Ultrasound-Guided Regional Anesthesia in Permanent Pacemaker Implantation: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
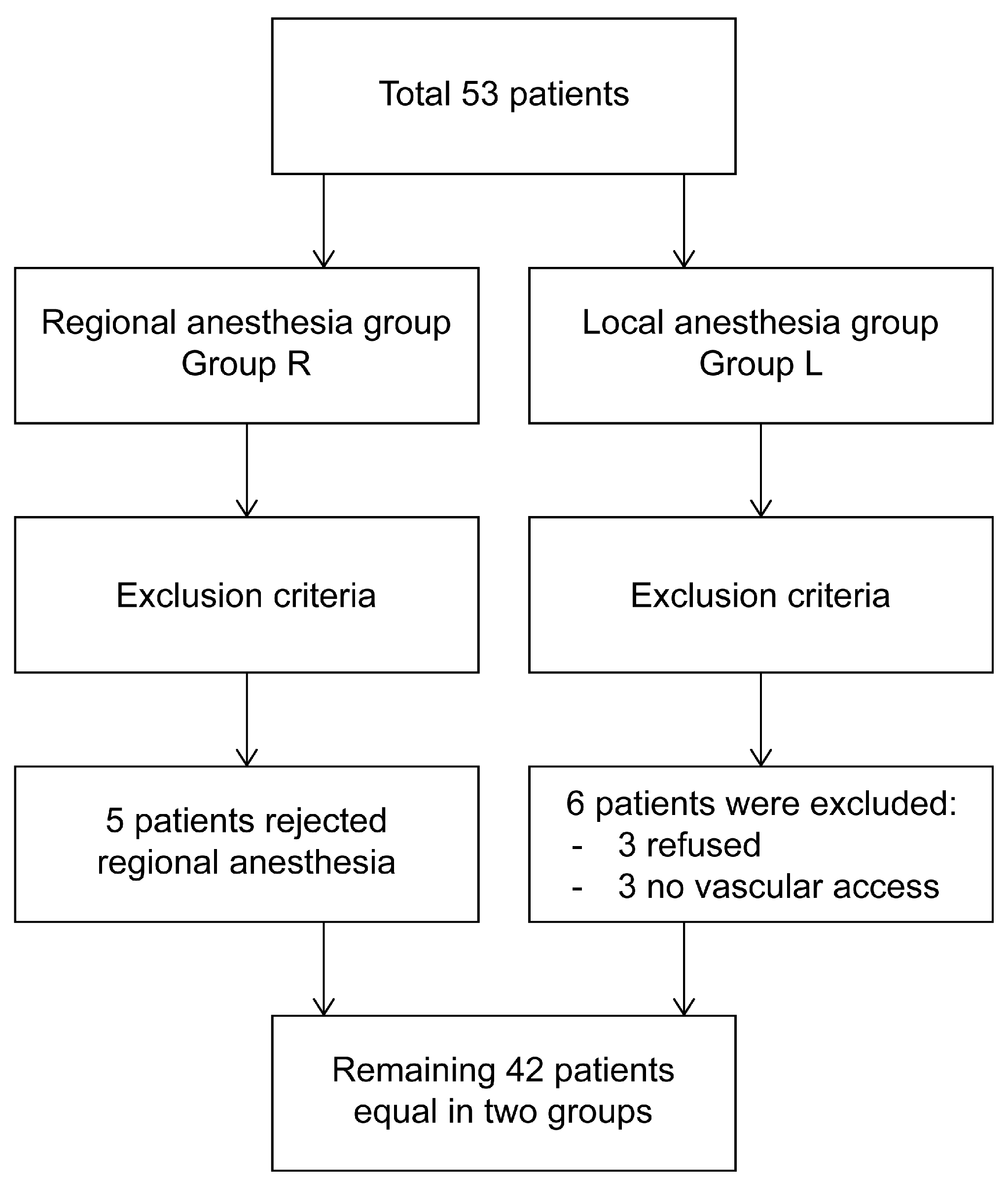
2.1. Patient Selection and Study Design
2.2. Randomization and Blinding
3. Objective and Aim
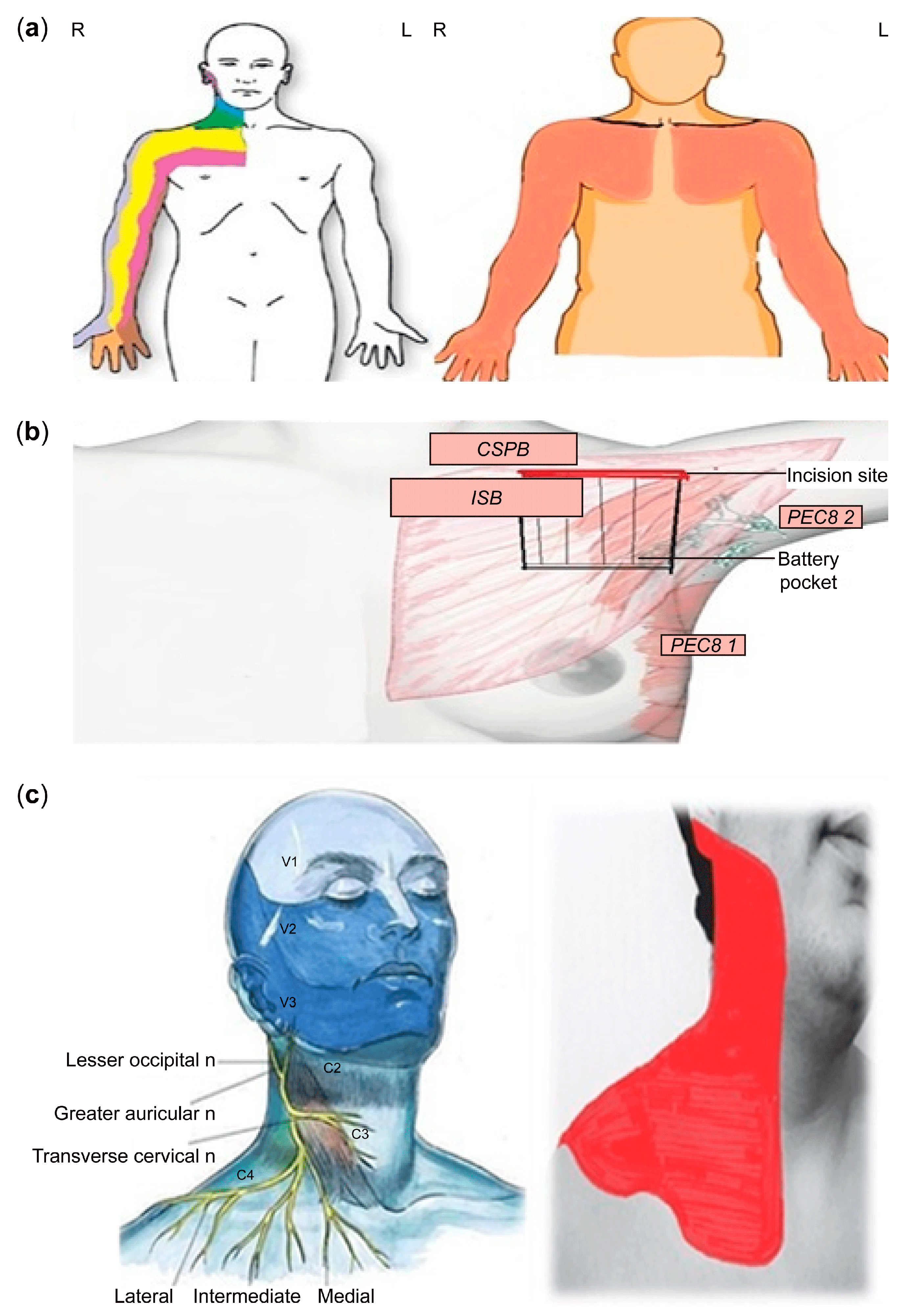
4. Anesthesia Preparation
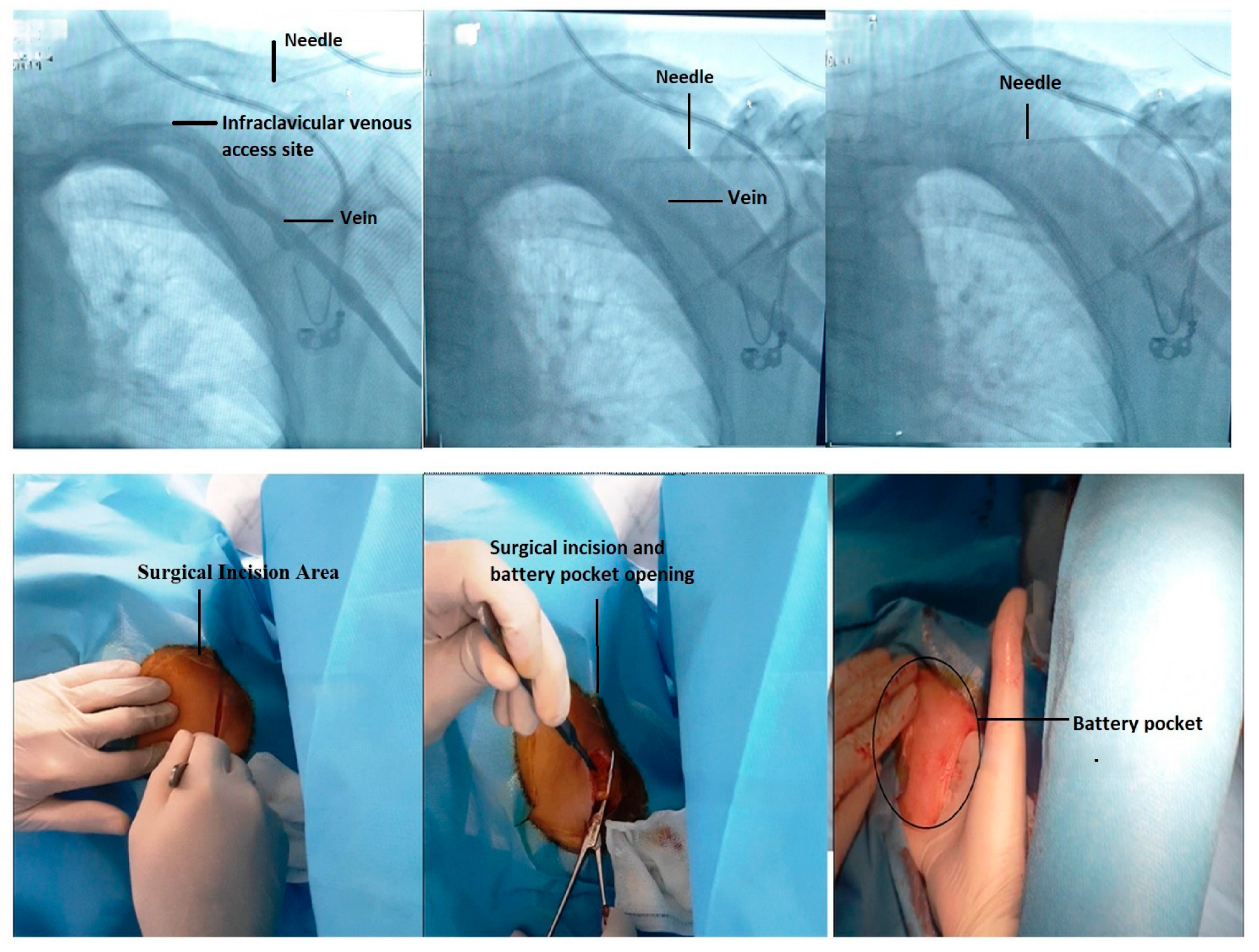
4.1. Fluoroscopic-Guided Vascular Puncture in Posterior–Anterior (PA) Projection
4.2. Postoperative Procedure
5. Statistical Methods
6. Results
7. Discussion
8. Study Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyers, G.F.O. Cardiac pacemaker and cardiac conduction system abnormalities. In Gibbon’s Surgery of the Chest, 4th ed.; Sabiston, D.C., Spencer, F.C., Eds.; Saunders: Philadelphia, PA, USA, 1983; Volume 11, pp. 1306–1355. [Google Scholar]
