Paired Remote Ischemic Preconditioning in Recipients and Living Donors Can Mitigate Cardiovascular Stress in Recipients After Living-Donor Kidney Transplantation: A Propensity-Score-Matching Analysis
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Considerations
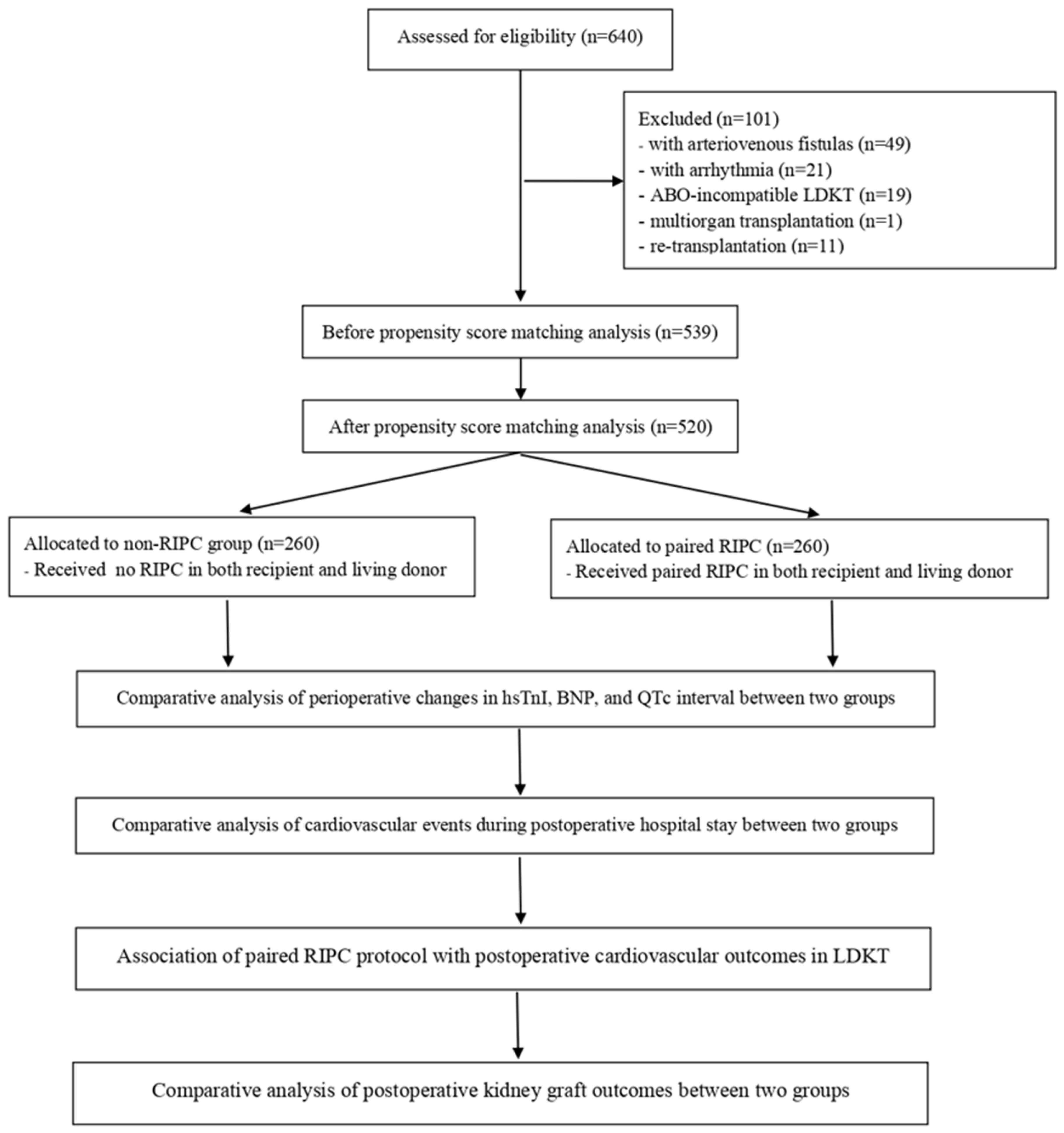
2.2. Study Population
2.3. LDKT and General Anesthesia
2.4. Paired-RIPC Intervention in Both Recipient and Living Donor
2.5. Measurement of High-Sensitivity Troponin I and B-Type Natriuretic Peptide and Corrected QT Interval
2.6. Clinical Variables
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Patients Undergoing LDKT
3.2. Comparison of Perioperative Factors Before and After PSM
3.3. Perioperative Changes in High-Sensitivity Troponin I, Brain Natriuretic Peptide, and QTc Interval in PS-Matched Patients
3.4. New Occurrence of Arrhythmia and Requirement for Cardiovascular Interventions Postoperatively in PS-Matched Patients
3.5. Association of the Paired-RIPC Protocol with Postoperative Cardiovascular Outcomes in LDKT in PS-Matched Patients
3.6. Postoperative Kidney Graft Outcomes in PS-Matched Patients
3.7. Comparison of Postoperative Cardiovascular Outcomes or Graft Function Between the Original and PSM Cohorts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Hurst, F.P.; Jindal, R.M.; Villines, T.C.; Kunz, J.S.; Yuan, C.M.; Hauptman, P.J.; Abbott, K.C. Cardiovascular risk assessment among potential kidney transplant candidates: Approaches and controversies. Am. J. Kidney Dis. 2010, 55, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Reed, A.; Kaplan, B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am. J. Transplant. 2004, 4, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Maclean, J.R.; Snyder, J.J. Acute myocardial infarction and kidney transplantation. J. Am. Soc. Nephrol. 2006, 17, 900–907. [Google Scholar] [CrossRef]
- Landesberg, G.; Beattie, W.S.; Mosseri, M.; Jaffe, A.S.; Alpert, J.S. Perioperative myocardial infarction. Circulation 2009, 119, 2936–2944. [Google Scholar] [CrossRef]
- Hart, A.; Weir, M.R.; Kasiske, B.L. Cardiovascular risk assessment in kidney transplantation. Kidney Int. 2015, 87, 527–534. [Google Scholar] [CrossRef]
- Lazaris, A.M.; Maheras, A.N.; Vasdekis, S.N.; Karkaletsis, K.G.; Charalambopoulos, A.; Kakisis, J.D.; Martikos, G.; Patapis, P.; Giamarellos-Bourboulis, E.J.; Karatzas, G.M.; et al. Protective effect of remote ischemic preconditioning in renal ischemia/reperfusion injury, in a model of thoracoabdominal aorta approach. J. Surg. Res. 2009, 154, 267–273. [Google Scholar] [CrossRef]
- Kierulf-Lassen, C.; Nieuwenhuijs-Moeke, G.J.; Krogstrup, N.V.; Oltean, M.; Jespersen, B.; Dor, F.J. Molecular Mechanisms of Renal Ischemic Conditioning Strategies. Eur. Surg. Res. 2015, 55, 151–183. [Google Scholar] [CrossRef]
