A Computed Tomography-Based Morphometric Assessment of the Foramen Lacerum in a Turkish Population Using the 3D Slicer Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cranial CT Protocol
2.3. Morphometric Analysis with 3D Slicer
2.4. Statistical Analysis
3. Results
3.1. FL and VC Measurements
3.2. Classification of FL Types
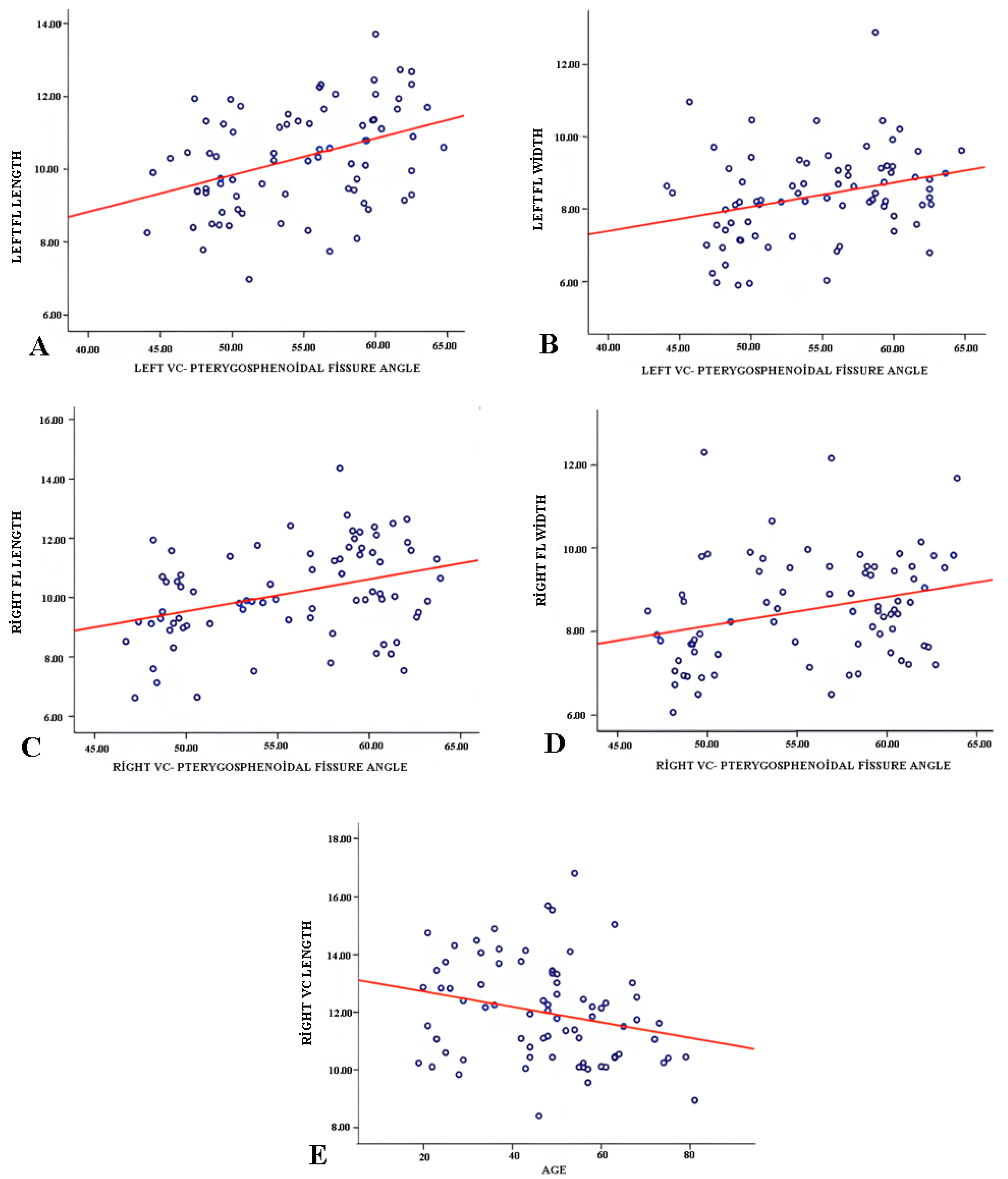
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP | Antero-posterior |
| CT | Computed tomography |
| FL | Foramen lacerum |
| ICA | Internal carotid artery |
| MRI | Magnetic resonance imaging |
| PACS | Picture archiving and communication system |
| SPSS | Statistical Package for the Social Sciences |
| VC | Vidian canal |
| 3D | Three-dimensional |
References
- Xu, Y.; Asmaro, K.; Mohyeldin, A.; Zhang, M.; Nunez, M.A.; Mao, Y.; Cohen-Gadol, A.A.; Fernandez-Miranda, J.C. The Pterygosphenoidal Triangle: Surgical Anatomy and Case Series in Endoscopic Endonasal Skull Base Surgery. Oper. Neurosurg. 2023, 24, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, A.T.; Little, A.S.; Vigo, V.; Benet, A.; Kakaizada, S.; Lawton, M.T. The pterygoclival ligament: A novel landmark for localization of the internal carotid artery during the endoscopic endonasal approach. J. Neurosurg. 2018, 130, 1699–1709. [Google Scholar] [CrossRef]
- Serioli, S.; Verstegen, M.J.; Kleijwegt, M.C.; Fiacchini, G.; van Furth, W.R.; Dallan, I. Extended TransnasalTranspterygoid Infra-Retropetrosal Approach for Resection of Skull Base Chondrosarcoma With Internal Carotid Artery Mobilization: 2-Dimensional Operative Video. Oper. Neurosurg. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Lieber, S.; Mathias, R.N.; Sun, X.; Gardner, P.A.; Snyderman, C.H.; Wang, E.W.; Fernandez-Miranda, J.C. The foramen lacerum: Surgical anatomy and relevance for endoscopic endonasal approaches. J. Neurosurg. 2019, 131, 1571–1582. [Google Scholar] [CrossRef]