- Nishimura, R.A.; Symanski, J.D.; Hurrell, D.G.; Trusty, J.M.; Hayes, D.L.; Tajik, A.J. Dual-Chamber Pacing for Cardiomyopathies: A 1996 Clinical Perspective. Mayo Clin. Proc. 1996, 71, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J. Cardiac pacemakers. In Textbook of Surgery: The Biological Basis of Modern Surgical Practice, 15th ed.; Sabiston, D.C., Ed.; Saunders: Philadelphia, PA, USA, 1997; pp. 2175–2198. [Google Scholar]
- Zipes, D.P.; Duffin, E.G. Cardiac pacemakers. In Heart Disease: A Textbook of Cardiovascular Medicine; Braunwald, E., Ed.; Saunders: Philadelphia, PA, USA, 1988; pp. 717–741. [Google Scholar]
- Gaitan, B.D.; Trentman, T.L.; Fassett, S.L.; Mueller, J.T.; Altemose, G.T. Sedation and Analgesia in the Cardiac Electrophysiology Laboratory: A National Survey of Electrophysiologists Investigating the Who, How, and Why? J. Cardiothorac. Vasc. Anesth. 2011, 25, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Sripriya, R.; Sivashanmugam, T.; Jayaraman, G.; Ravindran, C.; Ravishankar, M. Truncal injection brachial plexus block: A Description of a novel injection technique and dose finding study. Indian J. Anaesth. 2020, 64, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Lin, C.-P.; Hung, C.-Y.; Özçakar, L.; Wang, T.-G.; Chen, W.-S. Sonographic Nerve Tracking in the Cervical Region. Am. J. Phys. Med. Rehabil. 2016, 95, 862–870. [Google Scholar] [CrossRef]
- Mądro, P.; Dąbrowska, A.; Jarecki, J.; Garba, P. Znieczulenie do zabiegu endarterektomii tętnic szyjnych: Selektywna blokada elementów splotu szyjnego powierzchownego/pośredniego z wykorzystaniem ultrasonografii. Anaesthesiol. Intensiv. Ther. 2016, 48, 234–238. [Google Scholar] [CrossRef]
- Nagaraja, P.; Kumar, K.N.; Kalyane, R.N.; Singh, N.G.; Krishna, M.; Babu, B.; Varadaraju, R.; Sathish, N.; Manjunatha, N. Efficacy of bilateral pectoralis nerve block for ultrafast tracking and postoperative pain management in cardiac surgery. Ann. Card. Anaesth. 2018, 21, 333–338. [Google Scholar] [CrossRef]
- Medical Research Council. Aids to Examination of the Peripheral Nervous System; Memorandum No. 45; Her Majesty’s Stationary Office: London, UK, 1976.
