Morphofunctional Assessment of Malnutrition and Sarcopenia Using Nutritional Ultrasonography in Patients Undergoing Maintenance Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.2.1. Patient Characteristics and Analytic Variables
2.2.2. Anthropometric Variables
2.2.3. Bioelectrical Impedanciometry Variables
2.2.4. Muscle Strength Variables
2.2.5. Functional Physical Performance Variable
2.2.6. Nutritional Ultrasonography Variables
2.2.7. Malnutrition and Frailty Diagnosis
2.2.8. Sarcopenia Diagnosis
2.3. Statistical Analysis
3. Results
Baseline Patient Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Aziz, G.; Ebrahim, Z.; Esau, N.; Bazezew, M.M. Assessment Criteria to Diagnose Malnutrition (Undernutrition and Overnutrition) in Hemodialysis Patients. J. Ren. Nutr. 2024, 35, 328–336. [Google Scholar] [CrossRef]
- Rashid, I.; Bashir, A.; Tiwari, P.; D’Cruz, S.; Jaswal, S. Estimates of malnutrition associated with chronic kidney disease patients globally and its contrast with India: An evidence based systematic review and meta-analysis. Clin. Epidemiol. Glob. Health 2021, 12, 100855. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Rothenberg, E.; Barazzoni, R. A Clinically Relevant Diagnosis Code for “Malnutrition in Adults” Is Needed in ICD-11. J. Nutr. Health Aging 2022, 26, 314–315. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Sánchez Tocino, M.L.; Cigarrán, S.; Ureña, P.; González Casaus, M.L.; Mas-Fontao, S.; Gracia Iguacel, C.; Ortíz, A.; Gonzalez-Parra, E. Definición y evolución del concepto de sarcopenia. Nefrología 2024, 44, 323–330. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, F.; Carrero, J.J.; Rodrigues, J.C.D.; Bigogno, F.G.; Fetter, R.L.; Avesani, C.M. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: The impact of different diagnostic criteria. J. Nutr. Health Aging 2014, 18, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef]
- García Almeida, J.M.; García García, C.; Aguilar, I.M.V.; Castañeda, V.B.; Guerrero, D.B. Nuevo enfoque de la nutrición. Valoración del estado nutricional del paciente: Función y composición corporal. Nutr. Hosp. 2018, 35, 1–14. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, C.; Zhang, Z.; Su, B. Muscle ultrasound to diagnose sarcopenia in chronic kidney disease: A systematic review and bayesian bivariate meta-analysis. BMC Nephrol. 2024, 25, 12. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Guerrero, D.B.; Lesmes, I.B.; Madueño, F.J.T. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes. Nutr. 2023, 70 (Suppl. S1), 74–84. [Google Scholar] [CrossRef]
- International Society for the Advancement of Kinanthropometry. International Standards for Anthropometric Assessment—ScienceOpen. Available online: https://www.scienceopen.com/document?vid=ccde4859-d1c7-4ea6-92b8-37d48744f862 (accessed on 30 March 2025).
- Sartorio, A.; Malavolti, M.; Agosti, F.; Marinone, P.G.; Caiti, O.; Battistini, N.; Bedogni, G. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2005, 59, 155–160. [Google Scholar] [CrossRef]
- InBody S10 USER’S MANUAL. Available online: http://www.inbody.com (accessed on 30 March 2025).
- Lin, T.Y.; Wu, M.Y.; Chen, H.S.; Hung, S.C.; Lim, P.S. Development and validation of a multifrequency bioimpedance spectroscopy equation to predict appendicular skeletal muscle mass in hemodialysis patients. Clin. Nutr. 2021, 40, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Short Physical Performance Battery (SPPB)|National Institute on Aging. Available online: https://www.nia.nih.gov/research/labs/leps/short-physical-performance-battery-sppb (accessed on 30 March 2025).
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo De Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- A Formula to Estimate the Approximate Surface Area If Height and Weight Be Known. 1916. Available online: https://pubmed.ncbi.nlm.nih.gov/2520314/ (accessed on 31 March 2025).
- Sabatino, A.; Regolisti, G.; di Mario, F.; Ciuni, A.; Palumbo, A.; Peyronel, F.; Maggiore, U.; Fiaccadori, E. Validation by CT scan of quadriceps muscle thickness measurement by ultrasound in acute kidney injury. J. Nephrol. 2020, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Reliability of the 7-Point Subjective Global Assessment Scale in Assessing Nutritional Status of Dialysis Patients. Available online: https://pubmed.ncbi.nlm.nih.gov/10682107/ (accessed on 6 April 2025).
- Moreau-Gaudry, X.; Jean, G.; Genet, L.; Lataillade, D.; Legrand, E.; Kuentz, F.; Fouque, D. A simple protein-energy wasting score predicts survival in maintenance hemodialysis patients. J. Ren. Nutr. 2014, 24, 395–400. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Hakim, R.M.; Levin, N. Malnutrition in hemodialysis patients. Am. J. Kidney Dis. 1993, 21, 125–137. [Google Scholar] [CrossRef]
- Alp Ikizler, T.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Macedo, C.; Amaral, T.F.; Rodrigues, J.; Santin, F.; Avesani, C.M. Malnutrition and Sarcopenia Combined Increases the Risk for Mortality in Older Adults on Hemodialysis. Front. Nutr. 2021, 8, 721941. [Google Scholar] [CrossRef]
- Assessment of Methods to Identify Protein-Energy Wasting in Patients on Hemodialysis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21541464/ (accessed on 19 April 2025).