- Rassaf, T.; Totzeck, M.; Hendgen-Cotta, U.B.; Shiva, S.; Heusch, G.; Kelm, M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res. 2014, 114, 1601–1610. [Google Scholar] [CrossRef]
- Pickard, J.M.; Bøtker, H.E.; Crimi, G.; Davidson, B.; Davidson, S.M.; Dutka, D.; Ferdinandy, P.; Ganske, R.; Garcia-Dorado, D.; Giricz, Z.; et al. Remote ischemic conditioning: From experimental observation to clinical application: Report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res. Cardiol. 2015, 110, 453. [Google Scholar] [CrossRef]
- Carini, R.; Albano, E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology 2003, 125, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G15–G26. [Google Scholar] [CrossRef] [PubMed]
- Souza Filho, M.V.; Loiola, R.T.; Rocha, E.L.; Simão, A.F.; Gomes, A.S.; Souza, M.H.; Ribeiro, R.A. Hind limb ischemic preconditioning induces an anti-inflammatory response by remote organs in rats. Braz. J. Med. Biol. Res. 2009, 42, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Franchello, A.; Gilbo, N.; David, E.; Ricchiuti, A.; Romagnoli, R.; Cerutti, E.; Salizzoni, M. Ischemic preconditioning (IP) of the liver as a safe and protective technique against ischemia/reperfusion injury (IRI). Am. J. Transplant. 2009, 9, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Veighey, K.V.; Nicholas, J.M.; Clayton, T.; Knight, R.; Robertson, S.; Dalton, N.; Harber, M.; Watson, C.J.E.; De Fijter, J.W.; Loukogeorgakis, S.; et al. Early remote ischaemic preconditioning leads to sustained improvement in allograft function after live donor kidney transplantation: Long-term outcomes in the REnal Protection Against Ischaemia-Reperfusion in transplantation (REPAIR) randomised trial. Br. J. Anaesth. 2019, 123, 584–591. [Google Scholar] [CrossRef]
- Soendergaard, P.; Krogstrup, N.V.; Secher, N.G.; Ravlo, K.; Keller, A.K.; Toennesen, E.; Bibby, B.M.; Moldrup, U.; Ostraat, E.O.; Pedersen, M.; et al. Improved GFR and renal plasma perfusion following remote ischaemic conditioning in a porcine kidney transplantation model. Transpl. Int. 2012, 25, 1002–1012. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Li, Y.; Liao, D.; Hu, L.; Peng, K.; Liu, H.; Ji, F.; Shan, X. Remote ischemic conditioning may improve graft function following kidney transplantation: A systematic review and meta-analysis with trial sequential analysis. BMC Anesthesiol. 2024, 24, 168. [Google Scholar] [CrossRef]
- Moreno-Alarcón, C.; López-Cubillana, P.; López-González, P.; Prieto-González, A.; Ruiz-Morcillo, J.C.; Olarte-Barragán, E.H.; Pinzón-Navarrete, C.P.; Gutiérrez-Gutiérrez, P.F.; Gómez-Gómez, G. Lich-Gregoir technique and routine use of double J catheter as the best combination to avoid urinary complications in kidney transplantation. Transplant. Proc. 2014, 46, 167–169. [Google Scholar] [CrossRef]