- Demir, B.T. Morphological Analysis of Foramen Ovale and Foramen Lacerum in terms of Percutaneous and Endoscopic Endonasal Approaches. Chron. Precis. Med. Res. 2023, 4, 39–44. [Google Scholar] [CrossRef]
- Naeem, A.E.; Farid, A. A morphologic and morphometric study of the foramen Lacerum in adult human skulls: An osteological study in upper Egypt. Sohag Med. J. 2019, 23, 219–221. [Google Scholar] [CrossRef]
- Alonso, F.; Bernard, S.; Irwin, P.A.; Tubbs, R.I.; Iwanaga, J.; Loukas, M.; Oskouian, R.J.; Tubbs, R.S. The relationship between the carotid canal and mandibular condyle: An anatomical study with application to surgical approaches to the skull base via the infratemporal fossa. Anatomy 2016, 10, 193–199. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Kurt, M.H.; Bozkurt, P.; Bilecenoglu, B.; Kolsuz, M.E.; Orhan, K. Morphometric analysis of vidian canal and its relations with surrounding anatomic structures by using cone-beam computed tomography. Folia Morphol. 2020, 79, 366–373. [Google Scholar] [CrossRef]
- Kassam, A.B.; Vescan, A.D.; Carrau, R.L.; Prevedello, D.M.; Gardner, P.; Mintz, A.H.; Snyderman, C.H.; Rhoton, A.L. Expanded endonasal approach: Vidian canal as a landmark to the petrous internal carotid artery. J. Neurosurg. 2008, 108, 177–183. [Google Scholar] [CrossRef]
- Klein, B.M.; Bordoni, B. Anatomy, head and neck, emissary veins. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Mortazavi, M.M.; Tubbs, R.S.; Riech, S.; Verma, K.; Shoja, M.M.; Zurada, A.; Benninger, B.; Loukas, M.; Gadol, A.A.C. Anatomy and pathology of the cranial emissary veins: A review with surgical implications. Neurosurgery 2012, 70, 1312–1319. [Google Scholar] [CrossRef]
- Dwight, T.; McMurrich, J.P.; Hamann, C.A.; White, J.W. Human Anatomy: Including Structure and Development and Practical Considerations; JB Lippincott Company: Philadelphia, PA, USA, 1913; Volume 2. [Google Scholar]
- Gruber, W. BeiträgezurAnatomie des Schädelgrundes; Commissionnnaires de l’AcademieImpériale des Sciences: St. Petersbourg, Russia, 1869; Volume 1. [Google Scholar]