- De Mutsert, R.; Grootendorst, D.C.; Boeschoten, E.W.; Brandts, H.; Van Manen, J.G.; Krediet, R.T.; Dekker, F.W. Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients 1–4. Am. J. Clin. Nutr. 2009, 89, 787–793. [Google Scholar] [CrossRef]
- Sohrabi, Z.; Kohansal, A.; Mirzahosseini, H.; Naghibi, M.; Zare, M.; Haghighat, N.; Akbarzadeh, M. Comparison of the Nutritional Status Assessment Methods for Hemodialysis Patients. Clin. Nutr. Res. 2021, 10, 219. [Google Scholar] [CrossRef]
- Jansen, M.A.M.; Korevaar, J.C.; Dekker, F.W.; Jager, K.J.; Boeschoten, E.W.; Krediet, R.T. Renal function and nutritional status at the start of chronic dialysis treatment. J. Am. Soc. Nephrol. 2001, 12, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Iguacel, C.; González-Parra, E.; Mahillo, I.; Ortiz, A. Criteria for classification of protein-energy wasting in dialysis patients: Impact on prevalence. Br. J. Nutr. 2019, 121, 1271–1278. [Google Scholar] [CrossRef]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Arias-Guillén, M.; Collado, S.; Coll, E.; Carreras, J.; Betancourt, L.; Romano, B.; Fernández, M.; Duarte, V.; Garro, J.; Soler, J.; et al. Prevalence of Protein-Energy Wasting in Dialysis Patients Using a Practical Online Tool to Compare with Other Nutritional Scores: Results of the Nutrendial Study. Nutrients 2022, 14, 3375. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, A.Y.; Do, J.Y. Association of sarcopenia and its components with clinical outcomes in patients undergoing peritoneal dialysis. Kidney Res. Clin. Pract. 2022, 41, 741–752. [Google Scholar] [CrossRef]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Bárány, P.; Heimbürger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef]
- Zicarelli, M.; Duni, A.; Leivaditis, K.; Lin, Y.L.; Baciga, F.; Pugliese, S.; Fiorentino, M.; Hsu, B.-G.; Roumeliotis, S.; Battaglia, Y.; et al. Comprehensive Insights into Sarcopenia in Dialysis Patients: Mechanisms, Assessment, and Therapeutic Approaches. Medicina 2025, 61, 449. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.R.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of sarcopenia in patients with chronic kidney disease: A global systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef]
- Wathanavasin, W.; Banjongjit, A.; Avihingsanon, Y.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S.; Susantitaphong, P. Prevalence of Sarcopenia and Its Impact on Cardiovascular Events and Mortality among Dialysis Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4077. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.R.; Choi, M.J.; Kim, S.G.; Lee, Y.K.; Noh, J.W.; Kim, H.J.; Song, Y.R. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin. Nutr. 2014, 33, 64–68. [Google Scholar] [CrossRef] [PubMed]
- García-Menéndez, E.; Portolés, J.; Pérez Rodrigo, I.; Tato Ribera, A.; Yuste Lozano, C.; Ossorio González, M.; López, M.J.Á.; Sánchez, P.L.; Marín, D.J. Ecografía POCUS: Una herramienta para la detección y seguimiento de sarcopenia en diálisis peritoneal. Nefrología 2025, 45, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Lin, T.; Wang, H.; Zhao, Y.; Jiang, T.; Peng, X.; Yue, J. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Slee, A.; McKeaveney, C.; Adamson, G.; Davenport, A.; Farrington, K.; Fouque, D.; Kalantar-Zadeh, K.; Mallett, J.; Maxwell, A.P.; Mullan, R.; et al. Estimating the Prevalence of Muscle Wasting, Weakness, and Sarcopenia in Hemodialysis Patients. J. Ren. Nutr. 2020, 30, 313–321, Erratum in J. Ren. Nutr. 2021, 31, e5. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Wong, B.; Goldet, G.; Davenport, A. Differences in Prevalence of Muscle Wasting in Patients Receiving Peritoneal Dialysis per Dual-Energy X-Ray Absorptiometry Due to Variation in Guideline Definitions of Sarcopenia. Nutr. Clin. Pract. 2017, 32, 539–544. [Google Scholar] [CrossRef]
