A Narrative Review of the Odyssey of Thyroid Cancer Diagnosis: Can 99mTc-SESTAMIBI Molecular Imaging Replace Fine Needle Aspiration Biopsy?
Abstract
1. Introduction
2. The Cicones: Ultrasound
3. Polyphemus the Cyclops: Fine Needle Aspiration Biopsy
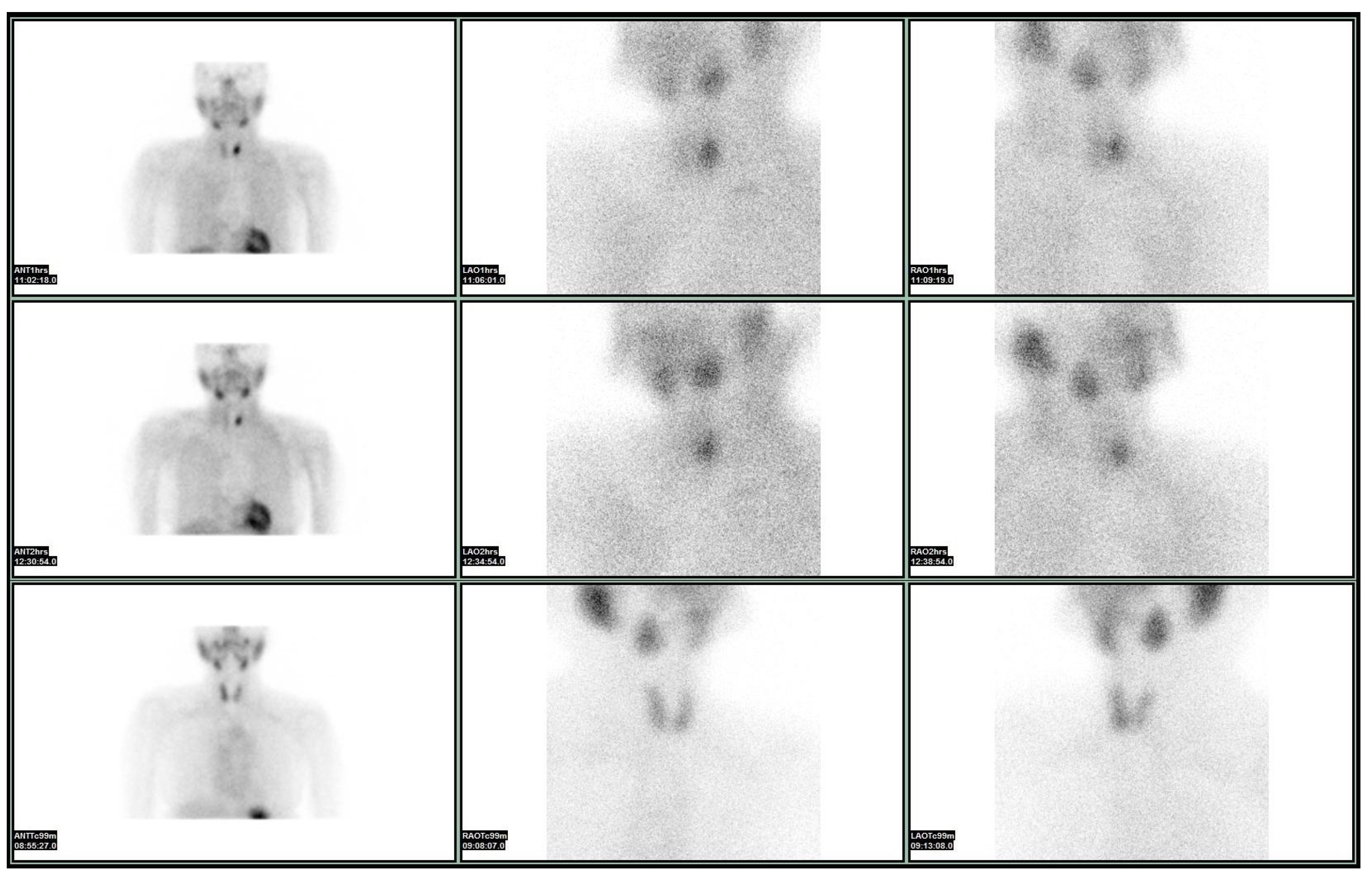
4. The Phaeacians: Scintigraphy
5. Ithaca: Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSH | Thyroid stimulating hormone |
| US | Ultrasound |
| FNAB ACR-TIRADS | Fine needle aspiration biopsy American College of Radiology-Thyroid imaging reporting and data system |
| TI-RADS | Thyroid imaging reporting and data system |
| EU-TIRADS | European Thyroid Association TI-RADS |
| RSS | Risk stratification systems |
| Sestamibi | 99mTc-SESTAMIBI |
| SPECT-CT | Single-Photon Emission Computed Tomography with Computed Tomography |
| PET-CT | Positron emission tomography scan and a computed tomography scan |
References
- Giannoula, E.; Melidis, C.; Frangos, S.; Papadopoulos, N.; Koutsouki, G.; Iakovou, I. Ecological Study on Thyroid Cancer Incidence and Mortality in Association with European Union Member States’ Air Pollution. Int. J. Environ. Res. Public Health 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.S.; Gharib, H. Epidemiology of thyroid nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Farrell, S.G.; Grossmann, M. Thyroid nodules: Diagnosis and management. Med. J. Aust. 2018, 209, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. JACR 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Mariani, G.; Tonacchera, M.; Grosso, M.; Fiore, E.; Falcetta, P.; Montanelli, L.; Bagattini, B.; Vitti, P.; Strauss, H.W. The Role of Nuclear Medicine in the Clinical Management of Benign Thyroid Disorders, Part 2: Nodular Goiter, Hypothyroidism, and Subacute Thyroiditis. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 886–895. [Google Scholar] [CrossRef]
- Mistry, R.; Hillyar, C.; Nibber, A.; Sooriyamoorthy, T.; Kumar, N. Ultrasound Classification of Thyroid Nodules: A Systematic Review. Cureus 2020, 12, e7239. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Rossi, E.D.; Adeniran, A.J.; Faquin, W.C. Pitfalls in Thyroid Cytopathology. Surg. Pathol. Clin. 2019, 12, 865–881. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, Y.; Xu, G.; Fan, Z.; Ren, W. Causes of misdiagnoses by thyroid fine-needle aspiration cytology (FNAC): Our experience and a systematic review. Diagn. Pathol. 2020, 15, 1. [Google Scholar] [CrossRef]
- Parameswaran, R.; Shulin Hu, J.; Min En, N.; Tan, W.B.; Yuan, N.K. Patterns of metastasis in follicular thyroid carcinoma and the difference between early and delayed presentation. Ann. R. Coll. Surg. Engl. 2017, 99, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Rosai, J. Handling of thyroid follicular patterned lesions. Endocr. Pathol. 2005, 16, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. Off. J. Am. Thyroid Assoc. 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Valderrabano, P.; McIver, B. Evaluation and Management of Indeterminate Thyroid Nodules: The Revolution of Risk Stratification Beyond Cytological Diagnosis. Cancer Control J. Moffitt Cancer Cent. 2017, 24, 1073274817729231. [Google Scholar] [CrossRef]