- Kiyak, H.; Hohl, T.; West, R.A.; McNeill, R. Psychologic changes in orthognathic surgery patients: A 24-month follow up. J. Oral. Maxillofac. Surg. 1984, 42, 506–512. [Google Scholar] [CrossRef]
- Scott, A.A.; Hatch, J.P.; Rugh, J.D.; Hoffman, T.J.; Rivera, S.M.; Dolce, C.; Bays, R.A. Psychosocial predictors of satisfaction among orthognathic surgery patients. Int. J. Adult Orthodon. Orthognath. Surg. 2000, 15, 7–15. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Tobias, J.D.; Froyshteter, A.B.; Bhalla, T.; Cambier, G.S.; Mckee, C.T. Pectoralis blocks for insertion of an implantable cardioverter defibrillator in two patients with Duchenne muscular dystrophy. Saudi J. Anaesth. 2018, 12, 324–327. [Google Scholar] [CrossRef]
- Sivashanmugam, T.; Areti, A.; Selvum, E.; Diwan, S.; Pandian, A. Selective blockade of supraclavicular nerves and upper trunk of brachial plexus: The SCUT block. Indian J. Anaesth. 2021, 65, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, M.K.; Mark, G.E.; Aasbo, J.D.; Joyner, C.A.; Sharma, S.; Decena, B.F.; Bolin, E.D.; Weiss, R.; Burke, M.C.; McClernon, T.R.; et al. Anesthesia for subcutaneous implantable cardioverter-defibrillator implantation: Perspectives from the clinical experience of a U.S. panel of physicians. Pacing Clin. Electrophysiol. 2018, 41, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Bozyel, S.; Yalnız, A.; Aksu, T.; Guler, T.E.; Genez, S. Ultrasound-guided combined pectoral nerve block and axillary venipuncture for the implantation of cardiac implantable electronic devices. Pacing Clin. Electrophysiol. 2019, 42, 1026–1031. [Google Scholar] [CrossRef]
- Biocic, M.; Vidosevic, D.; Boric, M.; Boric, T.; Giunio, L.; Fabijanic, D.; Puljak, L. Anesthesia and perioperative pain management during cardiac electronic device implantation. J. Pain Res. 2017, 10, 927–932. [Google Scholar] [CrossRef]
- Bode, K.; Breithardt, O.-A.; Kreuzhuber, M.; Mende, M.; Sommer, P.; Richter, S.; Doering, M.; Dinov, B.; Rolf, S.; Arya, A.; et al. Patient discomfort following catheter ablation and rhythm device surgery. EP Eur. 2015, 17, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Anger, T.; Pabst, P.; Linnemann, S.; Schneider, M.; Jorbenadze, R.; Oberhoff, M.; Döffert, J. Ultrasound-Guided Ropivacaine-Driven Pectoralis-Blockage 1 to avoid Significant and Severe Pain during Pacemaker-Implantation. J. Integr. Cardiol. 2019, 5, 1000274. [Google Scholar] [CrossRef]
- Kisanuki, M.; Fujita, K.; Moriyama, S.; Irie, K.; Yosida, C.; Fukata, M.; Arita, T.; Yokoyama, T.; Odashiro, K.; Maruyama, T.; et al. Complex regional pain syndrome induced by pacemaker implantation for sick sinus syndrome. J. Arrhythmia 2017, 33, 643–645. [Google Scholar] [CrossRef]