- Ng, J.K.C.; Lau, S.L.F.; Chan, G.C.K.; Tian, N.; Li, P.K.T. Nutritional Assessments by Bioimpedance Technique in Dialysis Patients. Nutrients 2023, 16, 15. [Google Scholar] [CrossRef]
- Cioffi, I.; Marra, M.; Imperatore, N.; Pagano, M.C.; Santarpia, L.; Alfonsi, L.; Testa, A.; Sammarco, R.; Contaldo, F.; Castiglione, F.; et al. Assessment of bioelectrical phase angle as a predictor of nutritional status in patients with Croh’s disease: A cross sectional study. Clin. Nutr. 2020, 39, 1564–1571. [Google Scholar] [CrossRef]
- Tan, R.-S.; Liang, D.-H.; Liu, Y.; Zhong, X.-S.; Zhang, D.-S.; Ma, J. Bioelectrical Impedance Analysis-Derived Phase Angle Predicts Protein-Energy Wasting in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2019, 29, 295–301. [Google Scholar] [CrossRef]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwée, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef]
- Ticinesi, A.; Narici, M.V.; Lauretani, F.; Nouvenne, A.; Colizzi, E.; Mantovani, M.; Corsonello, A.; Landi, F.; Meschi, T.; Maggio, M. Assessing sarcopenia with vastus lateralis muscle ultrasound: An operative protocol. Aging Clin. Exp. Res. 2018, 30, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.M.; van der Schans, C.P. The reliability and validity of ultrasound to quantify muscles in older adults: A systematic review. J. Cachexia Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, W.; Beek Lter Hobbelen, J.S.M.; van der Vaart, H.; Wempe, J.B.; van der Schans, C.P.; Jager-Wittenaar, H. The added value of ultrasound muscle measurements in patients with COPD: An exploratory study. Clin. Nutr. ESPEN 2019, 30, 152–158. [Google Scholar] [CrossRef]
- López-Gómez, J.J.; García-Beneitez, D.; Jiménez-Sahagún, R.; Izaola-Jauregui, O.; Primo-Martín, D.; Ramos-Bachiller, B.; Gómez-Hoyos, E.; Delgado-García, E.; Pérez-López, P.; De Luis-Román, D.A. Nutritional Ultrasonography, a Method to Evaluate Muscle Mass and Quality in Morphofunctional Assessment of Disease Related Malnutrition. Nutrients 2023, 15, 3923. [Google Scholar] [CrossRef]
- de Luis Roman, D.; García Almeida, J.M.; Bellido Guerrero, D.; Guzmán Rolo, G.; Martín, A.; Primo Martín, D.; García-Delgado, Y.; Guirado-Peláez, P.; Palmas, F.; Tejera Pérez, C.; et al. Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients 2024, 16, 1552. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.H.; Singh, B.K.S.; Sabatino, A.; Fiaccadori, E.; Daud, Z.A.M.; Ali, M.S.; Narayanan, S.S.; Tallman, D.; Chinna, K.; et al. Association of Ultrasound-Derived Metrics of the Quadriceps Muscle with Protein Energy Wasting in Hemodialysis Patients: A Multicenter Cross-Sectional Study. Nutrients 2020, 12, 3597. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Samaan, E.; El-Gamal, M.; Shamsuddin, M.; Tharwat, S. Concordance between muscle mass assessed by bioelectrical impedance analysis and by muscle ultrasound: A cross-sectional study in a cohort of patients on chronic hemodialysis. BMC Nephrol. 2024, 25, 49. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Yamamoto, S.; Suzuki, Y.; Imamura, K.; Harada, M.; Matsunaga, A.; Tamaki, A.; Fukui, T.; Shimokado, K. The clinical applicability of ultrasound technique for diagnosis of sarcopenia in hemodialysis patients. Clin. Nutr. 2021, 40, 1161–1167. [Google Scholar] [CrossRef]
- García-García, C.; Vegas-Aguilar, I.M.; Rioja-Vázquez, R.; Cornejo-Pareja, I.; Tinahones, F.J.; García-Almeida, J.M. Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial). Nutrients 2023, 15, 522. [Google Scholar] [CrossRef]