- Samman Tahhan, A.; Sandesara, P.; Hayek, S.S.; Hammadah, M.; Alkhoder, A.; Kelli, H.M.; Topel, M.; O’Neal, W.T.; Ghasemzadeh, N.; Ko, Y.A.; et al. High-Sensitivity Troponin I Levels and Coronary Artery Disease Severity, Progression, and Long-Term Outcomes. J. Am. Heart Assoc. 2018, 7, e007914. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Kwon, A.; Choi, H.J.; Chung, H.S.; Hong, S.H.; Park, C.S.; Choi, J.H.; Chae, M.S. The 2016 ASE/EACVI recommendations may be able to more accurately identify patients at risk for diastolic dysfunction in living donor liver transplantation. PLoS ONE 2019, 14, e0215603. [Google Scholar] [CrossRef]
- O’Hare, M.; Maldonado, Y.; Munro, J.; Ackerman, M.J.; Ramakrishna, H.; Sorajja, D. Perioperative management of patients with congenital or acquired disorders of the QT interval. Br. J. Anaesth. 2018, 120, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Ikitimur, B.; Cosansu, K.; Karadag, B.; Cakmak, H.A.; Avci, B.K.; Erturk, E.; Seyahi, N.; Ongen, Z. Long-Term Impact of Different Immunosuppressive Drugs on QT and PR Intervals in Renal Transplant Patients. Ann. Noninvasive Electrocardiol. 2015, 20, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.M.; Le, V.; Rotter, D.; Battiprolu, P.K.; Grinsfelder, B.; Tannous, P.; Burchfield, J.S.; Czubryt, M.; Backs, J.; Olson, E.N.; et al. Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ. Res. 2011, 109, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrøm, K.L.; Bjerrum, E.; Gögenur, I.; Burcharth, J.; Ekeloef, S. Effect of remote ischaemic preconditioning on mortality and morbidity after non-cardiac surgery: Meta-analysis. BJS Open 2021, 5, zraa026. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Kwon, H.M.; Jung, K.W.; Kim, K.S.; Shin, W.J.; Jun, I.G.; Song, J.G.; Hwang, G.S. Preoperative high-sensitivity troponin I and B-type natriuretic peptide, alone and in combination, for risk stratification of mortality after liver transplantation. Korean J. Anesthesiol. 2021, 74, 242–253. [Google Scholar] [CrossRef]
- Liu, P.; Wang, L.; Han, D.; Sun, C.; Xue, X.; Li, G. Acquired long QT syndrome in chronic kidney disease patients. Ren. Fail. 2020, 42, 54–65. [Google Scholar] [CrossRef]
- Kosiuk, J.; Langenhan, K.; Stegmann, C.; Uhe, T.; Dagres, N.; Dinov, B.; Kircher, S.; Richter, S.; Sommer, P.; Bertagnolli, L.; et al. Effect of remote ischemic preconditioning on electrophysiological parameters in nonvalvular paroxysmal atrial fibrillation: The RIPPAF Randomized Clinical Trial. Heart Rhythm. 2020, 17, 3–9. [Google Scholar] [CrossRef]
- Caru, M.; Lalonde, F.; Gravel, H.; Daigle, C.; Tournoux, F.; Jacquemet, V.; Curnier, D. Remote ischaemic preconditioning shortens QT intervals during exercise in healthy subjects. Eur. J. Sport Sci. 2016, 16, 1005–1013. [Google Scholar] [CrossRef]