- Chaudhary, S.; Kumar, S.; Chaudhary, S.; Munjal, S. Morphometric Variations of the Foramen Lacerum in Dry Skulls and its Clinical Significance: A Cross-sectional Study. Int. J. Anat. Radiol. Surg. 2023, 12, AO08–AO10. [Google Scholar] [CrossRef]
- Nayak, S.B. Multiple variations at the base of an adult skull: Implications in radiology and skull base surgery. J. Craniofacial Surg. 2019, 30, 254–255. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R. Variations in the morphology of foramen lacerum. J. Craniofacial Surg. 2020, 31, 1848–1850. [Google Scholar] [CrossRef]
- Borghei-Razavi, H.; Truong, H.Q.; Fernandes Cabral, D.T.; Sun, X.; Celtikci, E.; Wang, E.; Snyderman, C.; Gardner, P.A.; Fernandez-Miranda, J.C. Endoscopic Endonasal Petrosectomy: Anatomical Investigation, Limitations, and Surgical Relevance. Oper. Neurosurg. 2019, 16, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Çelik, N.G.; Akman, B. Anatomical analysis of foramen magnum: A 3D Slicer CT study. Med. Rec. 2023, 5, 182–186. [Google Scholar]
- Ma, J.; Su, S.; Yue, S.; Zhao, Y.; Li, Y.; Chen, X.; Ma, H. Preoperative visualization of cranial nerves in skull base tumor surgery using diffusion tensor imaging technology. Turk. Neurosurg. 2016, 26, 805–812. [Google Scholar] [CrossRef]
- Liang, C.; Li, M.; Gong, J.; Zhang, B.; Lin, C.; He, H.; Zhang, K.; Guo, Y. A new application of ultrasound-magnetic resonance multimodal fusion virtual navigation in glioma surgery. Ann. Transl. Med. 2019, 7, 736. [Google Scholar] [CrossRef]
- Ganesh, V.; Dharanipathy, S.; Pavana, V.; Kumar, A.; Sebastian, L.J.D.; Garg, A. A computed tomography (CT)-based morphometric study of various skull base parameters and their anatomical relationships relevant to endoscopic endonasal skull base surgery. Surg. Neurol. Int. 2024, 15, 68. [Google Scholar] [CrossRef]
- Xu, Y.; Mohyeldin, A.; Lee, C.K.; Nunez, M.A.; Mao, Y.; Cohen-Gadol, A.A.; Fernandez-Miranda, J.C. Endoscopic endonasal approach to the ventral petroclival fissure: Anatomical findings and surgical techniques. J. Neurol. Surg. Part B Skull Base 2024, 85, 420–430. [Google Scholar] [CrossRef]