- Liu, J.; Singh, B.; Tallini, G.; Carlson, D.L.; Katabi, N.; Shaha, A.; Tuttle, R.M.; Ghossein, R.A. Follicular variant of papillary thyroid carcinoma: A clinicopathologic study of a problematic entity. Cancer 2006, 107, 1255–1264. [Google Scholar] [CrossRef]
- Suster, S. Thyroid tumors with a follicular growth pattern: Problems in differential diagnosis. Arch. Pathol. Lab. Med. 2006, 130, 984–988. [Google Scholar] [CrossRef]
- Sahin, M.; Gursoy, A.; Tutuncu, N.B.; Guvener, D.N. Prevalence and prediction of malignancy in cytologically indeterminate thyroid nodules. Clin. Endocrinol. 2006, 65, 514–518. [Google Scholar] [CrossRef]
- Trimboli, P.; Treglia, G.; Guidobaldi, L.; Saggiorato, E.; Nigri, G.; Crescenzi, A.; Romanelli, F.; Orlandi, F.; Valabrega, S.; Sadeghi, R.; et al. Clinical characteristics as predictors of malignancy in patients with indeterminate thyroid cytology: A meta-analysis. Endocrine 2014, 46, 52–59. [Google Scholar] [CrossRef]
- Castro, M.R.; Espiritu, R.P.; Bahn, R.S.; Henry, M.R.; Gharib, H.; Caraballo, P.J.; Morris, J.C. Predictors of malignancy in patients with cytologically suspicious thyroid nodules. Thyroid Off. J. Am. Thyroid Assoc. 2011, 21, 1191–1198. [Google Scholar] [CrossRef]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1343–1421. [Google Scholar] [CrossRef]
- Stember, J.N.; Sheikh, A.; Perez, E.; Divgi, C.; Hamacher, K.; Jambawalikar, S.; Yeh, R. A threshold-based method to predict thyroid nodules on scintigraphy scans. Biomed. Phys. Eng. Express 2020, 6, 015019. [Google Scholar] [CrossRef] [PubMed]
- Meller, J.; Becker, W. The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound. Eur. J. Nucl. Med. Mol. Imaging 2002, 29 (Suppl. S2), S425–S438. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I.; Gillenwater, A.M. Diagnostic Evaluation of the Solitary Thyroid Nodule. In Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Hamilton, ON, USA, 2003. [Google Scholar]
- Underwood, S.R. A History of Radionuclide Studies in the UK: 50th Anniversary of the British Nuclear Medicine Society; McCready, R., Gnanasegaran, G., Bomanji, J.B., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Márián, T.; Balkay, L.; Szabó, G.; Krasznai, Z.T.; Hernádi, Z.; Galuska, L.; Szabó-Péli, J.; Esik, O.; Trón, L.; Krasznai, Z. Biphasic accumulation kinetics of [99mTc]-hexakis-2-methoxyisobutyl isonitrile in tumour cells and its modulation by lipophilic P-glycoprotein ligands. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2005, 25, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Kato, T.; Shioi, T.; Okuda, J.; Kawashima, T.; Tamaki, Y.; Niizuma, S.; Tanada, Y.; Takemura, G.; Narazaki, M.; et al. Measurement of technetium-99m sestamibi signals in rats administered a mitochondrial uncoupler and in a rat model of heart failure. PLoS ONE 2015, 10, e0117091. [Google Scholar] [CrossRef]
