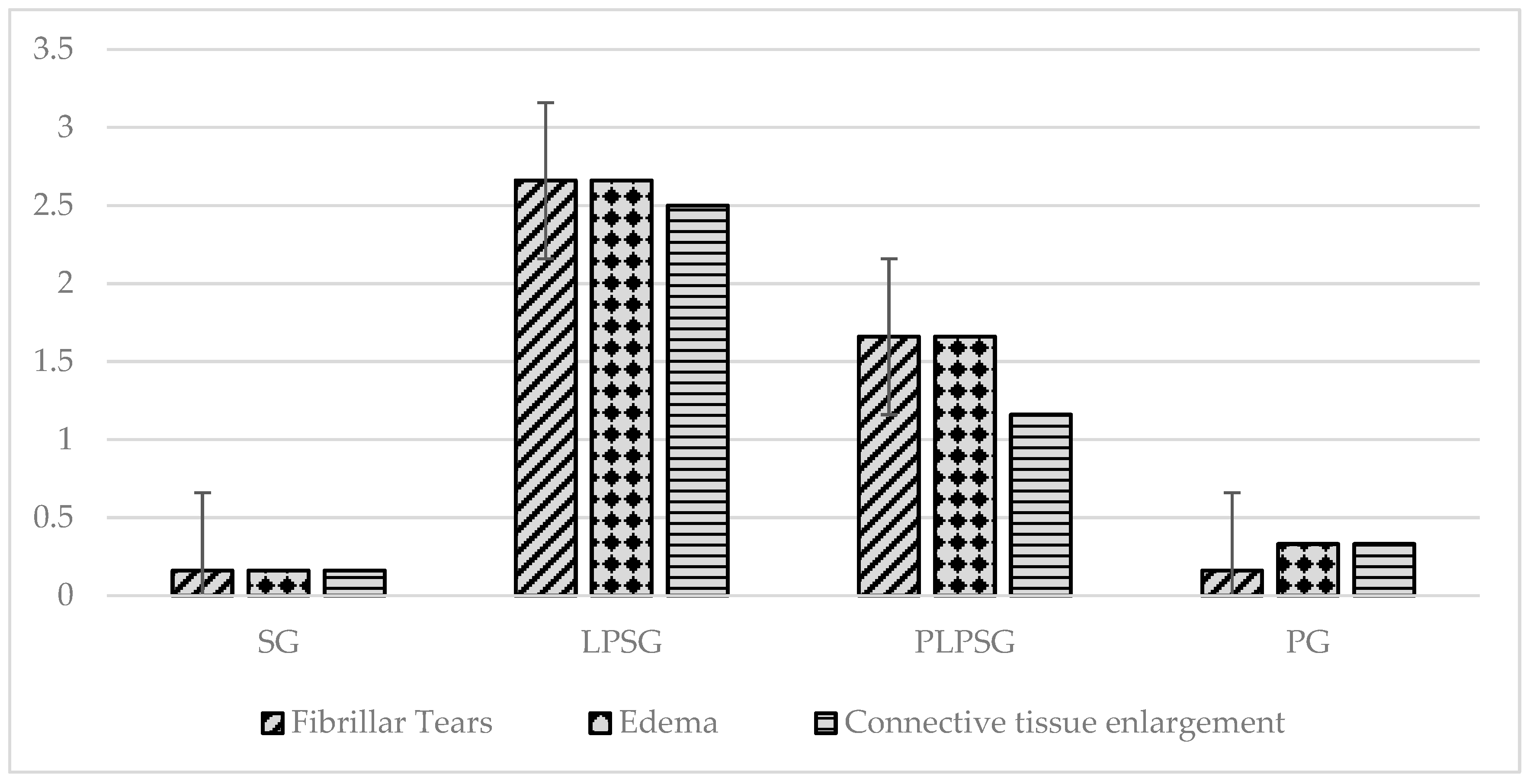
| Fibrillar tears | 0.16 ± 0.40 | 2.66 ± 0.51 | 1.66 ± 0.51 | 0.16 ± 0.40 | <0.001 | <0.001 | 1.000 | <0.01 | <0.001 | <0.001 |
| Edema | 0.16 ± 0.40 | 2.66 ± 0.51 | 1.66 ± 0.51 | 0.33 ± 0.51 | <0.001 | <0.001 | 0.935 | <0.05 | <0.001 | <0.01 |
| Connective tissue enlargement | 0.16 ± 0.40 | 2.50 ± 0.54 | 1.16 ± 0.40 | 0.33 ± 0.51 | <0.001 | <0.01 | 0.928 | <0.001 | <0.001 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taş, N.G.; Aktaş, O.; Taş, H.G.; Zırh, S.; Kurt, N.; Uslu, H. Protective Effect of Probiotics on Cardiac Damage in Experimental Sepsis Model Induced by Lipopolysaccharide in Rats. Medicina 2025, 61, 589. https://doi.org/10.3390/medicina61040589
Taş NG, Aktaş O, Taş HG, Zırh S, Kurt N, Uslu H. Protective Effect of Probiotics on Cardiac Damage in Experimental Sepsis Model Induced by Lipopolysaccharide in Rats. Medicina. 2025; 61(4):589. https://doi.org/10.3390/medicina61040589
Chicago/Turabian StyleTaş, Necip Gökhan, Osman Aktaş, Hakan Gökalp Taş, Selim Zırh, Nezahat Kurt, and Hakan Uslu. 2025. "Protective Effect of Probiotics on Cardiac Damage in Experimental Sepsis Model Induced by Lipopolysaccharide in Rats" Medicina 61, no. 4: 589. https://doi.org/10.3390/medicina61040589
APA StyleTaş, N. G., Aktaş, O., Taş, H. G., Zırh, S., Kurt, N., & Uslu, H. (2025). Protective Effect of Probiotics on Cardiac Damage in Experimental Sepsis Model Induced by Lipopolysaccharide in Rats. Medicina, 61(4), 589. https://doi.org/10.3390/medicina61040589