- Vogt, B.P.; Borges, M.C.C.; Goés CRde Caramori, J.C.T. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin. Nutr. 2016, 35, 1429–1433. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Neri, S.G.R.; Oliveira, J.S.; Bennett, P.N.; Viana, J.L.; Lima, R.M. Association between sarcopenia and clinical outcomes in chronic kidney disease patients: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Avesani, C.M.; de Abreu, A.M.; Ribeiro, H.S.; Brismar, T.B.; Stenvinkel, P.; Sabatino, A.; Lindholm, B. Muscle fat infiltration in chronic kidney disease: A marker related to muscle quality, muscle strength and sarcopenia. J. Nephrol. 2023, 36, 895. [Google Scholar] [CrossRef]
- Bunout, D.; Gonzalez, S.; Canales, M.; Barrera, G.; Hirsch, S. Ultrasound assessment of rectus femoris pennation angle and echogenicity. Their association with muscle functional measures and fat infiltration measured by CT scan. Clin. Nutr. ESPEN 2023, 55, 420–424. [Google Scholar] [CrossRef]
- Mañago, M.M.; Seamon, B.A.; Boncella, K.L.; Wallin, M.T.; Maloni, H.; Hoover, B.; Blackman, M.R.; Harris-Love, M.O. Ultrasound measures of muscle morphology in people with multiple sclerosis are associated with muscle performance and functional mobility. Mult. Scler. Relat. Disord. 2023, 75, 104759. [Google Scholar] [CrossRef] [PubMed]
- Corona, L.P.; de Oliveira Duarte, Y.A.; Lebrão, M.L. Markers of nutritional status and mortality in older adults: The role of anemia and hypoalbuminemia. Geriatr. Gerontol. Int. 2018, 18, 177–182. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Sabatino, A.; Barazzoni, R.; Carrero, J.J.; Cupisti, A.; De Waele, E.; Jonckheer, J.; Singer, P.; Cuerda, C. ESPEN Guideline ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin. Nutr. 2021, 40, 1644–1668. [Google Scholar] [CrossRef]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Sy, J.; Johansen, K.L. The impact of frailty on outcomes in dialysis. Curr. Opin. Nephrol. Hypertens. 2017, 26, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, Y.; Kanda, E.; Ishibashi, Y.; Yoshida, M. Sarcopenia and Frailty in PD: Impact on Mortality, Malnutrition, and Inflammation. Perit. Dial. Int. 2018, 38, 447–454. [Google Scholar] [CrossRef]
- Hu, X.; Wu, B.; Yang, Y.; Zhang, L.; Xue, C. Sarcopenia in Peritoneal Dialysis: Prevalence, Pathophysiology, and Management Strategies. Kidney Med. 2025, 75, 100989. [Google Scholar] [CrossRef]
- Liu, P.J.; Guo, J.; Zhang, Y.; Wang, F.; Yu, K. Effects of oral nutritional supplements on the nutritional status and inflammatory markers in patients on maintenance dialysis: A systematic review and meta-analysis of randomized clinical trials. Clin. Kidney J. 2023, 16, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, R.; Yamamoto, S.; Suzuki, Y.; Abe, Y.; Harada, M.; Shimoda, T.; Imamura, K.; Yamabe, S.; Ito, H.; Yoshikoshi, S.; et al. The effects of amino acid/protein supplementation in patients undergoing hemodialysis: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN. 2021, 44, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar]
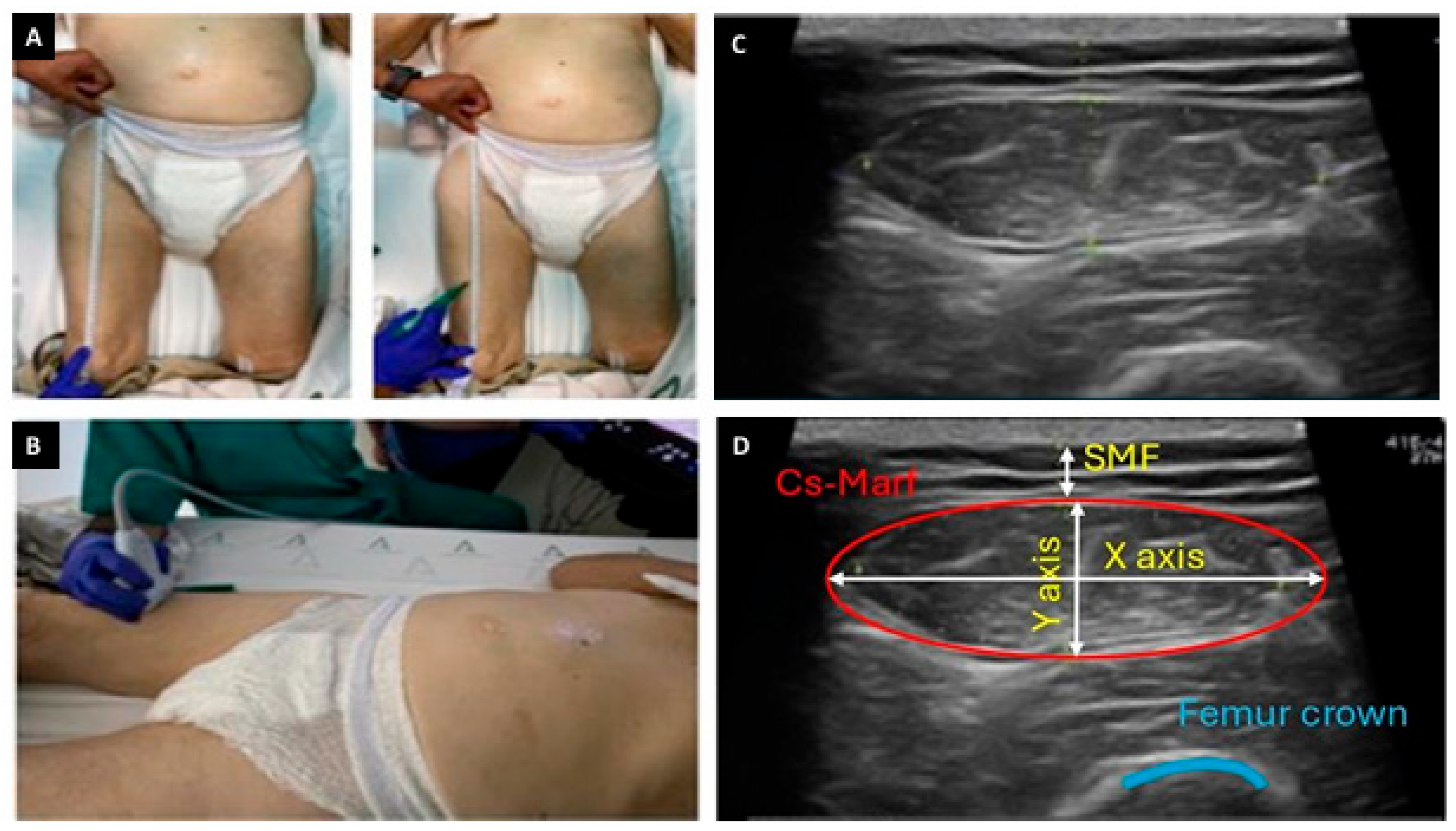
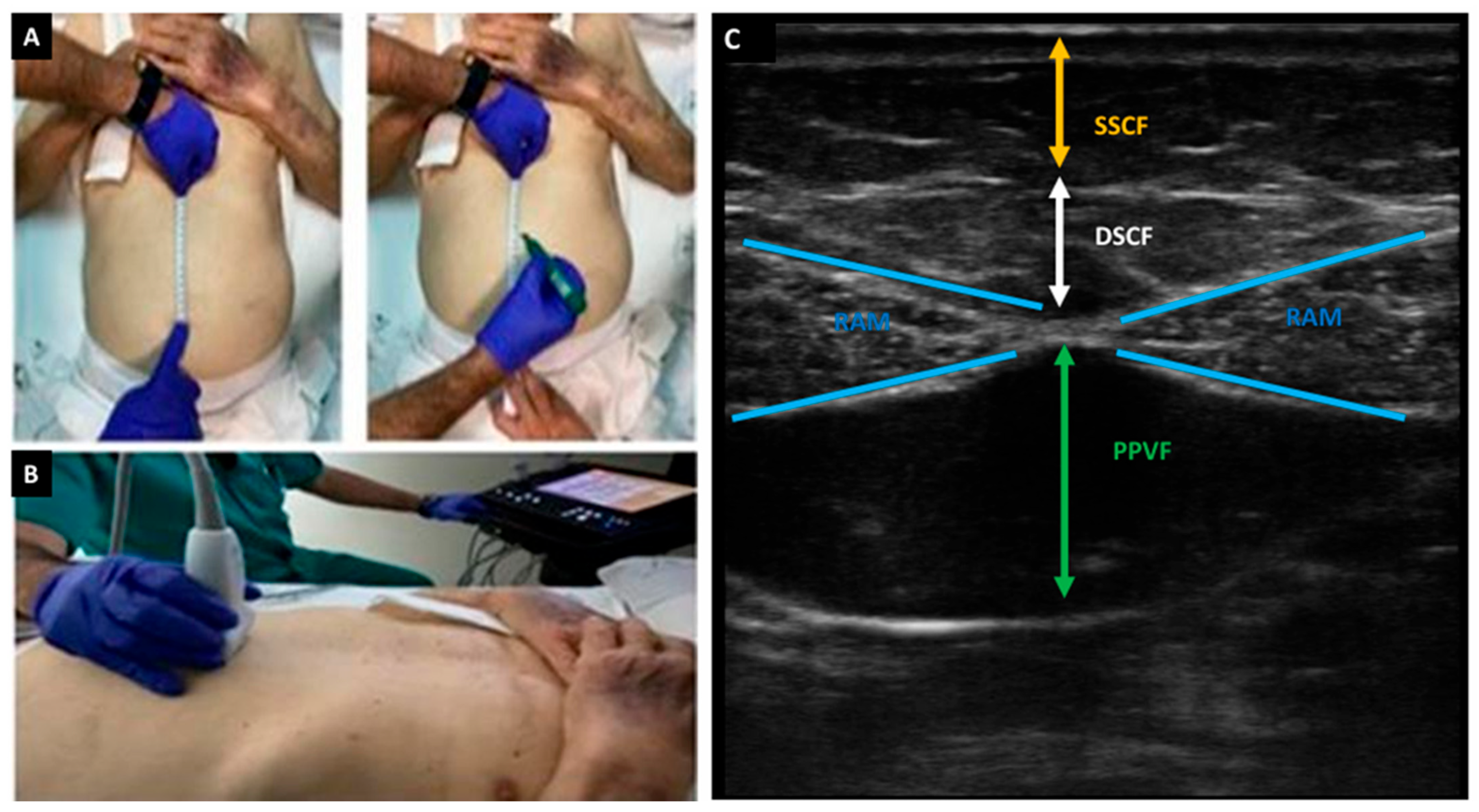

| (A) | ||||
| Women n = 22 29.7% | Men n = 52 70.3% | Total n = 74 100% | p-Value | |
| Sociodemographic and anthropometric | ||||
| Age (years), mean (SD) | 69.5 (19.8) | 74.7 (13.3) | 73.1 (15.5) | 0.2 |
| BMI (kg/m2), mean (SD) | 26.1 (5.1) | 24.4 (3.7) | 24.9 (4.2) | 0.11 |
| Arm circumference (cm), mean (SD) | 28.9 (3.4) | 28.7 (8.6) | 28.8 (7.4) | 0.91 |
| Calf circumference (cm), mean (SD) | 81.8 (12.9) | 76.1 (15.3) | 77.8 (14.8) | 0.13 |
| Triceps skinfold (mL), mean (SD) | 15.1 (5.8) | 11.2 (6.0) | 12.4 (6.2) | 0.01 |
| Suprailiac skinfold (mL), mean (SD) | 17.9 (8.1) | 16.3 (7.9) | 16.7 (8.0) | 0.42 |