- Rizk, T.H.; Nagalli, S. Technetium 99m sestamibi. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bongiovanni, M.; Paone, G.; Ceriani, L.; Pusztaszeri, M.J.C.; Imaging, T. Cellular and molecular basis for thyroid cancer imaging in nuclear medicine. Clin. Trans. Imaging 2013, 1, 149–161. [Google Scholar] [CrossRef]
- Sundram, F.X.; Mack, P. Evaluation of thyroid nodules for malignancy using 99Tcm-sestamibi. Nucl. Med. Commun. 1995, 16, 687–693. [Google Scholar] [CrossRef]
- Treglia, G.; Caldarella, C.; Saggiorato, E.; Ceriani, L.; Orlandi, F.; Salvatori, M.; Giovanella, L. Diagnostic performance of (99m)Tc-MIBI scan in predicting the malignancy of thyroid nodules: A meta-analysis. Endocrine 2013, 44, 70–78. [Google Scholar] [CrossRef]
- Leidig-Bruckner, G.; Cichorowski, G.; Sattler, P.; Bruckner, T.; Sattler, B. Evaluation of thyroid nodules--combined use of (99m)Tc-methylisobutylnitrile scintigraphy and aspiration cytology to assess risk of malignancy and stratify patients for surgical or nonsurgical therapy--a retrospective cohort study. Clin. Endocrinol. 2012, 76, 749–758. [Google Scholar] [CrossRef]
- Giovanella, L.; Suriano, S.; Maffioli, M.; Ceriani, L.; Spriano, G. (99m)Tc-sestamibi scanning in thyroid nodules with nondiagnostic cytology. Head Neck 2010, 32, 607–611. [Google Scholar] [CrossRef]
- Hurtado-López, L.M.; Arellano-Montaño, S.; Torres-Acosta, E.M.; Zaldivar-Ramirez, F.R.; Duarte-Torres, R.M.; Alonso-De-Ruiz, P.; Martínez-Duncker, I.; Martínez-Duncker, C. Combined use of fine-needle aspiration biopsy, MIBI scans and frozen section biopsy offers the best diagnostic accuracy in the assessment of the hypofunctioning solitary thyroid nodule. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1273–1279. [Google Scholar] [CrossRef]
- Beristain Hernández, J.L.; Servín Torres, E.; Sosa Caballero, A.; Velázquez García, J.A.; Pozzo Bobarín, R.; Delgadillo Teyer, G.; Serrano Galeana, I.; Márquez Hernández, A.; Bevia Pérez, F.; Piscil Salazar, M.A.; et al. Determination of the diagnostic accuracy of 99mTc sestamibi scanning in patients with thyroid nodule and a definitive histopathological report. Endocrinol. Nutr. Organo Soc. Esp. Endocrinol. Nutr. 2010, 57, 460–466. [Google Scholar] [CrossRef]