- Yang, L.; Wang, G.; Du, Y.; Ji, B.; Zheng, Z. Remote ischemic preconditioning reduces cardiac troponin I release in cardiac surgery: A meta-analysis. J. Cardiothorac. Vasc. Anesth. 2014, 28, 682–689. [Google Scholar] [CrossRef]
- Javaherforoosh Zadeh, F.; Moadeli, M.; Soltanzadeh, M.; Janatmakan, F. Effect of Remote Ischemic Preconditioning on Troponin I in CABG. Anesth. Pain. Med. 2017, 7, e12549. [Google Scholar] [CrossRef]
- Han, X.; Zhang, S.; Chen, Z.; Adhikari, B.K.; Zhang, Y.; Zhang, J.; Sun, J.; Wang, Y. Cardiac biomarkers of heart failure in chronic kidney disease. Clin. Chim. Acta 2020, 510, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.M.; Jin, L.; Lv, L.C.; Zhang, B.J.; Wang, L.X. Changes in plasma B-type natriuretic peptide after allograft renal transplantation. Nephrology 2007, 12, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Nagele, P.; Pal, S.; Brown, F.; Blood, J.; Miller, J.P.; Johnston, J. Postoperative QT interval prolongation in patients undergoing noncardiac surgery under general anesthesia. Anesthesiology 2012, 117, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Chavira, H.; Hernández-Guedea, M.; Jiménez-Pérez, J.C.; Pérez-Rodríguez, E.; Muñoz-Espinosa, L.; Muñoz-Maldonado, G.; Cordero-Pérez, P. Modulation of Remote Ischemic Preconditioning by Proinflammatory Cytokines in Renal Transplant Recipients. J. Investig. Surg. 2019, 32, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gorjipour, F.; Saeedzadeh, T.; Toloueitabar, Y.; Kachoueian, N.; Bahlouli Ghashghaei, S.; Mortazian, M.; Dehghani Firoozabadi, M.; Jadbabaie, A.; Tirgarfakheri, K.; Motamednejad, A.; et al. Remote ischemic preconditioning effects on inflammatory markers and myocardial protection in coronary artery bypass graft surgery. Perfusion 2022, 37, 56–61. [Google Scholar] [CrossRef]
- Abel, F.; Murke, F.; Gaida, M.; Garnier, N.; Ochsenfarth, C.; Theiss, C.; Thielmann, M.; Kleinbongard, P.; Giebel, B.; Peters, J.; et al. Extracellular vesicles isolated from patients undergoing remote ischemic preconditioning decrease hypoxia-evoked apoptosis of cardiomyoblasts after isoflurane but not propofol exposure. PLoS ONE 2020, 15, e0228948. [Google Scholar] [CrossRef]
- Mandyam, M.C.; Soliman, E.Z.; Alonso, A.; Dewland, T.A.; Heckbert, S.R.; Vittinghoff, E.; Cummings, S.R.; Ellinor, P.T.; Chaitman, B.R.; Stocke, K.; et al. The QT interval and risk of incident atrial fibrillation. Heart Rhythm. 2013, 10, 1562–1568. [Google Scholar] [CrossRef]
- Karaman, K.; Karayakali, M.; Arisoy, A.; Akar, I.; Ozturk, M.; Yanik, A.; Yilmaz, S.; Celik, A. Is There any Relationship Between Myocardial Repolarization Parameters and the Frequency of Ventricular Premature Contractions? Arq. Bras. Cardiol. 2018, 110, 534–541. [Google Scholar] [CrossRef]
