Core Exercise as Non-Pharmacological Strategy for Improving Metabolic Health in Prediabetic Women
Abstract
1. Introduction
2. Materials and Methods
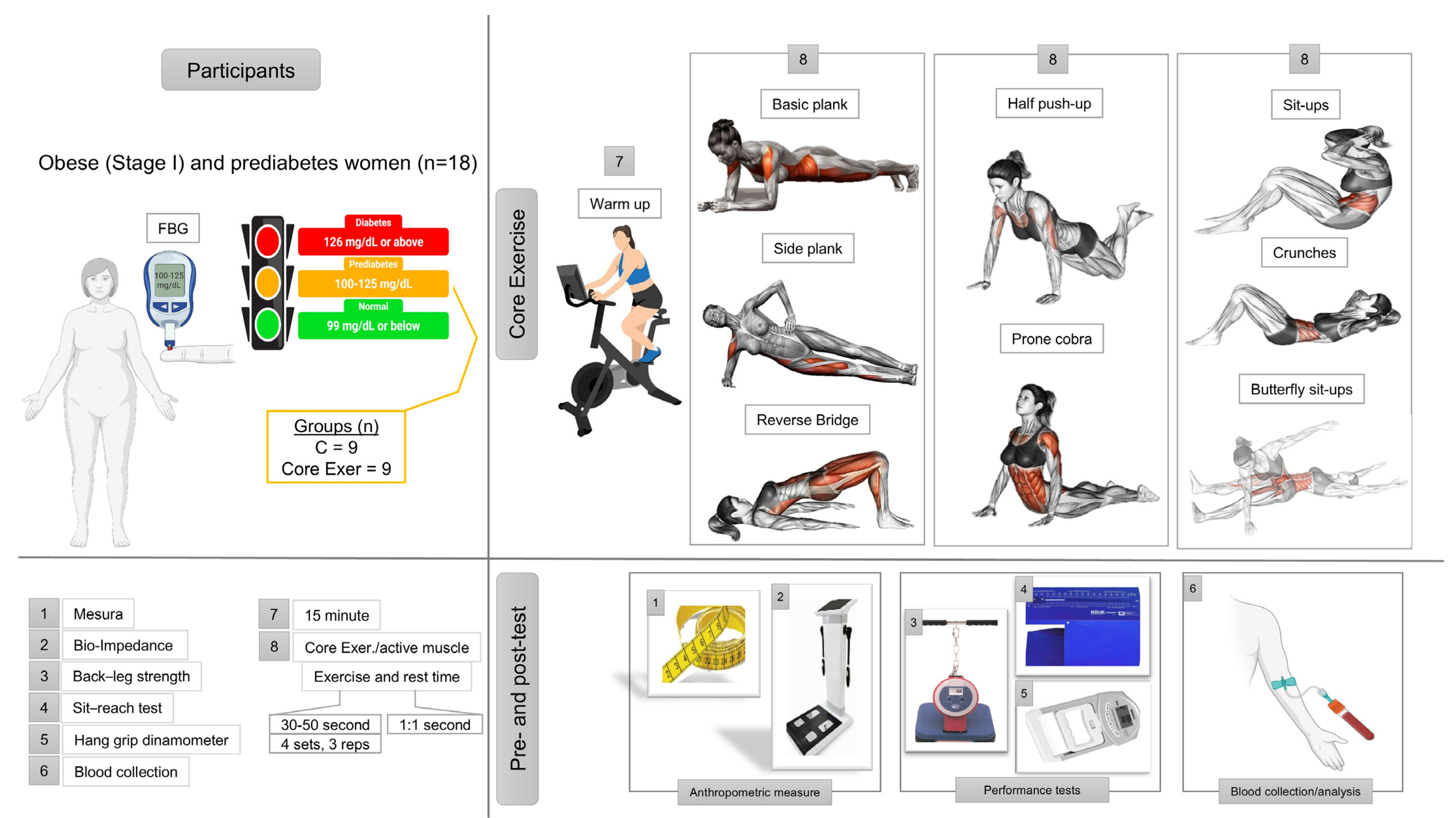
2.1. Participants
2.2. Experimental Design
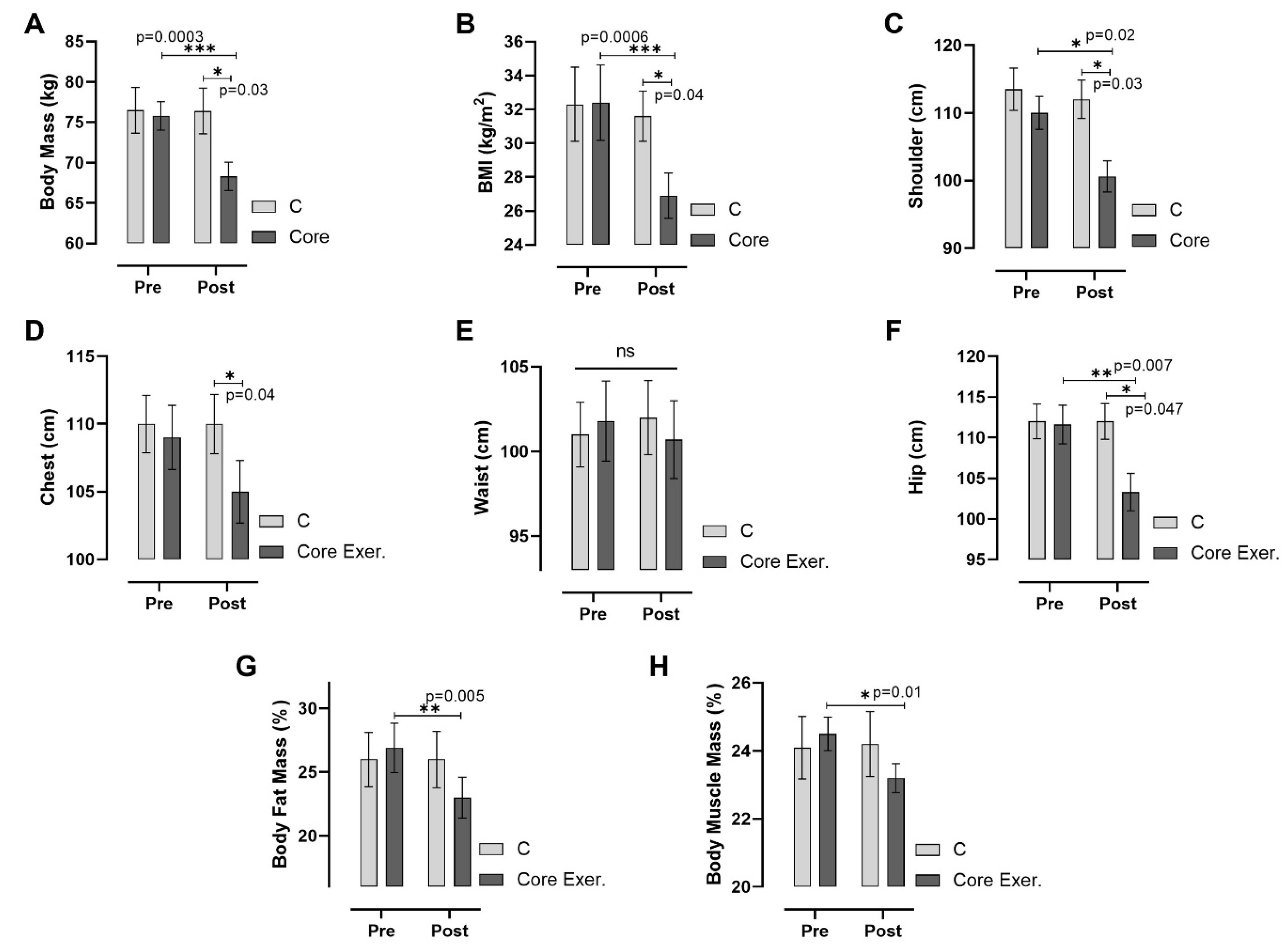
2.3. Anthropometric Measurements
2.4. Performance Test
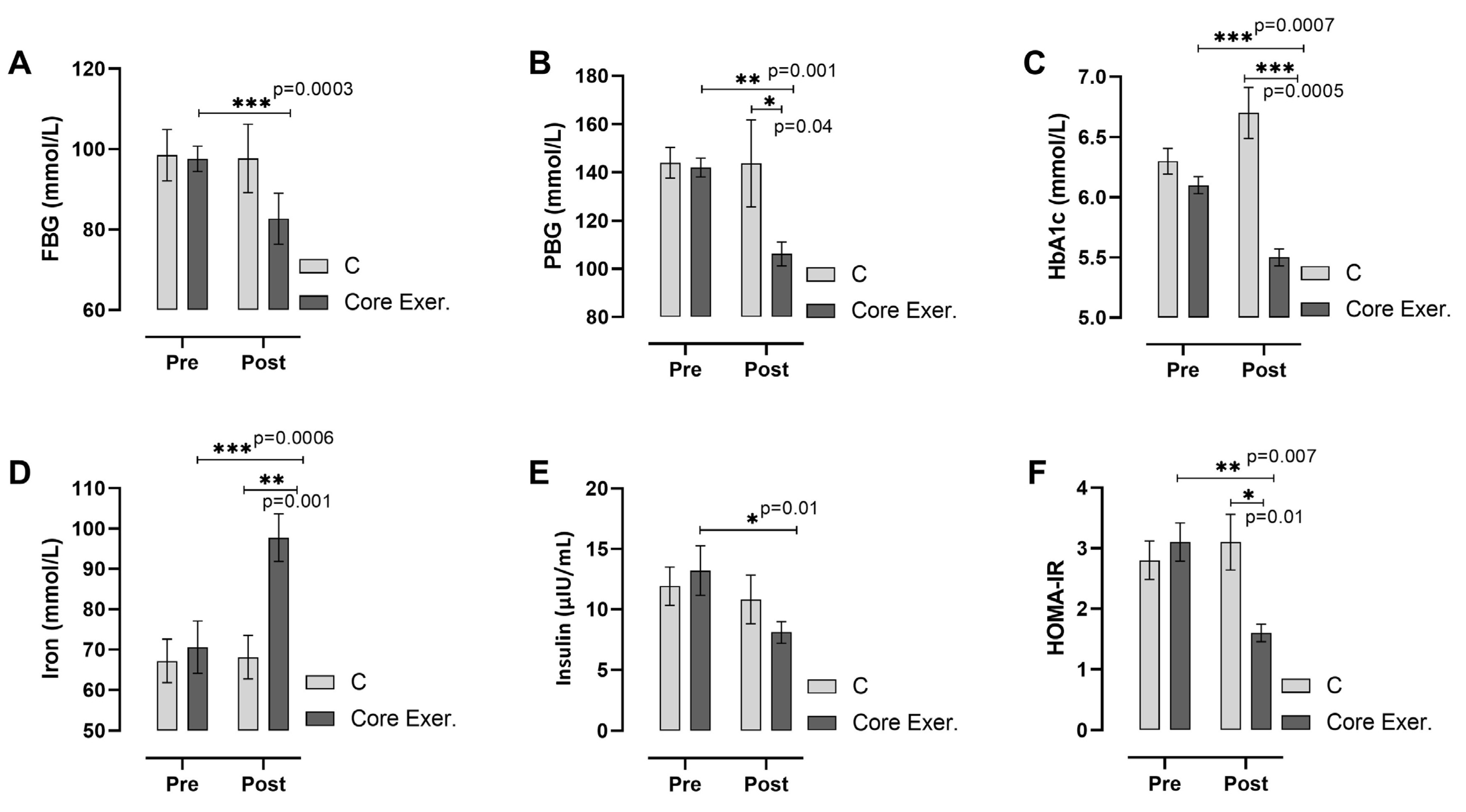
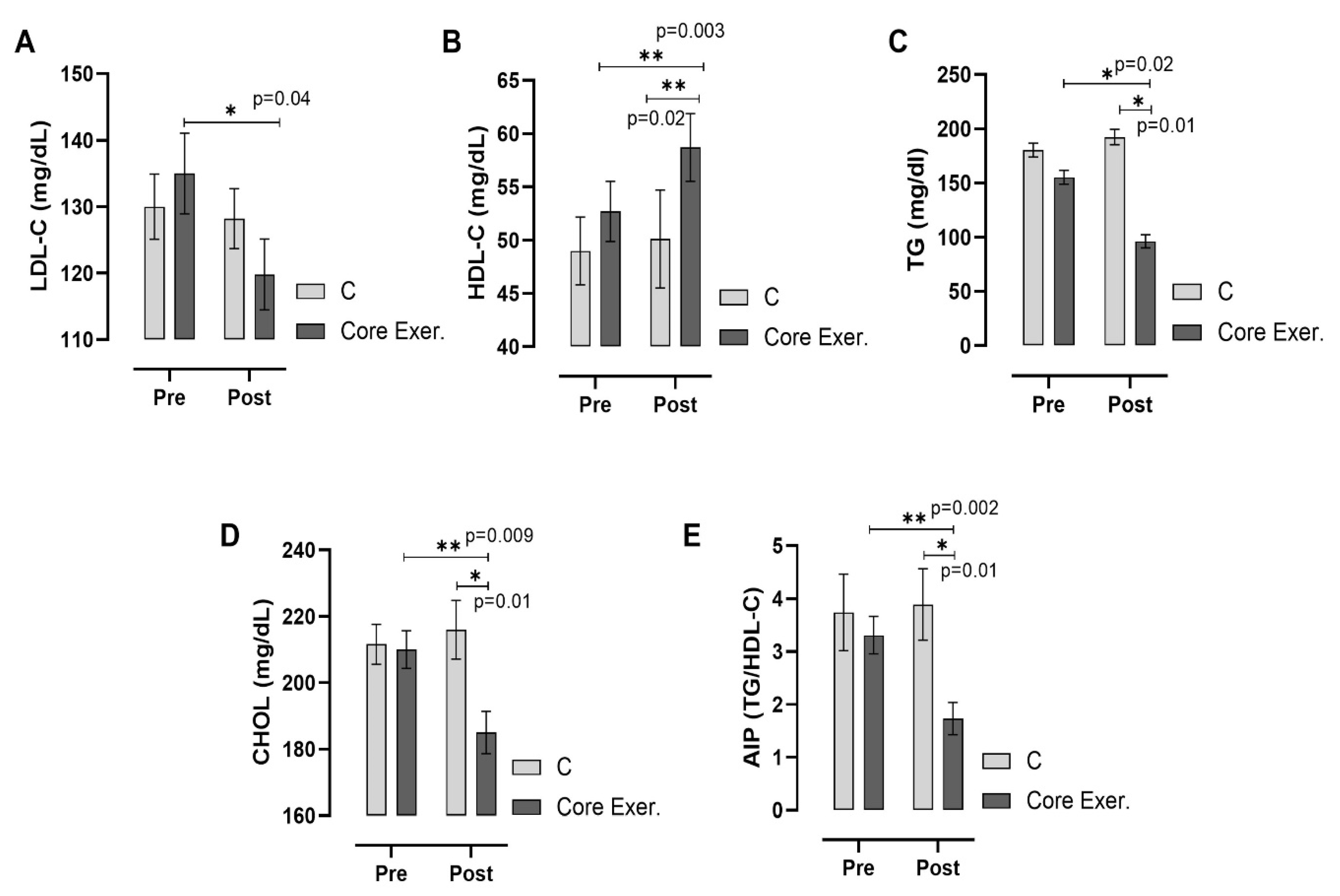
2.5. Biochemical Analyses
2.6. Statistical Analysis
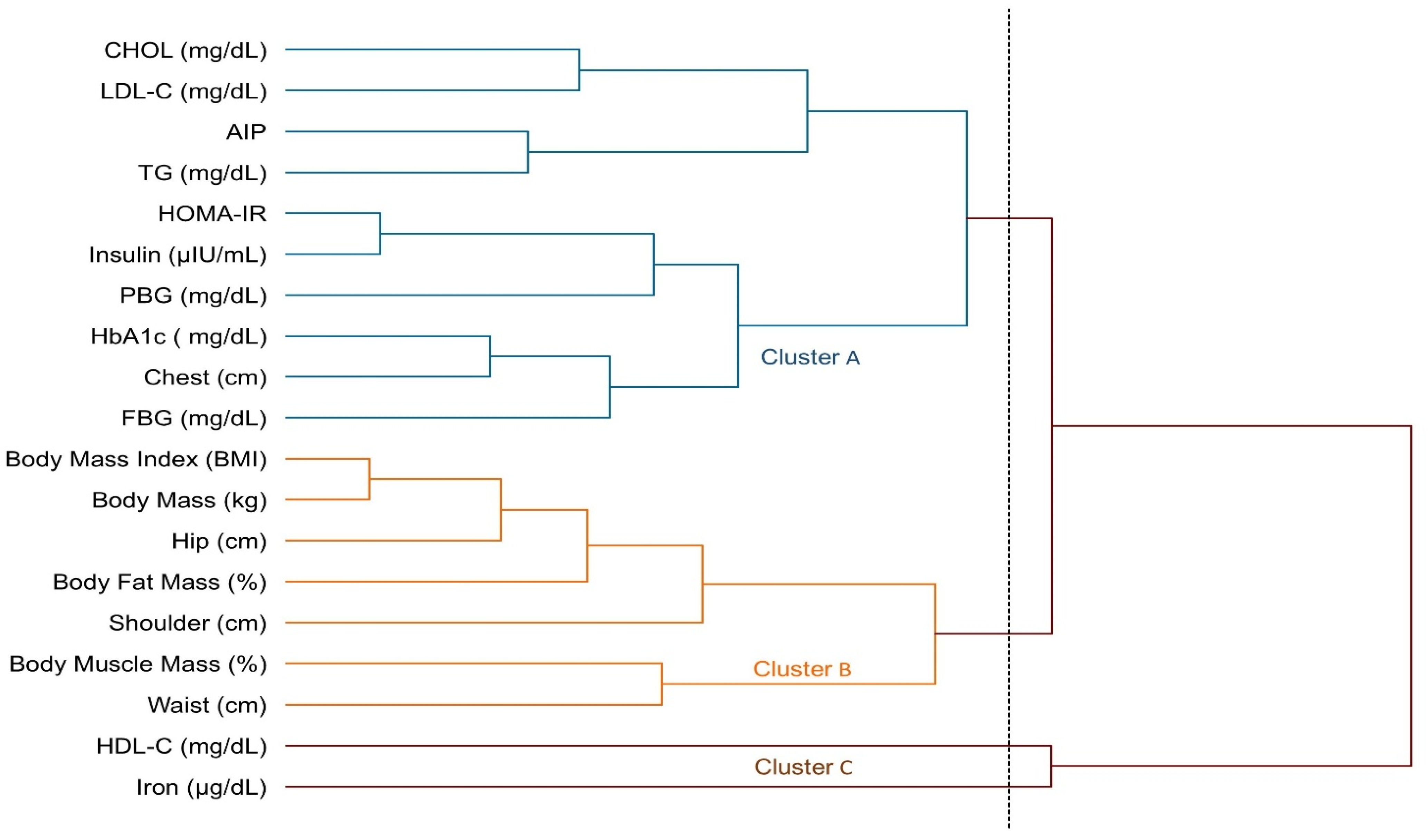
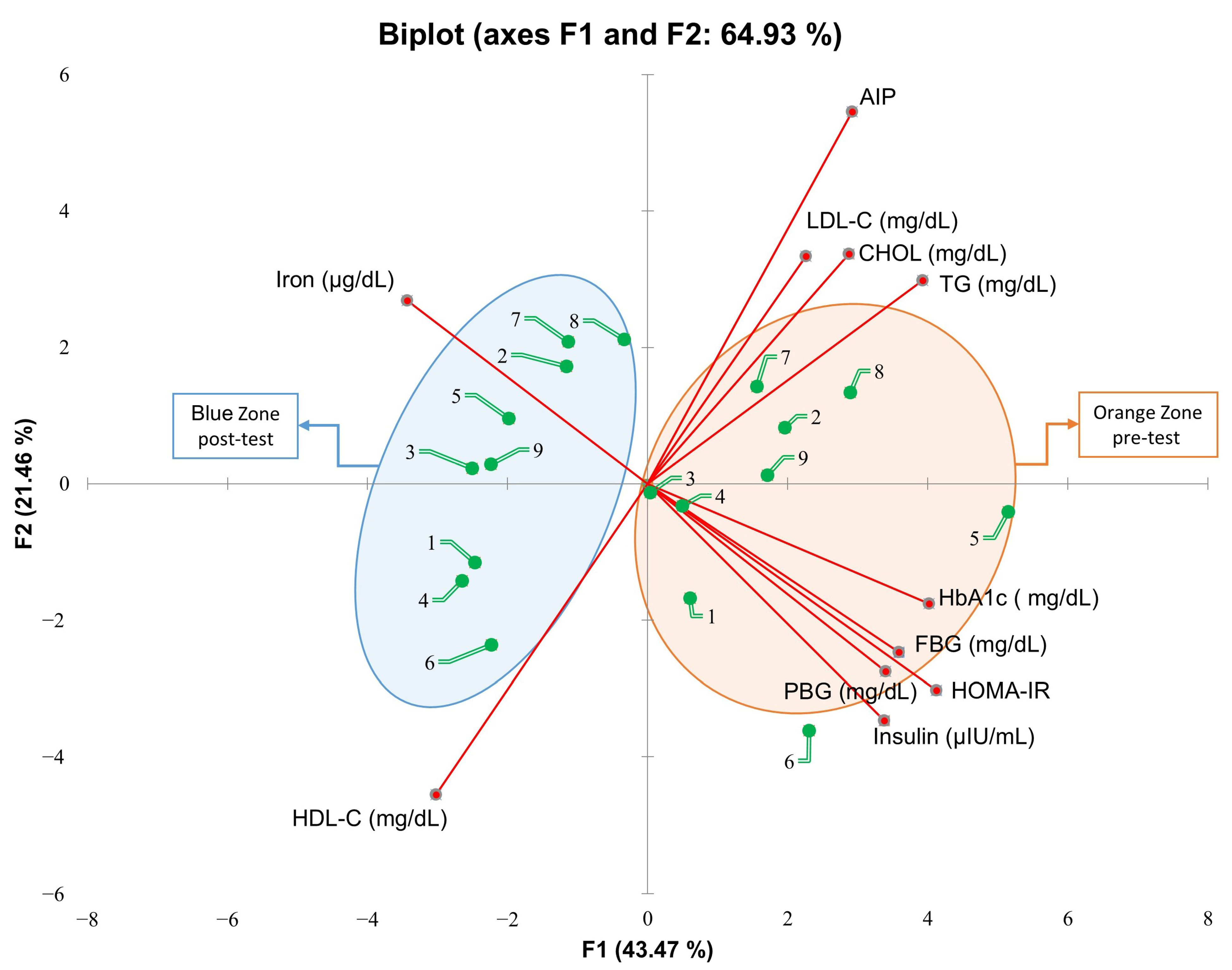
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Prediabetes |
| DM | Diabetes mellitus |
| BMI | Body mass index |
| FBG | Fasting blood glucose |
| PBG | Postprandial blood glucose |
| HbA1c | Hemoglobin A1c |
| HOMA-IR | homeostatic model assessment |
| LDL-C | low density lipoprotein cholesterol |
| HDL-C | high density lipoprotein cholesterol |
| TG | triglyceride |
| CHOL | Total cholesterol |