- Sathekge, M.M.; Mageza, R.B.; Muthuphei, M.N.; Modiba, M.C.; Clauss, R.C. Evaluation of thyroid nodules with technetium-99m MIBI and technetium-99m pertechnetate. Head Neck 2001, 23, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Riazi, A.; Kalantarhormozi, M.; Nabipour, I.; Eghbali, S.S.; Farzaneh, M.; Javadi, H.; Ostovar, A.; Seyedabadi, M.; Assadi, M. Technetium-99m methoxyisobutylisonitrile scintigraphy in the assessment of cold thyroid nodules: Is it time to change the approach to the management of cold thyroid nodules? Nucl. Med. Commun. 2014, 35, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Sager, S.; Vatankulu, B.; Erdogan, E.; Mut, S.; Teksoz, S.; Ozturk, T.; Sonmezoglu, K.; Kanmaz, B. Comparison of F-18 FDG-PET/CT and Tc-99m MIBI in the preoperative evaluation of cold thyroid nodules in the same patient group. Endocrine 2015, 50, 138–145. [Google Scholar] [CrossRef]
- Giovanella, L.; Campenni, A.; Treglia, G.; Verburg, F.A.; Trimboli, P.; Ceriani, L.; Bongiovanni, M. Molecular imaging with (99m)Tc-MIBI and molecular testing for mutations in differentiating benign from malignant follicular neoplasm: A prospective comparison. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1018–1026. [Google Scholar] [CrossRef]
- Piccardo, A.; Puntoni, M.; Treglia, G.; Foppiani, L.; Bertagna, F.; Paparo, F.; Massollo, M.; Dib, B.; Paone, G.; Arlandini, A.; et al. Thyroid nodules with indeterminate cytology: Prospective comparison between 18F-FDG-PET/CT, multiparametric neck ultrasonography, 99mTc-MIBI scintigraphy and histology. Eur. J. Endocrinol. 2016, 174, 693–703. [Google Scholar] [CrossRef]
- Psyhoumtakis, G.; Alexiou, S.; Makromichelaki Mintza, T. Homer’s Odyssey, 3rd ed.; University of Crete Press (UCP): Crete, Greece, 2010; pp. 421–447. [Google Scholar]
- Aschebrook-Kilfoy, B.; James, B.; Nagar, S.; Kaplan, S.; Seng, V.; Ahsan, H.; Angelos, P.; Kaplan, E.L.; Guerrero, M.A.; Kuo, J.H.; et al. Risk Factors for Decreased Quality of Life in Thyroid Cancer Survivors: Initial Findings from the North American Thyroid Cancer Survivorship Study. Thyroid Off. J. Am. Thyroid Assoc. 2015, 25, 1313–1321. [Google Scholar] [CrossRef]
- Pace-Asciak, P.; Russell, J.O.; Tufano, R.P. Review: Improving quality of life in patients with differentiated thyroid cancer. Front. Oncol. 2023, 13, 1032581. [Google Scholar] [CrossRef]
- Hoftijzer, H.C.; Heemstra, K.A.; Corssmit, E.P.; van der Klaauw, A.A.; Romijn, J.A.; Smit, J.W. Quality of life in cured patients with differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2008, 93, 200–203. [Google Scholar] [CrossRef]
- Singer, S.; Lincke, T.; Gamper, E.; Bhaskaran, K.; Schreiber, S.; Hinz, A.; Schulte, T. Quality of life in patients with thyroid cancer compared with the general population. Thyroid Off. J. Am. Thyroid Assoc. 2012, 22, 117–124. [Google Scholar] [CrossRef]