- Lang, J. Skull Base and Related Structures: Atlas of Clinical Anatomy; Schattauer Verlag: Stuttgart, Germany, 2001. [Google Scholar]
- Storey, C.; Barry, J.; Adkins, W.; Nanda, A.; Saenz-Cuellar, H. A Morphometric Analysis for the Feasibility of Percutaneous Translacerum Access of the Internal Carotid Artery Based on Computed Tomography Angiography. World Neurosurg. 2019, 121, e925–e930. [Google Scholar] [CrossRef] [PubMed]
- Mato, D.; Yokota, H.; Hirono, S.; Martino, J.; Saeki, N. The vidian canal: Radiological features in Japanese population and clinical implications. Neurol. Med.-Chir. 2015, 55, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Papavasileiou, G.; Hajiioannou, J.; Kapsalaki, E.; Bizakis, I.; Fezoulidis, I.; Vassiou, K. Vidian canal and sphenoid sinus: An MDCT and cadaveric study of useful landmarks in skull base surgery. Surg. Radiol. Anat. 2020, 42, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro-Neto, C.D.; Fernandez-Miranda, J.C.; Rivera-Serrano, C.M.; Paluzzi, A.; Snyderman, C.H.; Gardner, P.A.; Sennes, L.U. Endoscopic anatomy of the palatovaginal canal (palatosphenoidal canal) a landmark for dissection of the vidian nerve during endonasal transpterygoid approaches. Laryngoscope 2012, 122, 6–12. [Google Scholar] [CrossRef]
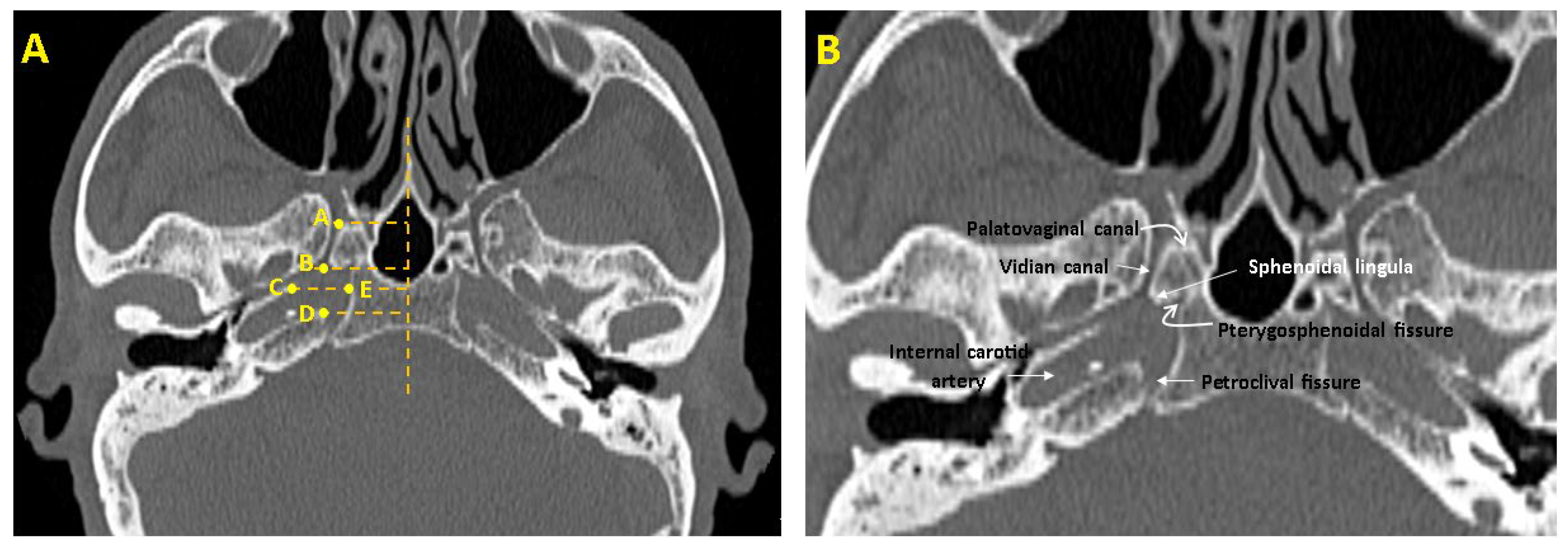

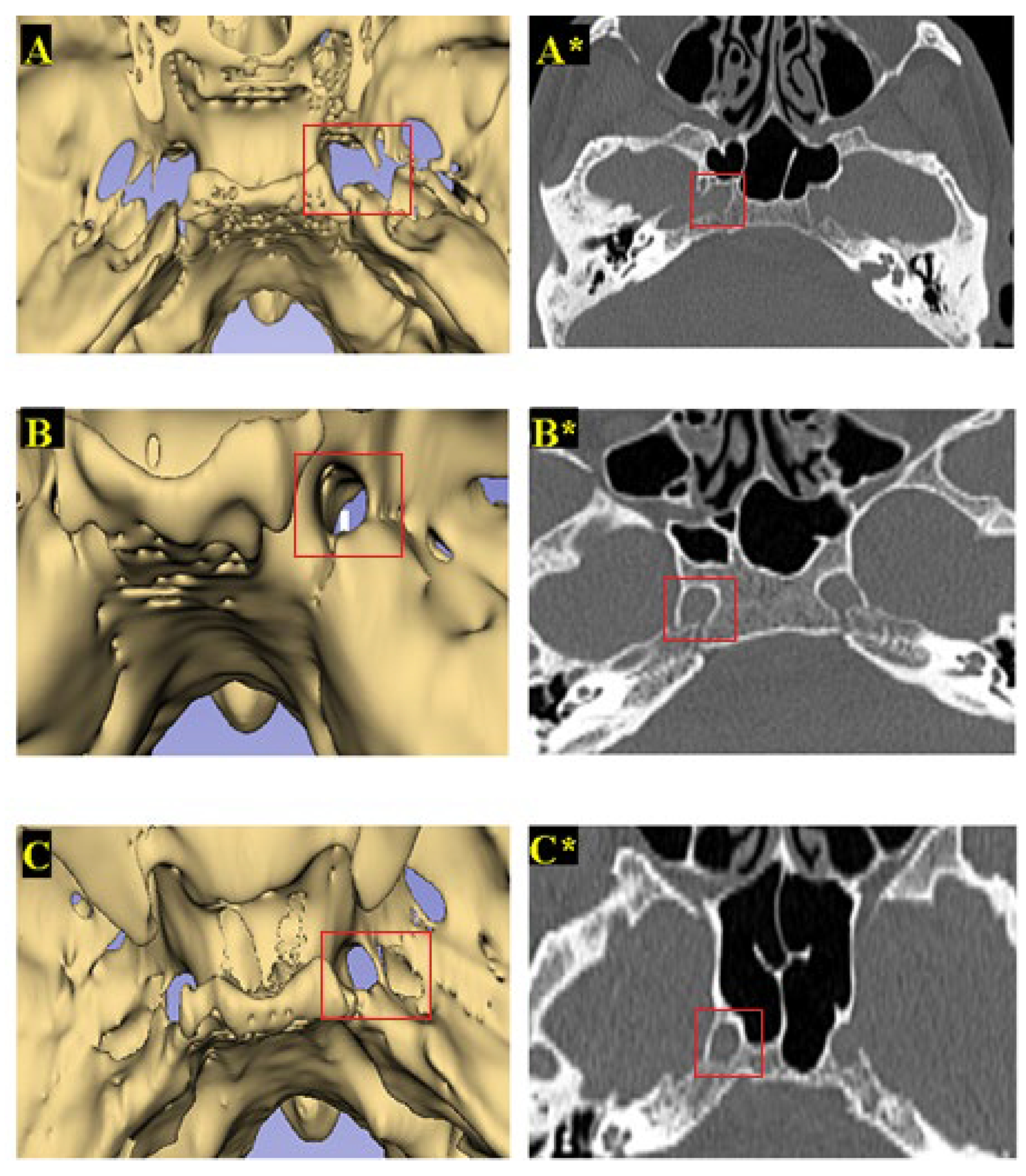
| Parameters | Definitions of Landmarks (Figure 1) |
|---|---|
| FL length(mm) | The distance between the posterior end of the VC opening into the FL and the petrous apex of the temporal bone (B–D). |
| FL width (mm) | The distance between the temporal bone’s petrous apex and the sphenoid bone’s corpus (C–E). |
| VC length (mm) | The distance between the anterior end of the VC opening into the pterygopalatine fossa and the posterior end of the VC opening into the FL (A,B). |