| HIIT | high-intensity interval training |
References
- Ghody, P.; Shikha, D.; Karam, J.; Bahtiyar, G. Identifying prediabetes–is it beneficial in the long run? Maturitas 2015, 81, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Thipsawat, S. Intervention for prevention of type 2 diabetes mellitus among prediabetes: A review of the literature. SAGE Open Nurs. 2023, 9, 23779608231175581. [Google Scholar] [CrossRef] [PubMed]
- Kindlovits, R.; Sousa, A.C.; Viana, J.L.; Milheiro, J.; Marques, F.; Teixeira, V.H. Combined low-carbohydrate diet and long-term exercise in hypoxia in type 2 diabetes: A randomized controlled trial protocol to assess glycemic control, cardiovascular risk factors and body composition. Nutr. Health 2024, 30, 5–13. [Google Scholar] [CrossRef]
- Teixeira, P.P.; Zucatti, K.P.; Matzenbacher, L.S.; Wayerbacher, L.F.; Zhang, M.; Colpani, V.; Gerchman, F. Long-term lifestyle intervention can reduce the development of type 2 diabetes mellitus in subjects with prediabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2024, 210, 111637. [Google Scholar] [CrossRef]
- Neeraja, M.; Vani, L.; Vijitha, P.A. Study on lipid profile and body mass index (BMI) in adult females with sedentary and active life styles. Eur. J. Cardiovasc. Med. 2023, 13, 247–251. [Google Scholar]
- Wang, Y.; Li, H.; Yang, D.; Wang, M.; Han, Y.; Wang, H. Effects of aerobic exercises in prediabetes patients: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1227489. [Google Scholar] [CrossRef]
- Gür, M.; Çınar, V.; Akbulut, T.; Bozbay, K.; Yücedal, P.; Aslan, M.; Migliaccio, G.M. Determining the levels of cortisol, testosterone, lactic acid and anaerobic performance in athletes using various forms of coffee. Nutrients 2024, 16, 3228. [Google Scholar] [CrossRef]