- Hedman, C.; Djärv, T.; Strang, P.; Lundgren, C.I. Effect of Thyroid-Related Symptoms on Long-Term Quality of Life in Patients with Differentiated Thyroid Carcinoma: A Population-Based Study in Sweden. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Gamper, E.; Becherer, A.; Hoffmann, M. Quality of life aspects in the management of thyroid cancer. Oral Oncol. 2015, 51, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.H.; Wong, C.K. A cost-minimization analysis comparing total thyroidectomy alone and total thyroidectomy with prophylactic central neck dissection in clinically nodal-negative papillary thyroid carcinoma. Ann. Surg. Oncol. 2014, 21, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Nasrollah, N.; Amendola, S.; Crescenzi, A.; Guidobaldi, L.; Chiesa, C.; Maglio, R.; Nigri, G.; Pontecorvi, A.; Romanelli, F.; et al. A cost analysis of thyroid core needle biopsy vs. diagnostic surgery. Gland Surg. 2015, 4, 307–311. [Google Scholar] [CrossRef]
| Sonographic Pattern | Estimated Risk of Malignancy, % |
|---|---|
| High suspicion | >70–90 |
| Intermediate suspicion | 10–20 |
| Low suspicion | 5–10 |
| Very low suspicion | <3 |
| Benign | <1 |
| Diagnostic Category | Estimated/Predicted Risk of Malignancy by the Bethesda System, % |
|---|---|
| Nondiagnostic or unsatisfactory | 1–4 |
| Benign | 0–3 |
| Atypia of undetermined significance or follicular lesion of undetermined significance | 5–15 |
| Follicular neoplasm or suspicious for a follicular neoplasm | 15–30 |
| Suspicious for malignancy | 60–75 |
| Malignant | 97–99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iakovou, I.; Papadopoulos, N.; Exadaktylou, P.; Melidis, C.; Koutsouki, G.; Katsadouros, I.; Frangos, S.; Koutelidakis, I.; Kotsa, K.; Giannoula, E. A Narrative Review of the Odyssey of Thyroid Cancer Diagnosis: Can 99mTc-SESTAMIBI Molecular Imaging Replace Fine Needle Aspiration Biopsy? Medicina 2025, 61, 1043. https://doi.org/10.3390/medicina61061043
Iakovou I, Papadopoulos N, Exadaktylou P, Melidis C, Koutsouki G, Katsadouros I, Frangos S, Koutelidakis I, Kotsa K, Giannoula E. A Narrative Review of the Odyssey of Thyroid Cancer Diagnosis: Can 99mTc-SESTAMIBI Molecular Imaging Replace Fine Needle Aspiration Biopsy? Medicina. 2025; 61(6):1043. https://doi.org/10.3390/medicina61061043
Chicago/Turabian StyleIakovou, Ioannis, Nikitas Papadopoulos, Paraskevi Exadaktylou, Christos Melidis, Georgia Koutsouki, Ilias Katsadouros, Savvas Frangos, Ioannis Koutelidakis, Kalliopi Kotsa, and Evanthia Giannoula. 2025. "A Narrative Review of the Odyssey of Thyroid Cancer Diagnosis: Can 99mTc-SESTAMIBI Molecular Imaging Replace Fine Needle Aspiration Biopsy?" Medicina 61, no. 6: 1043. https://doi.org/10.3390/medicina61061043
APA StyleIakovou, I., Papadopoulos, N., Exadaktylou, P., Melidis, C., Koutsouki, G., Katsadouros, I., Frangos, S., Koutelidakis, I., Kotsa, K., & Giannoula, E. (2025). A Narrative Review of the Odyssey of Thyroid Cancer Diagnosis: Can 99mTc-SESTAMIBI Molecular Imaging Replace Fine Needle Aspiration Biopsy? Medicina, 61(6), 1043. https://doi.org/10.3390/medicina61061043