- Markman, T.M.; Brown, C.R.; Yang, L.; Guandalini, G.S.; Hyman, M.C.; Arkles, J.S.; Santangeli, P.; Schaller, R.D.; Supple, G.E.; Deo, R.; et al. Persistent Opioid Use After Cardiac Implantable Electronic Device Procedures. Circulation 2021, 144, 1590–1597. [Google Scholar] [CrossRef]
- Lee, J.Z.; Pasha, A.K.; Glasgow, A.E.; Habermann, E.B.; Kusumoto, F.M.; McLeod, C.J.; Goel, V.; Sorajja, D.; Srivathsan, K.; Shen, W.-K.; et al. Postoperative opioid prescription patterns and new opioid refills following cardiac implantable electronic device procedures. Heart Rhythm 2019, 16, 1841–1848. [Google Scholar] [CrossRef]
- Essandoh, M.K.; Otey, A.J.; Abdel-Rasoul, M.; Stein, E.J.; Turner, K.R.; Joseph, N.C.; Daoud, E.G. Monitored Anesthesia Care for Subcutaneous Cardioverter-Defibrillator Implantation: A Single-Center Experience. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1228–1233. [Google Scholar] [CrossRef]
- Kaya, E.; Südkamp, H.; Lortz, J.; Rassaf, T.; Jánosi, R.A. Feasibility and safety of using local anaesthesia with conscious sedation during complex cardiac implantable electronic device procedures. Sci. Rep. 2018, 8, 7103. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, P.; LeQuang, J.A.; Pergolizzi, J.V. Postoperative Pain Control Following Cardiac Implantable Electronic Device Implantation; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Çevikkalp, E.; Yapici, F. Selective C5 nerve root block versus combined interscalene block for clavicle surgery. Eur. Res. J. 2022, 8, 434–441. [Google Scholar] [CrossRef]
- Raza, S.M.; Vasireddy, A.R.; Candido, K.D.; Winnie, A.P.; Masters, R.W. A complete regional anesthesia technique for cardiac pacemaker insertion. J. Cardiothorac. Vasc. Anesth. 1991, 5, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Komasawa, N.; Minami, T. Pectoral nerves (PECS) and intercostal nerve block for cardiac resynchronization therapy device implantation. SpringerPlus 2014, 3, 409. [Google Scholar] [CrossRef]
- Mavarez, A.C.; Ripat, C.I.; Suarez, M.R. Pectoralis Plane Block for Pacemaker Insertion: A Successful Primary Anesthetic. Front. Surg. 2019, 6, 64. [Google Scholar] [CrossRef]
- Tran, D.Q.; Tiyaprasertkul, W.; González, A.P. Analgesia for clavicular fracture and surgery: A call for evidence. Reg. Anesth. Pain Med. 2013, 38, 539–543. [Google Scholar] [CrossRef]
- Balaban, O.; Dülgeroğlu, T.C.; Aydın, T. Ultrasound-Guided Combined Interscalene-Cervical Plexus Block for Surgical Anesthesia in Clavicular Fractures: A Retrospective Observational Study. Anesthesiol. Res. Pract. 2018, 2018, 7842128. [Google Scholar] [CrossRef]