| COPD, n (%) | 5 (22.7) | 18 (34.6) | 23 (31.1) | 0.3 |
| Ischemic heart disease, n (%) | 9 (40.9) | 28 (53.9) | 37 (50.0) | 0.3 |
| Secondary hyperparathyroidism, n (%) | 21 (95.5) | 52 (100) | 73 (98.7) | 0.1 |
| Causes of CKD, n (%) | ||||
| Diabetic kidney disease | 7 (31.8) | 18 (34.6) | 25 (33.8) | 0.8 |
| Non-diabetic kidney disease | 15 (68.2) | 34 (65.4) | 49 (66.2) | |
| Hemodialysis parameters | ||||
| HD vintage (months), mean (SD) | 51.2 (41.7) | 33.5 (27.2) | 38.8 (32.9) | 0.03 |
| Dry weight (Kg), mean (SD) | 63.8 (13.2) | 70.1 (11.6) | 68.2 (12.3) | 0.047 |
| IDWG (kg)-mean (SD | 2.0 (0.6) | 2.1(0.7) | 2.1(0.7) | 0.39 |
| Kt/V urea, mean (SD) | 1.7 (0.3) | 1.6 (0.2) | 1.6 (0.2) | 0.02 |
| KT (L)-mean (SD) | 52.2 (5.9) | 52 (6.6) | 52 (6.4) | 0.88 |
| nPCR (g Urea/Kg/d), mean (SD) | 1.1 (0.4) | 1.1 (0.4) | 1.1 (0.4) | 0.63 |
| QB (mL/min), mean (SD) | 334.4 (22.6) | 335.4 (21.1) | 335.1 (21.4) | 0.86 |
| Inf.Vol. OL-HDF (L), mean (SD) | 24.9 (4.1) | 24.2 (4.5) | 24.4 (4.4) | 0.53 |
| APF (mL/min), mean (SD) | 182.1 (32.2) | 168.9 (37.4) | 172.8 (36.2) | 0.16 |
| VPF (mL/min), mean (SD) | 167.4 (22.6) | 160.2 (22.4) | 162.3 (22.5) | 0.21 |
| SBP (mmHg), mean (SD) | 136.1 (21.5) | 128 (24.4) | 130.4 (23.7) | 0.18 |
| DBP (mmHg), mean (SD) | 69.9 (14.9) | 66.5 (16.9) | 67.5 (16.3) | 0.41 |
| Sessions per week (day) | ||||
| Three times per week, n (%) | 18 (81.8) | 43 (82.7) | 61 (82.4) | 0.9 |
| iHD one or two times per week, n (%) | 4 (18.2) | 9 (17.3) | 13 (17.6) | |
| Vascular access type | ||||
| Arteriovenous fistula, n (%) | 6 (27.3) | 25 (48.1) | 31 (41.9) | 0.1 |
| Tunneled catheter, n (%) | 16 (72.7) | 27 (51.9) | 43 (58.1) | |
| (B) | ||||
| Women n = 22 29.7% | Men n = 52 70.3% | Total n = 74 100% | p-Value | |
| Muscle strength | ||||
| Handgrip strength (kg), mean (SD) | 11.9 (6.7) | 20.9 (8.1) | 18.2 (8.7) | <0.001 |
| 30 s Chair Stand Test (number of repeats), mean (SD) | 9 (5.5) | 10.1 (4.8) | 9.8 (5) | 0.41 |
| Functional performance | ||||
| SPPB (points) | 8.3 (2.2) | 8.9 (2.2) | 8.7 (2.2) | 0.3 |
| Low performance (SPPB ≤ 8), n (%) | 6 (26.9) | 15 (28.84) | 21 (28.4) | 0.7 |
| Muscle nutritional ultrasound | ||||
| Y-axis (mm), mean (SD) | 8.4 (2.2) | 9.2 (2.4) | 8.7 (2.3) | 0.18 |
| Y-axis/height (mm/m2), mean (SD) | 3 (0.9) | 3.8 (0.9) | 3.2 (1) | <0.001 |
| Y-axis/BSA (mm/m2), mean (SD) | 2.7 (0.8) | 3.5 (0.7) | 2.9 (0.9) | <0.001 |
| X-axis (mm), mean (SD) | 30.5 (5.8) | 30.7 (8.1) | 30.5 (6.5) | 0.89 |
| CS-MARF (cm2), mean (SD) | 2.5 (0.7) | 2.9 (0.9) | 2.6 (0.8) | 0.03 |
| MARFIh (cm2/m2), mean (SD) | 0.9 (0.3) | 1.2 (0.4) | 1 (0.3) | <0.001 |
| MARFIBSA (cm2/m2), mean (SD) | 0.8 (0.2) | 1.1 (0.3) | 0.9 (0.3) | <0.001 |
| X-axis/Y-axis ratio, mean (SD) | 3.6 (1.5) | 3.4 (0.9) | 3.8 (1.4) | 0.14 |
| SMF (mm), mean (SD) | 8.3 (2.5) | 6.6 (2.0) | 6.9 (2.2) | 0.01 |
| Visceral fat nutritional ultrasound | ||||
| Transverse PPVF, (cm) (SD) | 0.6 (0.3) | 0.6 (0.3) | 0.6 (0.3) | 0.71 |
| Transverse SSCF (cm), mean (SD) | 1.2 (0.5) | 1.0 (0.4) | 1.2 (0.5) | 0.1 |
| Transverse DSCF (cm), mean (SD) | 0.9 (0.3) | 0.8 (0.3) | 0.8 (0.3) | 0.3 |
| Bioimpedance parameters | ||||
| TBW (L), mean (SD) | 29 (3.9) | 37.7 (7.9) | 35.1 (8) | <0.001 |
| ICW (L), mean (SD) | 17.5 (2.6) | 24.1 (9) | 22.1 (8.2) | 0.001 |
| ECW (L), mean (SD) | 11.4 (1.5) | 15.3 (3.9) | 14.1 (3.8) | <0.001 |
| ECW/TBW ratio, mean (SD) | 0.4 (0) | 0.4 (0.1) | 0.4 (0) | 0.36 |
| BFM (kg), mean (SD) | 24.1 (11.4) | 19.3 (8.5) | 20.8 (9.6) | 0.05 |
| FFM (kg), mean (SD) | 39.5 (5.4) | 51.1 (11.2) | 47.7 (11.2) | <0.001 |
| LBM (kg), mean (SD) | 36.6 (5.4) | 48.2 (10.2) | 44.7 (10.5) | <0.001 |
| BCM (kg), mean (SD) | 30.5 (7.0) | 30.6 (6.5) | 30.5(6.9) | 0.97 |