| VC—pterygosphenoidal fissure angle (°) | The angle formed between the VC and the oblique line drawn from the pterygosphenoidal fissure. |
| VC—palatovaginal canal angle (°) | The angle formed between the VC and the oblique line drawn from the palatovaginal canal. |
| Parameters | Gender | Mean ± SD | SE | p-Value |
|---|---|---|---|---|
| Right FL length (mm) | Female | 9.76 ± 1.52 | 0.23 | 0.017 * |
| Male | 10.62 ± 1.53 | 0.25 | ||
| Left FL length (mm) | Female | 9.97 ± 1.36 | 0.21 | 0.022 * |
| Male | 10.69 ± 1.34 | 0.22 | ||
| Right FL width (mm) | Female | 8.44 ± 1.23 | 0.19 | 0.516 |
| Male | 8.63 ± 1.34 | 0.22 | ||
| Left FL width (mm) | Female | 8.09 ± 1.12 | 0.17 | 0.030 * |
| Male | 8.70 ± 1.30 | 0.22 | ||
| Right VC length(mm) | Female | 12.11 ± 1.70 | 0.26 | 0.508 |
| Male | 11.84 ± 1.75 | 0.29 | ||
| Left VC length (mm) | Female | 12.36 ± 1.40 | 0.21 | 0.949 |
| Male | 11.84 ± 1.90 | 0.32 | ||
| Right VC-pterygosphenoidal fissure angle (°) | Female | 53.80 ± 5.32 | 0.82 | 0.001 * |
| Male | 57.97 ± 4.16 | 0.70 | ||
| Left VC-pterygosphenoidal fissure angle (°) | Female | 52.55 ± 5.62 | 0.86 | 0.001 * |
| Male | 57.01 ± 4.15 | 0.70 | ||
| Right VC-palatovaginal canal angle (°) | Female | 50.04 ± 2.27 | 0.35 | 0.154 |
| Male | 51.03 ± 3.71 | 0.62 | ||
| Left VC-palatovaginal canal angle (°) | Female | 50.25 ± 2.27 | 0.35 | 0.139 |
| Male | 51.20 ± 3.25 | 0.54 |
| Parameters | Right (Mean ± SD) | Left (Mean ± SD) | Mean Difference | t | df | p-Value |
|---|---|---|---|---|---|---|
| FL length (mm) | 9.76 ± 1.53 | 9.97 ± 1.37 | −0.21 | −1.27 | 41 | 0.212 |
| FL width (mm) | 8.44 ± 1.24 | 8.09 ± 1.13 | 0.35 | 2.34 | 41 | 0.025 * |
| VC length (mm) | 12.11 ± 1.71 | 12.36 ± 1.41 | −0.25 | −1.19 | 41 | 0.242 |
| VC–pterygosphenoidal angle (°) | 53.81 ± 5.33 | 52.56 ± 5.63 | 1.25 | 2.34 | 41 | 0.024 * |
| VC–palatovaginal canal angle (°) | 50.04 ± 2.27 | 50.26 ± 2.27 | −0.22 | −0.63 | 41 | 0.530 |
| Parameter | Right (Mean ± SD) | Left (Mean ± SD) | Mean Difference | t | df | p-Value |