- Heusch, G.; Bøtker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef]
- White, S.K.; Frohlich, G.M.; Sado, D.M.; Maestrini, V.; Fontana, M.; Treibel, T.A.; Tehrani, S.; Flett, A.S.; Meier, P.; Ariti, C.; et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2015, 8, 178–188. [Google Scholar] [CrossRef]
- Hoole, S.P.; Heck, P.M.; Sharples, L.; Khan, S.N.; Duehmke, R.; Densem, C.G.; Clarke, S.C.; Shapiro, L.M.; Schofield, P.M.; O’Sullivan, M.; et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: A prospective, randomized control trial. Circulation 2009, 119, 820–827. [Google Scholar] [CrossRef]
- Lau, J.K.; Roy, P.; Javadzadegan, A.; Moshfegh, A.; Fearon, W.F.; Ng, M.; Lowe, H.; Brieger, D.; Kritharides, L.; Yong, A.S. Remote Ischemic Preconditioning Acutely Improves Coronary Microcirculatory Function. J. Am. Heart Assoc. 2018, 7, e009058. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Kim, S.G.; Oh, J.; Kim, S.O.; Go, Y.J.; Hwang, G.S.; Song, J.G. Impact of Remote Ischemic Preconditioning Conducted in Living Kidney Donors on Renal Function in Donors and Recipients Following Living Donor Kidney Transplantation: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Bøtker, H.E.; Kharbanda, R.; Schmidt, M.R.; Bøttcher, M.; Kaltoft, A.K.; Terkelsen, C.J.; Munk, K.; Andersen, N.H.; Hansen, T.M.; Trautner, S.; et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet 2010, 375, 727–734. [Google Scholar] [CrossRef] [PubMed]
| Before Propensity Score Matching | After Propensity Score Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | No-RIPC | Paired-RIPC | p | SD | No-RIPC | Paired-RIPC | p | SD |
| n | 270 | 269 | 260 | 260 | ||||
| Preoperative recipient factors | ||||||||
| Sex (female) | 124 (45.9%) | 138 (51.3%) | 0.212 | 0.107 | 118 (45.4%) | 132 (50.8%) | 0.219 | 0.108 |
| Age (years) | 50.0 (40.0–57.0) | 52.0 (41.5–59.0) | 0.126 | 0.115 | 50.0 (41.0–57.0) | 52.0 (42.0–59.0) | 0.135 | 0.113 |
| Body mass index (kg/m2) | 22.9 (20.4–25.4) | 22.8 (20.6–26.0) | 0.676 | 0.071 | 23.0 (20.3–25.5) | 23.0 (20.6–26.1) | 0.557 | 0.082 |
| Dialysis duration (day) | 1.0 (0.0–11.0) | 1.0 (0.0–7.5) | 0.9 | −0.081 | 0.5 (0.0–10.0) | 1.0 (0.0–6.0) | 0.86 | −0.080 |
| Comorbidity | ||||||||
| Diabetes mellitus | 86 (31.9%) | 112 (41.6%) | 0.018 | 0.213 | 84 (32.3%) | 105 (40.4%) | 0.056 | 0.179 |
| Hypertension | 132 (48.9%) | 146 (54.3%) | 0.211 | 0.108 | 127 (48.8%) | 142 (54.6%) | 0.188 | 0.116 |
| Vital sign | ||||||||
| Systolic blood pressure (mmHg) | 130.0 (120.0–140.0) | 132.0 (124.0–141.0) | 0.033 | 0.178 | 130.0 (120.0–140.0) | 131.0 (124.0–140.0) | 0.063 | 0.150 |
| Diastolic blood pressure (mmHg) | 80.0 (79.0–90.0) | 80.0 (80.0–90.0) | 0.256 | 0.092 | 80.0 (80.0–90.0) | 80.0 (80.0–90.0) | 0.38 | 0.065 |