| BTM (kCals/24 h), mean (SD) | 1222.7 (116) | 1486.7 (218.6) | 1408.2 (228.1) | <0.001 |
| Skeletal muscle mass (kg), mean (SD) | 20.9 (3.4) | 28 (5.8) | 25.9 (6.1) | <0.001 |
| Bone mineral content (kg), mean (SD) | 2.4 (0.3) | 3.1 (0.6) | 2.9 (0.6) | <0.001 |
| TBW/FFM (%), mean (SD) | 73.6 (0.5) | 73.6 (0.9) | 73.6 (0.8) | 0.82 |
| Protein (Kg), mean (SD) | 7.6 (1.1) | 9.9 (1.9) | 9.2 (2) | <0.001 |
| Minerals (Kg) mean (SD) | 2.8 (0.4) | 3.7 (0.7) | 3.4 (0.7) | <0.001 |
| Visceral fat area (cm2), mean (SD) | 122.8 (67) | 80.1 (46.3) | 92.8 (56.3) | 0.002 |
| ASMI using Lin’s formula, kg/m2, mean (SD) | 5.9 (0.8) | 6.8 (1.2) | 6.6 (1.2) | 0.003 |
| Phase angle (◦), mean (SD) | 4.6 (1.4) | 4.9 (1.5) | 4.8 (1.5) | 0.38 |
| Biochemical parameters | ||||
| Hb (g/L), mean (SD) | 10.7 (1.4) | 11.4 (1.6) | 11.2 (1.6) | 0.07 |
| Lymphocytes (103/µL), mean (SD) | 1.3 (0.4) | 1 (0.5) | 1.1 (0.5) | 0.08 |
| Fe (mg/dL), mean (SD) | 70.3 (42.5) | 69.7 (29) | 69.8 (33.3) | 0.94 |
| Transferrin (mg/dL), mean (SD) | 169.5 (28.9) | 169.3 (29.8) | 169.4 (29.4) | 0.97 |
| TSAT (%), | 32.5 (18.6) | 32.6 (13.6) | 32.6 (15.1) | 0.98 |
| Ferritin (ng/mL), mean (SD) | 541.5 [383–688] | 679.5 [447–1101.5] | 638.5 [413–1017] | 0.28 |
| Calcium serum (mg/dL), mean (SD | 8.9 (0.8) | 8.8 (0.7) | 8.9 (0.7) | 0.62 |
| Magnesium serum (mg/dL), mean (SD) | 2.2 (0.3) | 2.2 (0.3) | 2.2 (0.3) | 0.75 |
| Phosphorus serum (mg/dL), mean (SD) | 4.2 (1.7) | 4.3 (1.5) | 4.3 (1.5) | 0.69 |
| 25OHD serum (ng/mL), mean (SD) | 26.5 (14.4) | 25.1 (13.8) | 25.5 (13.9) | 0.7 |
| PTH serum (pg/mL), mean (SD) | 137 [73.7–415] | 241 [109.5–340.8] | 217.5 [86.6–346] | 0.84 |
| Cholesterol (mg/dL), mean (SD) | 150.5 (38.2) | 121.2 (24) | 129.9 (31.7) | <0.001 |
| Triglycerides (mg/dL), mean (SD) | 130 (50.3) | 119.8 (74.9) | 122.8 (68.3) | 0.56 |
| HDL (mg/dL), mean (SD) | 50.2 (21.9) | 47.5 (16.3) | 48.3 (18) | 0.56 |
| Non-HDL cholesterol (mg/dL), mean (SD) | 100.3 (32.7) | 73.6 (20.7) | 81.6 (27.5) | <0.001 |
| LDL (mg/dL), mean (SD) | 80.6 (29.3) | 53.7 (18.2) | 61.7 (25.2) | <0.001 |
| C-reactive protein (mg/L), mean (SD) | 2.9 (7.4) | 2 (3) | 2.3 (4.8) | 0.42 |
| Albumin (g/dL), mean (SD) | 3.3 (0.5) | 3.2 (0.5) | 3.2 (0.5) | 0.77 |
| Prealbumin (mg/dL), mean (SD) | 26.5 (6.3) | 26.5 (6.9) | 26.5 (6.7) | 0.99 |
| Total proteins (g/dL), mean (SD) | 6.4 (0.4) | 6.4 (0.7) | 6.4 (0.6) | 0.8 |
| Urea serum (mmol/mL), mean (SD) | 111.9 (48.4) | 122.3 (54.4) | 119.2 (52.6) | 0.44 |
| Cr serum (g/dL), mean (SD) | 5.7 (2.5) | 6.2 (2.1) | 6 (2.2) | 0.42 |
| Sodium serum (mmol/L), mean (SD) | 138.1 (3.2) | 138.2 (3.5) | 138.2 (3.4) | 0.97 |
| Potassium serum (mmol/L), mean (SD) | 4.6 (0.9) | 4.5 (0.7) | 4.5 (0.8) | 0.92 |
| Chlorine serum (mmol/L), mean (SD) | 101.9 (3.1) | 101.6 (4) | 101.7 (3.7) | 0.73 |
| Bicarbonate (mEq/L), mean (SD) | 22.5 (2.6) | 23.6 (2.3) | 23.3 (2.4) | 0.09 |
| Scales of risk malnutrition | ||||
| 7 –points SGA scale | ||||
| Well-nourished, n (%) | 13 (59) | 24 (46) | 37 (50) | 0.06 |
| Mild–moderate–severely malnourished, n (%) | 9 (41) | 28 (54) | 37 (50) | |
| MIS (points) | 7.9 (3.3) | 7.5 (4.1) | 7.6 (3.9) | 0.7 |
| Patients with MIS ≥ 8 points, n (%) | 9 (40.9) | 21 (40.4) | 30 (40.5) | 0.97 |
| MST ≥ 2 points, n (%) | 3 (13.6) | 13 (25) | 16 (21.6) | 0.3 |
| PEW (score) | ||||
| PEW (score 0–3), n (%) | 19 (86.4) | 47 (90.4) | 66 (89.2) | 0.6 |
| No PEW (score 4), n (%) | 3 (13.6) | 5 (9.61) | 8 (10.8) | |
| FRAIL scale | ||||
| No frailty (score 0 points), n (%) | 7 (31.8) | 23 (44.2) | 30 (40.5) | 0.5 |
| Risk of frailty (score 1–2 points), n (%) | 6 (27.3) | 9 (17.3) | 15 (20.3) | |
| Frail (score ≥ 3 points), n (%) | 9 (40.9) | 20 (38.5) | 29 (39.2) | |