|---|---|---|---|---|---|---|
| FL length (mm) | 10.62 ± 1.53 | 10.70 ± 1.35 | −0.08 | −0.54 | 34 | 0.591 |
| FL width (mm) | 8.63 ± 1.34 | 8.71 ± 1.30 | −0.07 | −0.37 | 34 | 0.712 |
| VC length (mm) | 11.85 ± 1.75 | 12.36 ± 1.91 | −0.54 | −2.39 | 34 | 0.022 * |
| VC-pterygosphenoidal angle (°) | 57.97 ± 4.17 | 57.02 ± 4.15 | 0.96 | 1.48 | 34 | 0.148 |
| VC–palatovaginal canal angle (°) | 51.03 ± 3.72 | 51.20 ± 3.25 | −0.17 | −0.25 | 34 | 0.807 |
| FL Types | Total (%/n) | Female (%/n) | Male (%/n) |
|---|---|---|---|
| Right FL | |||
| Type 1 (Normal) | %70.1 (54) | %64.3 (27) | %77.1 (27) |
| Type 2 (Canal-shaped) | %15.6(12) | %26.2 (11) | %2.9 (1) |
| Type 3 (Bridged) | %14.3 (11) | %9.5 (4) | %20.0 (7) |
| Left FL | |||
| Type 1 (Normal) | %72.7 (56) | %73.8 (31) | %71.4 (25) |
| Type 2 (Canal-shaped) | %11.7 (9) | %16.7 (7) | %5.7 (2) |
| Type 3 (Bridged) | %15.6 (12) | %9.5 (4) | %22.9 (8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muslu, M.; Karaca, Ö.; Kökçe, A.; Acer, N. A Computed Tomography-Based Morphometric Assessment of the Foramen Lacerum in a Turkish Population Using the 3D Slicer Method. Medicina 2025, 61, 943. https://doi.org/10.3390/medicina61050943
Muslu M, Karaca Ö, Kökçe A, Acer N. A Computed Tomography-Based Morphometric Assessment of the Foramen Lacerum in a Turkish Population Using the 3D Slicer Method. Medicina. 2025; 61(5):943. https://doi.org/10.3390/medicina61050943
Chicago/Turabian StyleMuslu, Merve, Ömür Karaca, Aybars Kökçe, and Niyazi Acer. 2025. "A Computed Tomography-Based Morphometric Assessment of the Foramen Lacerum in a Turkish Population Using the 3D Slicer Method" Medicina 61, no. 5: 943. https://doi.org/10.3390/medicina61050943
APA StyleMuslu, M., Karaca, Ö., Kökçe, A., & Acer, N. (2025). A Computed Tomography-Based Morphometric Assessment of the Foramen Lacerum in a Turkish Population Using the 3D Slicer Method. Medicina, 61(5), 943. https://doi.org/10.3390/medicina61050943