| Heart rate (beats/min) | 80.0 (73.0–88.0) | 80.0 (73.0–88.0) | 0.583 | 0.042 | 80.0 (73.0–88.0) | 62.0 (58.8–64.9) | 0.492 | −0.021 |
| Echocardiography | ||||||||
| Ejection fraction (%) | 62.0 (57.3–64.6) | 62.0 (58.7–64.8) | 0.314 | 0.086 | 62.0 (58.0–64.7) | 62.0 (58.8–64.9) | 0.484 | 0.037 |
| Left ventricular mass index (g/m2) | 119.1 (102.0–144.0) | 119.1 (101.0–140.3) | 0.376 | −0.109 | 119.1 (101.0–142.0) | 118.7 (99.0–140.3) | 0.477 | −0.097 |
| E/e’ ratio | 10.0 (7.8–12.7) | 10.3 (8.7–12.9) | 0.17 | 0.005 | 10.0 (7.8–12.5) | 10.3 (8.7–12.6) | 0.142 | 0.008 |
| Laboratory variables | ||||||||
| White blood cell count (×109/L) | 6.3 (4.8–7.8) | 6.2 (4.8–8.1) | 0.983 | −0.026 | 6.3 (4.8–7.8) | 6.2 (4.7–8.1) | 0.945 | −0.027 |
| Neutrophil (%) | 67.2 (60.7–82.9) | 70.5 (61.6–85.4) | 0.284 | 0.099 | 67.4 (60.6–82.9) | 70.3 (61.5–85.2) | 0.367 | 0.087 |
| Lymphocyte (%) | 19.7 (11.6–26.3) | 18.4 (10.9–25.2) | 0.211 | −0.106 | 19.7 (11.8–26.2) | 18.7 (10.9–25.1) | 0.261 | −0.099 |
| Hemoglobin (g/dL) | 10.6 (9.4–11.6) | 10.7 (9.6–11.5) | 0.508 | 0.025 | 10.6 (9.5–11.6) | 10.7 (9.6–11.5) | 0.649 | 0.016 |
| Platelet count (×109/L) | 178.5 (141.0–230.3) | 178.0 (140.5–218.0) | 0.466 | −0.114 | 180.0 (141.0–230.8) | 178.0 (140.3–216.8) | 0.333 | −0.132 |
| Albumin (g/dL) | 4.1 (3.8–4.3) | 4.1 (3.8–4.3) | 0.54 | 0.053 | 4.1 (3.8–4.4) | 4.1 (3.8–4.3) | 0.868 | −0.014 |
| Sodium (mEq/L) | 138.0 (135.0–140.0) | 138.0 (135.0–140.0) | 0.891 | 0.021 | 138.0 (135.0–140.0) | 138.0 (135.0–140.0) | 0.865 | 0.001 |
| Potassium (mEq/L) | 4.7 (4.3–5.2) | 4.7 (4.2–5.2) | 0.577 | −0.025 | 4.7 (4.3–5.2) | 4.7 (4.2–5.2) | 0.462 | −0.045 |
| Creatinine (mg/dL) | 7.6 (5.9–9.3) | 7.1 (6.0–9.1) | 0.371 | −0.060 | 7.5 (5.9–9.1) | 7.2 (6.0–9.1) | 0.632 | −0.015 |
| B-type natriuretic peptide (pg/mL) | 82.4 (36.1–218.7) | 78.7 (31.6–177.2) | 0.166 | −0.391 | 76.7 (34.1–194.3) | 77.0 (31.6–176.8) | 0.44 | −0.159 |
| High-sensitivity troponin I (pg/mL) | 20.5 (10.4–46.3) | 21.4 (11.1–44.6) | 0.631 | −0.150 | 20.4 (10.4–46.3) | 21.1 (11.0–44.2) | 0.589 | −0.057 |
| Corrected QT interval (ms) | 452.0 (432.0–475.0) | 450.0 (431.0–469.5) | 0.431 | −0.069 | 452.0 (432.0–473.0) | 450.0 (430.3–469.0) | 0.497 | −0.061 |
| Hourly urine output (mL/kg/h) | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 0.162 | 0.131 | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 0.242 | 0.108 |
| Intraoperative recipient factors | ||||||||
| Operation time (min) | 225.0 (190.0–260.0) | 225.0 (195.0–255.0) | 0.677 | 0.015 | 223.5 (190.0–260.0) | 225.0 (195.0–255.0) | 0.533 | 0.037 |
| Hourly fluid infusion (mL/kg/h) | 9.3 (7.3–11.6) | 9.0 (7.4–11.4) | 0.567 | −0.062 | 9.3 (7.3–11.6) | 8.9 (7.2–11.3) | 0.344 | −0.094 |
| Donor and graft factors | ||||||||
| Sex (female) | 176 (65.2%) | 163 (60.6%) | 0.27 | −0.094 | 168 (64.6%) | 160 (61.5%) | 0.467 | −0.063 |