| SARC-F score | 3.4 (2.9) | 2.6 (2.9) | 2.9 (2.9) | 0.28 |
| SARC-F ≥ 4 points, n (%) | 9 (40.9) | 17 (32.7) | 26 (35.1) | 0.5 |
| EWGSOP2 | ||||
| Confirmed sarcopenia, n (%) | 5 (22.7) | 25 (48.1) | 30 (40.5) | 0.01 |
| Risk of sarcopenia, n (%) | 10 (45.5) | 8 (15.4) | 18 (24.3) | |
| Non-sarcopenia, n (%) | 7 (31.8) | 19 (36.5) | 26 (35.1) | |
| (A) | ||||
| N (%) | Non-Sarcopenia 26 (35.1) | Risk-Sarcopenia 18 (24.3) | Confirmed-Sarcopenia 30 (40.5) | p-Value |
| Anthropometry variables | ||||
| Age—years, mean (SD) | 64.8 (16.9) | 71.3 (9.7) | 81.5 (15.5) | <0.001 |
| Sex—male, (%) | 73.1 | 44.4 | 83.3 | 0.02 |
| Weight (kg), mean (SD) | 73.3 (10.4) | 72.4 (10.1) | 61.3 (12.3) | <0.001 |
| BMI (kg/m2), mean (SD) | 26.9 (4.1) | 28.1 (4.1) | 22.1 (2.5) | <0.001 |
| Triceps skinfold (mL), mean (SD) | 13.1 (6.7) | 15.4 (4.8) | 9.9 (6.2) | 0.006 |
| Suprailiac skinfold (mL), mean (SD) | 19.7 (8.5) | 19.5 (4.9) | 12.5 (8) | <0.001 |
| Muscle strength | ||||
| Handgrip strength (kg), mean (SD) | 26.8 (6.8) | 12 (6.1) | 14.5 (8.7) | <0.001 |
| Functional performance | ||||
| SPPB (points) | 10.3 (1.5) | 8.2 (2) | 7.6 (2) | <0.001 |
| Low performance (SPPB ≤ 8), (%) | 3.9 | 27.8 | 50 | 0.001 |
| Hemodialysis parameters | ||||
| HD vintage (months), mean (SD) | 33 (28.5) | 39 (37.2) | 43.6 (32.9) | 0.49 |
| nPCR (g Urea/Kg/d), mean (SD) | 1.3 (0.2) | 0.9 (0.4) | 1 (0.4) | 0.001 |
| Vascular access type | ||||
| Arteriovenous fistula, (%) | 46.2 | 44.4 | 36.7 | 0.75 |
| Tunneled catheter, (%) | 53.9 | 55.6 | 63.3 | |
| Kt/V urea, mean (SD) | 1.6 (0.2) | 1.6 (0.2) | 1.6 (0.2) | 0.95 |
| KT (L), mean (SD) | 54 (5.8) | 53.2 (5.3) | 49.7 (6.4) | 0.02 |
| HD conventional (three sessions/week), n (%) | 20 (76.9) | 16 (88.9) | 25 (83.3) | 0.6 |
| iHD (one or two sessions/week), n (%) | 6 (23.1) | 2 (11.1) | 5 (16.7) | |
| Biochemical parameters | ||||
| Hb (g/L), mean (SD) | 11 (1.6) | 10.7 (1.5) | 11.7 (1.6) | 0.06 |
| Lymphocytes (103/µL), mean (SD) | 1.1 (0.5) | 1.2 (0.5) | 1.1 (0.5) | 0.6 |
| Ferritin (ng/mL), mean (SD) | 657.6 (386.3) | 841.7 (709.7) | 848.6 (520.1) | 0.3 |
| sCr (g/dL), mean (SD) | 7.1 (2.3) | 5.8 (2) | 5.3 (2.2) | 0.007 |
| Cholesterol (mg/dL), mean (SD) | 122.3 (37.3) | 138.2 (30.7) | 131.5 (31.7) | 0.25 |
| Triglycerides (mg/dL), mean (SD) | 133.7 (91.4) | 151.6 (34.4) | 96.2 (68.3) | 0.01 |
| C-reactive protein (mg/L), mean (SD) | 2.2 (3.8) | 2.3 (3.4) | 2.3 (4.8) | 0.99 |
| Albumin (g/dL), mean (SD) | 3.4 (0.5) | 3.1 (0.5) | 3.2 (0.5) | 0.13 |
| Prealbumin (mg/dL), mean (SD) | 27.7 (5.8) | 26.1 (5.4) | 25.6 (6.7) | 0.51 |
| (B) | ||||
| N (%) | Non-Sarcopenia 26 (35.1) | Risk-Sarcopenia 18 (24.3) | Confirmed-Sarcopenia 30 (40.5) | p-Value |
| Bioimpedance parameters | ||||
| TBW (L), mean (SD) | 39.4 (9.5) | 34.7 (5.7) | 31.7 (8) | 0.001 |
| BFM (kg), mean (SD) | 19.7 (10) | 26.8 (6.9) | 18.1 (9.6) | 0.007 |
| FFM (kg), mean (SD) | 53.5 (12.4) | 47 (9.2) | 43 (11.2) | 0.002 |
| LBM (kg), mean (SD) | 49.4 (12.4) | 43.7 (7.3) | 41.3 (10.5) | 0.01 |
| BCM (kg), mean (SD) | 34.3 (7.4) | 29.8 (5.5) | 27.6 (6.9) | 0.001 |
| Skeletal muscle mass (kg), mean (SD) | 29.1 (6.7) | 25.2 (4.8) | 23.4 (6.1) | 0.001 |
| Visceral fat area (cm2), mean (SD) | 85.3 (63.4) | 124.1 (43.6) | 80.4 (56.3) | 0.02 |
| ASMI using Lin’s formula (kg/m2), mean (SD) | 7.2 (1.2) | 7 (0.7) | 5.8 (1.2) | <0.001 |
| ASMI < 5.5 kg/m2 (♀) and < 7 kg/m2 (♂), n (%) | 1 (3.8) | 7 (38.9) | 18 (60) | <0.001 |
| Phase angle (◦), mean (SD) | 5.2 (2) | 5.1 (0.8) | 4.3 (1.5) | 0.03 |
| Nutritional ultrasonography parameters | ||||
| Y-axis (mm), mean (SD) | 9.6 (2.8) | 8.8 (2) | 7.8 (2.3) | 0.01 |
| Y-axis/height (mm/m2), mean (SD) | 3.4 (1.3) | 3.5 (0.7) | 2.9 (1) | 0.03 |