- Bengin, E.; Kırtepe, A.; Çınar, V.; Akbulut, T.; Russo, L.; Aydemir, İ.; Migliaccio, G.M. Leptin, ghrelin, irisin, asprosin and subfatin changes in obese women: Effect of exercise and different nutrition types. Medicina 2024, 60, 1118. [Google Scholar] [CrossRef]
- Şengün, N.; Pala, R.; Çınar, V.; Akbulut, T.; Larion, A.; Padulo, J.; Migliaccio, G.M. Alterations in biomarkers associated with cardiovascular health and obesity with short-term lifestyle changes in overweight women: The Role of Exercise and Diet. Medicina 2024, 60, 2019. [Google Scholar] [CrossRef]
- Bozbay, K.; Çinar, V.; Akbulut, T.; Aydemir, I.; Yasul, Y.; Aytac, K.Y.; Migliaccio, G.M. Effects of exercise and pomegranate–black carrot juice interventions on mineral metabolism and fatty acids. Appl. Sci. 2024, 14, 7284. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Hua, G.; Guo, C.; Gong, S.; Li, M.; Yang, Y. Exercise training modalities in prediabetes: A systematic review and network meta-analysis. Front. Endocrinol. 2024, 15, 1308959. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.B.; Slentz, C.A.; Willis, L.H.; Hoselton, A.; Huebner, J.L.; Kraus, V.B.; Kraus, W.E. Rejuvenation of neutrophil functions in association with reduced diabetes risk following ten weeks of low-volume high intensity interval walking in older adults with prediabetes–a pilot study. Front. Immunolo. 2020, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Bennasar-Veny, M.; Malih, N.; Galmes-Panades, A.M.; Hernandez-Bermudez, I.C.; Garcia-Coll, N.; Ricci-Cabello, I.; Yañez, A.M. Effect of physical activity and different exercise modalities on glycemic control in people with prediabetes: A systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1233312. [Google Scholar] [CrossRef]
- Hou, L.; Wang, Q.; Pan, B.; Li, R.; Li, Y.; He, J.; Yang, K. Exercise modalities for type 2 diabetes: A systematic review and network meta-analysis of randomized trials. Diabetes Metab. Res. Rev. 2023, 39, e3591. [Google Scholar] [CrossRef]