- Lee, C.C.M.; Beh, Z.Y.; Lua, C.B.; Peng, K.; Fathil, S.M.; Hou, J.-D.; Lin, J.-A. Regional Anesthetic and Analgesic Techniques for Clavicle Fractures and Clavicle Surgeries: Part 1—A Scoping Review. Healthcare 2022, 10, 1487. [Google Scholar] [CrossRef]
- Lee, C.C.M.; Lua, C.B.; Peng, K.; Beh, Z.Y.; Fathil, S.M.; Hou, J.-D.; Lin, J.-A. Regional Anesthetic and Analgesic Techniques for Clavicle Fractures and Clavicle Surgeries: Part 2—A Retrospective Study. Healthcare 2022, 10, 1987. [Google Scholar] [CrossRef]
- Abu Sabaa, M.A.; Elbadry, A.A.; El Malla, D.A. Ultrasound-Guided Clavipectoral Block for Postoperative Analgesia of Clavicular Surgery: A Prospective Randomized Trial. Anesthesiol. Pain. Med. 2022, 12, e121267. [Google Scholar] [CrossRef]
- Kukreja, P.; Davis, C.J.; MacBeth, L.; Feinstein, J.; Kalagara, H. Ultrasound-Guided Clavipectoral Fascial Plane Block for Surgery Involving the Clavicle: A Case Series. Cureus 2020, 12, e9072. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, R.; Rashidi, M.; Gousheh, M.; Olapour, A.; Tasbihi, B. Comparison of the Ketamine-Lidocaine and Fentanyl-Lidocaine in Postoperative Analgesia in Axillary Block in Upper Limb Fractures by Ultrasound Guidance. Anesthesiol. Pain. Med. 2019, 9, e92695. [Google Scholar] [CrossRef] [PubMed]
- Prasad, G.K.; Khanna, S.; Jaishree, S. Review of adjuvants to local anesthetics in peripheral nerve blocks: Current and future trends. Saudi J. Anaesth. 2020, 14, 77–84. [Google Scholar] [CrossRef]
- Sultan, P.; Murphy, C.; Halpern, S.; Carvalho, B. The effect of low concentrations versus high concentrations of local anesthetics for labour analgesia on obstetric and anesthetic outcomes: A meta-analysis. Can. J. Anaesth. 2013, 60, 840–854. [Google Scholar] [CrossRef]
- Lam, K.; Leung, M.K.; Irwin, M.G. Labour analgesia: Update and literature review. Hong Kong Med. J. 2020, 26, 413–420. [Google Scholar] [CrossRef] [PubMed]
- de Aragão, F.F.; de Aragão, P.W.; Martins, C.A.; Leal, K.F.C.S.; Tobias, A.F. Neuraxial labor analgesia: A literature review. Braz. J. Anesthesiol. 2019, 69, 291–298. [Google Scholar] [CrossRef]
- Hamed, M.A.; Ghaber, S.; Reda, A. Dexmedetomidine and fentanyl as an adjunct to bupivacaine 0.5% in supraclavicular nerve block: A randomized controlled study. Anesth. Essays Res. 2018, 12, 475–479. [Google Scholar] [CrossRef]

| Score | Description |
|---|---|
| 0 | No contraction |
| 1 | Flicker or trace of contraction |
| 2 | Active movement, with gravity eliminated |
| 3 | Active movement against gravity |
| 4 | Active movement against gravity and resistance |
| 5 | Normal power |
| Post-Treatment Questionnaire Please Circle Your Response to Each Question | |||||||
|---|---|---|---|---|---|---|---|
| Question | 1. Not at All | 2 | 3 | 4. Neutral | 5 | 6 | 7. Very Likely |