| Y-axis/BSA (mm/m2), mean (SD) | 2.9 (1.1) | 3 (0.8) | 2.9 (0.9) | 0.91 |
| X-axis (mm), mean (SD) | 31.6 (6) | 29.5 (6.5) | 30.2 (6.5) | 0.54 |
| X-axis/Y-axis ratio, mean (SD) | 1.2 (0.3) | 1.4 (0.7) | 1.5 (0.5) | 0.09 |
| CS-MARF (cm2), mean (SD) | 2.9 (1) | 2.6 (0.6) | 2.4 (0.8) | 0.03 |
| MARFIh (cm2/m2), mean (SD) | 1 (0.4) | 1 (0.3) | 0.9 (0.3) | 0.13 |
| MARFIBSA (cm2/m2), mean (SD) | 0.9 (0.3) | 0.9 (0.3) | 0.9 (0.3) | 0.88 |
| SMF (mm), mean (SD) | 7.5 (2.1) | 7.3 (1.8) | 6 (2.2) | 0.02 |
| Transverse PPVF (cm), mean (SD) | 0.7 (0.3) | 0.6 (0.2) | 0.5 (0.3) | 0.05 |
| Transverse SSCF (cm), mean (SD) | 0.9 (0.3) | 0.8 (0.2) | 0.7 (0.3) | 0.04 |
| Transverse DSCF (cm), mean (SD) | 1.1 (0.6) | 1.4 (0.4) | 1.1 (0.5) | 0.05 |
| Scales of malnutrition, frailty, and sarcopenia | ||||
| 7-point SGA (malnutrition), n (%) | 3 (11.5) | 12 (66.7) | 22 (73.3) | <0.001 |
| MIS ≥ 8 points, n (%) | 3 (11.5) | 9 (50) | 18 (60) | 0.009 |
| MST ≥ 2 points, n (%) | 2 (7.7) | 4 (22.2) | 10 (33.3) | 0.07 |
| Frailty (score ≥ 3 points), n (%) | 3 (11.5) | 8 (44.4) | 18 (60) | <0.001 |
| Severe PEW (score 0–2), n (%) | 6 (23.1) | 7 (38.9) | 20 (66.7) | 0.004 |
| SARC-F ≥ 4 points, n (%) | 3 (11.5) | 7 (38.9) | 16 (53.3) | 0.04 |
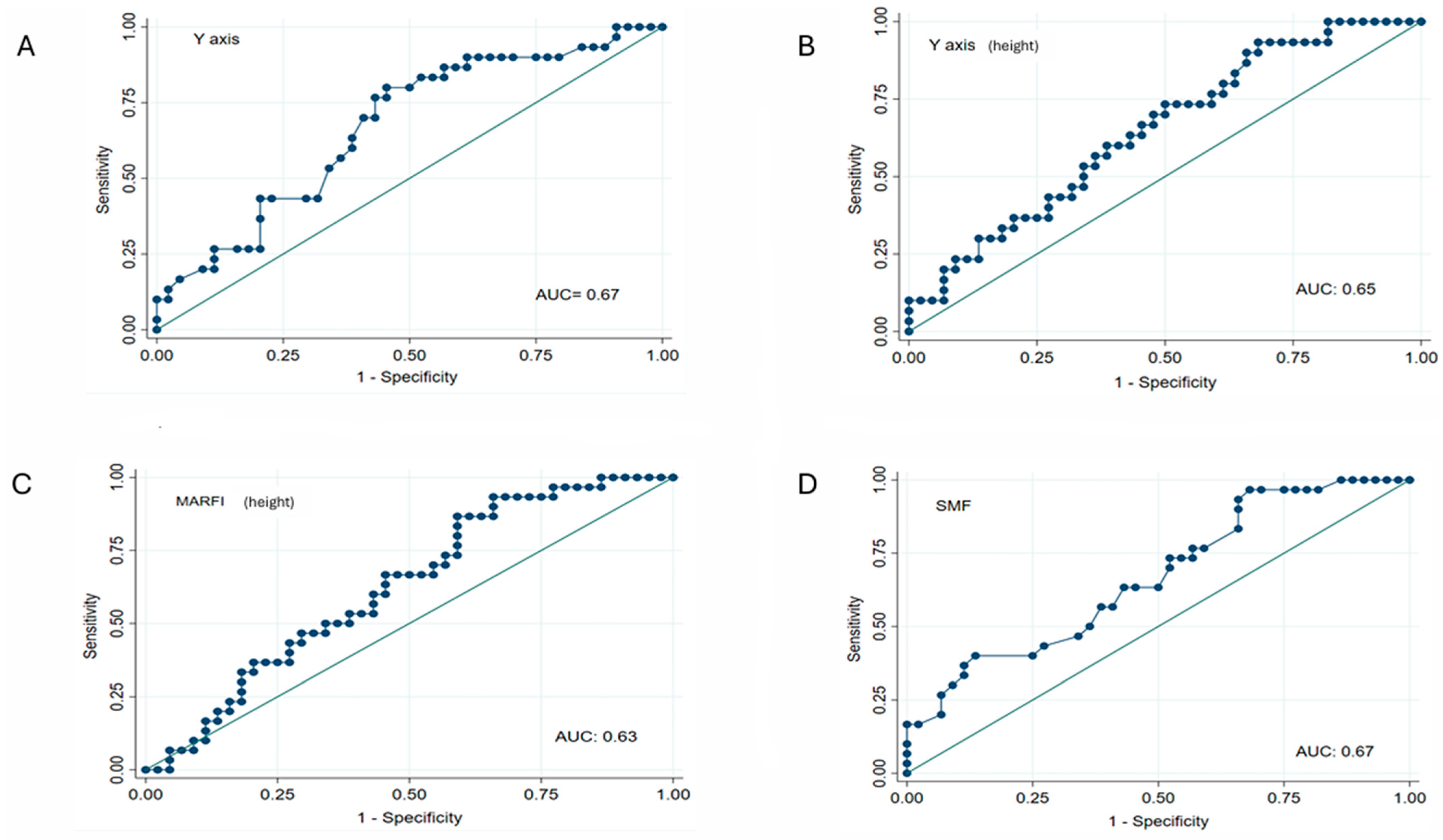
| AUC | 95% CI | Sign | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Y-axis (mm) | 0.67 | 0.54–0.79 | p < 0.05 | 76.7 | 56.8 |
| Y-axis/height (mm/m2) | 0.65 | 0.52–0.77 | p < 0.05 | 60 | 45.5 |
| Y-axis/BSA (mm/m2) | 0.51 | 0.37–0.65 | NS | 73.3 | 50 |
| MARFIh (cm2/m2) | 0.63 | 0.50–0.75 | p < 0.05 | 86.7 | 40.9 |
| MARFIBSA (cm2/m2) | 0.50 | 0.36–0.63 | NS | 70 | 34.1 |
| SMF (mm) | 0.67 | 0.54–0.79 | p < 0.05 | 96.7 | 31.8 |
| Transverse PPVF (cm) | 0.63 | 0.49–0.75 | NS | 53.3 | 65.9 |
| Transverse SSCF (cm) | 0.66 | 0.53–0.79 | p < 0.05 | 53.3 | 70.5 |
| Transverse DSCF (cm) | 0.60 | 0.47–0.73 | NS | 60 | 56.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Flor, J.C.; García-Menéndez, E.; Romero-González, G.; Rodríguez Tudero, C.; Jiménez Mayor, E.; Florit Mengual, E.; Moral Berrio, E.; Soria Morales, B.; Cieza Terrones, M.; Cigarrán Guldris, S.; et al. Morphofunctional Assessment of Malnutrition and Sarcopenia Using Nutritional Ultrasonography in Patients Undergoing Maintenance Hemodialysis. Medicina 2025, 61, 1044. https://doi.org/10.3390/medicina61061044
De La Flor JC, García-Menéndez E, Romero-González G, Rodríguez Tudero C, Jiménez Mayor E, Florit Mengual E, Moral Berrio E, Soria Morales B, Cieza Terrones M, Cigarrán Guldris S, et al. Morphofunctional Assessment of Malnutrition and Sarcopenia Using Nutritional Ultrasonography in Patients Undergoing Maintenance Hemodialysis. Medicina. 2025; 61(6):1044. https://doi.org/10.3390/medicina61061044
Chicago/Turabian StyleDe La Flor, José C., Estefanya García-Menéndez, Gregorio Romero-González, Celia Rodríguez Tudero, Elena Jiménez Mayor, Enrique Florit Mengual, Esperanza Moral Berrio, Beatriz Soria Morales, Michael Cieza Terrones, Secundino Cigarrán Guldris, and et al. 2025. "Morphofunctional Assessment of Malnutrition and Sarcopenia Using Nutritional Ultrasonography in Patients Undergoing Maintenance Hemodialysis" Medicina 61, no. 6: 1044. https://doi.org/10.3390/medicina61061044
APA StyleDe La Flor, J. C., García-Menéndez, E., Romero-González, G., Rodríguez Tudero, C., Jiménez Mayor, E., Florit Mengual, E., Moral Berrio, E., Soria Morales, B., Cieza Terrones, M., Cigarrán Guldris, S., & Hernández Vaquero, J. (2025). Morphofunctional Assessment of Malnutrition and Sarcopenia Using Nutritional Ultrasonography in Patients Undergoing Maintenance Hemodialysis. Medicina, 61(6), 1044. https://doi.org/10.3390/medicina61061044