| Age (years) | 51.0 (41.0–57.0) | 51.0 (38.0–59.0) | 0.941 | −0.023 | 51.0 (41.0–57.0) | 51.0 (38.0–59.0) | 0.983 | −0.024 |
| Body mass index (kg/m2) | 23.6 (21.7–25.6) | 23.5 (21.9–26.1) | 0.602 | 0.068 | 23.7 (21.8–25.7) | 23.4 (21.9–26.1) | 0.911 | 0.038 |
| Hemoglobin (g/dL) | 13.7 (12.8–14.9) | 13.9 (12.9–15.1) | 0.54 | 0.035 | 13.7 (12.8–14.9) | 13.8 (12.9–15.1) | 0.647 | 0.024 |
| Left kidney graft | 162 (60.0%) | 174 (64.7%) | 0.262 | −0.098 | 156 (60.0%) | 169 (65.0%) | 0.239 | −0.104 |
| Graft weight (g) | 178.0 (150.0–204.5) | 174.0 (150.0–208.0) | 0.81 | −0.001 | 178.0 (152.0–205.5) | 174.0 (150.0–208.0) | 0.635 | −0.017 |
| Total ischemic time (min) | 55.0 (43.8–67.3) | 54.0 (45.0–67.5) | 0.885 | −0.041 | 55.0 (43.3–67.0) | 54.5 (45.0–68.0) | 0.899 | −0.004 |
| Group | No-RIPC | Paired-RIPC | p |
|---|---|---|---|
| n | 260 | 260 | |
| Cardiac enzymes | |||
| hsTnI (pg/mL) | |||
| Preoperative day | 20.4 (10.4–46.1) | 21.0 (11.0–44.2) | 0.57 |
| 30 min after reperfusion | 21.3 (11.5–53.4) | 21.8 (12.3–47.2) | 0.877 |
| POD 1 | 40.2 (19.2–81.3) | 21.1 (10.1–43.0) | <0.001 |
| hsTnI ≥ 15 pg/mL for female and ≥36 pg/mL for male | |||
| Preoperative day | 119 (45.8%) | 125 (48.1%) | 0.598 |
| 30 min after reperfusion | 127 (48.8%) | 126 (48.5%) | >0.999 |
| POD 1 | 176 (67.7%) | 119 (45.8%) | <0.001 |
| BNP (pg/mL) | |||
| Preoperative day | 76.7 (34.1–194.3) | 77.0 (31.6–176.8) | 0.44 |
| 30 min after reperfusion | 164.3 (88.0–331.1) | 173.2 (90.2–336.9) | 0.703 |
| POD 1 | 129.1 (74.2–259.6) | 116.9 (79.2–227.7) | 0.391 |
| BNP ≥ 100 pg/mL | |||
| Preoperative day | 109 (41.9%) | 110 (42.3%) | 0.929 |
| 30 min after reperfusion | 177 (68.1%) | 181 (69.6%) | 0.705 |
| POD 1 | 162 (62.3%) | 160 (61.5%) | 0.857 |
| Electrocardiogram | |||
| QTc (ms) | |||
| Preoperative day | 452.0 (432.0–473.0) | 450.0 (430.3–469.0) | 0.497 |
| POD 1 | 498.5 (477.0–515.0) | 467.0 (444.8–492.0) | <0.001 |
| QTc ≥ 460 ms for female and ≥450 ms for male | |||
| Preoperative day | 119 (45.8%) | 103 (39.6%) | 0.156 |
| POD 1 | 230 (88.5%) | 170 (65.4%) | <0.001 |
| Group | No-RIPC | Paired-RIPC | p |
|---|---|---|---|
| n | 260 | 260 | |
| New occurrence of arrhythmia | |||
| Atrial fibrillation | 26 (10.0%) | 7 (2.7%) | 0.001 |
| Ventricular premature complex | 32 (12.3%) | 9 (3.5%) | <0.001 |
| Requirement of cardiovascular interventions | |||
| Percutaneous coronary intervention | 12 (4.6%) | 4 (1.5%) | 0.042 |
| Cardiopulmonary resuscitation | 2 (0.8%) | 1 (0.4%) | >0.999 |
| β | Odds Ratio | 95% CI | p | |
|---|---|---|---|---|
| Paired RIPC adjusted for PS | ||||
| hsTnI ≥ 15 pg/mL for female and ≥36 pg/mL for male on POD 1 | −0.909 | 0.403 | 0.282–0.575 | <0.001 |
| BNP ≥ 100 pg/mL on POD 1 | −0.033 | 0.968 | 0.679–1.379 | 0.857 |