| If you had to make the decision again, how likely would you be to undergo this same surgery? | |||||||
| How likely would you be to recommend this same surgery to others? | |||||||
| Considering everything, how satisfied are you now with the results of surgery? | |||||||
| Overall, how satisfied are you with your current bite? | |||||||
| Overall, how satisfied are you with your current speech articulation? | |||||||
| Overall, how satisfied are you with your current lip posture and lip closure? | |||||||
| Overall, how satisfied are you with your current breathing? | |||||||
| Overall, how accepting are you with your current level of TMJ/facial pain? | |||||||
| Overall, how accepting are you with your current level of lower lip/chin sensation? | |||||||
| 1 | I strongly disagree |
|---|---|
| 2 | I disagree |
| 3 | I partially disagree |
| 4 | Neither agree nor disagree |
| 5 | Partially agree |
| 6 | Agree |
| 7 | Strongly agree |
| Measure | Group L (n = 21) | Group R (n = 21) | p |
|---|---|---|---|
| Age (years) | 70.4 ± 11.6 | 62.1 ± 18.4 | 0.088 |
| Sex (male/female) | 11/10 | 12/9 | 0.757 |
| Processing time (minutes) | 46.6 ± 11.4 | 43.9 ± 10.5 | 0.428 |
| Need for additional dose (yes/no) | 3/18 | 2/19 | 0.634 |
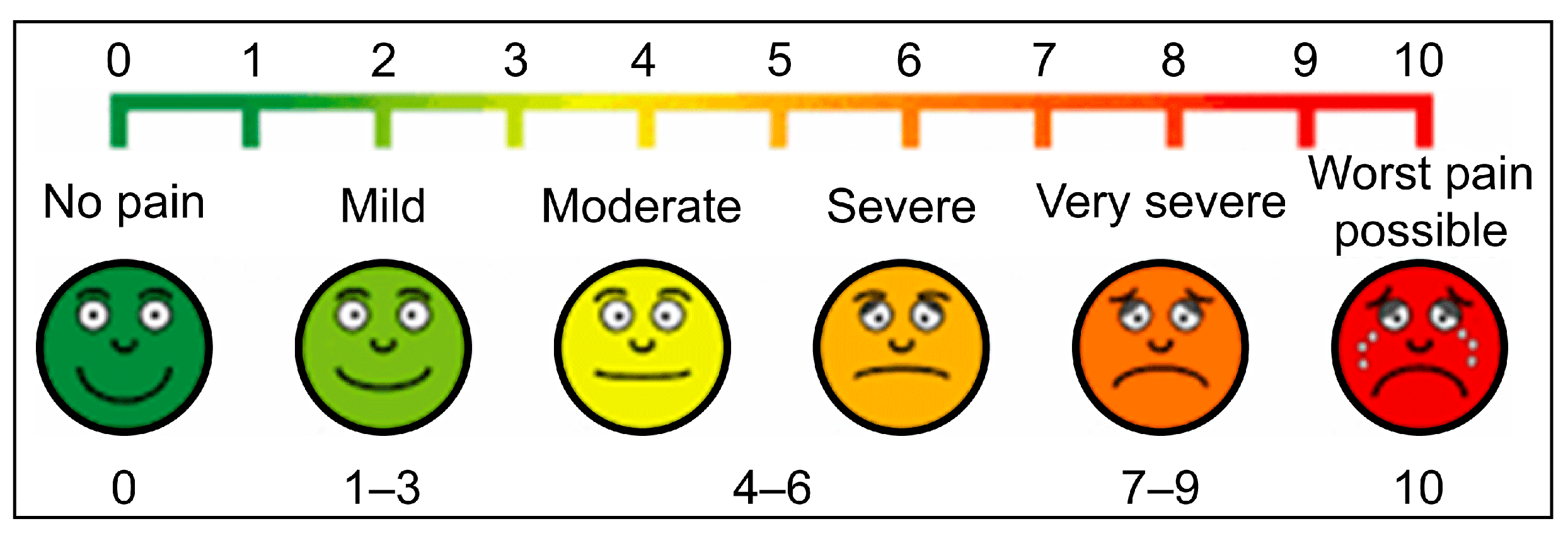
| VAS Score, median (Q1–Q3) | 4.0 (2.5–7.5) | 1.0 (0–2.0) | <0.001 * |
| MRC Muscle Power Scale | 5.0 ± 0 | 3.3 ± 1.0 | <0.001 * |
| PSPSQ QUESTION 1 | 4.7 ± 1.6 | 6.7 ± 0.5 | <0.001 * |
| PSPSQ QUESTION 2 | 4.1 ± 1.7 | 6.7 ± 0.5 | <0.001 * |
| PSPSQ QUESTION 3 | 4.4 ± 1.9 | 6.8 ± 0.6 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akelma, H.; Çelik, E.; İpek, Y.; Turgut, M.A.; Tanırcan, M.R.; Aktan, A.; Karahan, M.Z. Ultrasound-Guided Regional Anesthesia in Permanent Pacemaker Implantation: An Observational Study. Medicina 2025, 61, 1001. https://doi.org/10.3390/medicina61061001
Akelma H, Çelik E, İpek Y, Turgut MA, Tanırcan MR, Aktan A, Karahan MZ. Ultrasound-Guided Regional Anesthesia in Permanent Pacemaker Implantation: An Observational Study. Medicina. 2025; 61(6):1001. https://doi.org/10.3390/medicina61061001
Chicago/Turabian StyleAkelma, Hakan, Enes Çelik, Yusuf İpek, Mehmet Ali Turgut, Muhammed Raşit Tanırcan, Adem Aktan, and Mehmet Zülküf Karahan. 2025. "Ultrasound-Guided Regional Anesthesia in Permanent Pacemaker Implantation: An Observational Study" Medicina 61, no. 6: 1001. https://doi.org/10.3390/medicina61061001
APA StyleAkelma, H., Çelik, E., İpek, Y., Turgut, M. A., Tanırcan, M. R., Aktan, A., & Karahan, M. Z. (2025). Ultrasound-Guided Regional Anesthesia in Permanent Pacemaker Implantation: An Observational Study. Medicina, 61(6), 1001. https://doi.org/10.3390/medicina61061001