| QTc ≥ 460 ms for female and ≥450 ms for male on POD 1 | −1.401 | 0.246 | 0.156–0.39 | <0.001 |
| New occurrence of arrhythmia during postoperative hospital stay | ||||
| Atrial fibrillation | −1.39 | 0.249 | 0.106–0.585 | 0.001 |
| Ventricular premature complex | −1.365 | 0.255 | 0.119–0.547 | <0.001 |
| Requirement of cardiovascular interventions during postoperative hospital stay | ||||
| Percutaneous coronary intervention | −1.13 | 0.323 | 0.103–1.015 | 0.053 |
| Cardiopulmonary resuscitation | −0.697 | 0.498 | 0.045–5.527 | 0.57 |
| Group | No-RIPC | Paired-RIPC | p |
|---|---|---|---|
| n | 260 | 260 | |
| Administration period (days) | |||
| ICU stay | 4.0 (2.0–5.0) | 2.0 (2.0–3.0) | <0.001 |
| hospital stay | 14.0 (12.0–15.0) | 13.0 (12.0–15.0) | 0.351 |
| Requirement of rescue dialysis | 26 (10.0%) | 13 (5.0%) | 0.03 |
| Requirement of re-operation | 3 (1.2%) | 3 (1.2%) | >0.999 |
| Serum creatinine (mg/dL) | |||
| Preoperative day | 7.5 (5.9–9.1) | 7.2 (6.0–9.1) | 0.632 |
| POD 1 | 2.7 (1.9–3.5) | 2.6 (1.8–3.4) | 0.438 |
| POD 2 | 1.3 (1.0–1.8) | 1.3 (0.9–1.9) | 0.981 |
| POD 3 | 1.1 (0.8–1.5) | 1.1 (0.8–1.5) | 0.887 |
| POD 7 | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) | 0.792 |
| Hourly urine output (mL/kg/h) | |||
| Preoperative day | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 0.242 |
| POD 1 | 6.4 (5.0–8.5) | 6.6 (5.0–8.4) | 0.823 |
| POD 2 | 4.5 (3.6–5.6) | 4.6 (3.6–5.7) | 0.807 |
| POD 3 | 4.0 (3.3–5.1) | 4.0 (3.1–5.0) | 0.422 |
| POD 7 | 2.2 (1.8–2.8) | 2.3 (1.9–2.8) | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, J.; Chae, M.S. Paired Remote Ischemic Preconditioning in Recipients and Living Donors Can Mitigate Cardiovascular Stress in Recipients After Living-Donor Kidney Transplantation: A Propensity-Score-Matching Analysis. Medicina 2024, 60, 1826. https://doi.org/10.3390/medicina60111826
Huh J, Chae MS. Paired Remote Ischemic Preconditioning in Recipients and Living Donors Can Mitigate Cardiovascular Stress in Recipients After Living-Donor Kidney Transplantation: A Propensity-Score-Matching Analysis. Medicina. 2024; 60(11):1826. https://doi.org/10.3390/medicina60111826
Chicago/Turabian StyleHuh, Jaewon, and Min Suk Chae. 2024. "Paired Remote Ischemic Preconditioning in Recipients and Living Donors Can Mitigate Cardiovascular Stress in Recipients After Living-Donor Kidney Transplantation: A Propensity-Score-Matching Analysis" Medicina 60, no. 11: 1826. https://doi.org/10.3390/medicina60111826
APA StyleHuh, J., & Chae, M. S. (2024). Paired Remote Ischemic Preconditioning in Recipients and Living Donors Can Mitigate Cardiovascular Stress in Recipients After Living-Donor Kidney Transplantation: A Propensity-Score-Matching Analysis. Medicina, 60(11), 1826. https://doi.org/10.3390/medicina60111826