- Yasul, Y.; Akcinar, F.; Yigiter, N.; Yasul, M.E. Comprasion of physical activity levels of working women and housewives according to some variables. JROLSS 2023, 4, 1010–1023. [Google Scholar]
- Otman, S.A.; Köse, N. Anthropometric Measurements: Fundamental Assessment Principles in Therapeutic Movements; Yucel Ofset Publications: Ankara, Turkey, 2008; pp. 49–57. [Google Scholar]
- Yasul, M.E.; Akbulut, T.; Yasul, Y.; Çınar, V. The effects of short-term electromyostimulation exercises and different diet types on body composition in obese women. J. Educ. Recreat. Patterns 2024, 5, 130–142. [Google Scholar] [CrossRef]
- Yang, S.; Chen, C.; Tang, Y.; Li, K.; Yu, X.; Tan, J.; Luo, F. The effects of back extensor strength in different body positions on health-related quality of life in patients with degenerative spinal deformity. J. Back. Musculoskeletal Rehabil. 2023, 37, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Güder, F.; Canbolat, B.; Gunay, M. Investigation of the relationship between body composition, strength, and flexibility in 12–14-year-old taekwondo athletes. Mediterr. J. Soc. Sci. 2022, 5, 166–175. [Google Scholar]
- Asan, S.; Altuğ, T.; Çingöz, Y.E. An Investigation of the effect of 12-week gymnastics and ballet training on balance and flexibility skills in preschool children. Educ. Q. Rev. 2021, 4, 2007–2013. [Google Scholar] [CrossRef]
- Bonn, S.E.; Alexandrou, C.; Hjörleifsdottir Steiner, K.; Wiklander, K.; Östenson, C.G.; Löf, M.; Trolle Lagerros, Y. App-technology to increase physical activity among patients with diabetes type 2-the diaCert-study, a randomized controlled trial. BMC Public. Health 2018, 18, 119. [Google Scholar] [CrossRef]
- Öner, S.; Yasul, Y.; Akçinar, F. The effects of high-intensity interval training on body composition and lipid profile. Pak. J. Med. Health Sci. 2021, 15, 641–645. [Google Scholar]
- Mitchai, M.; Suwansaksri, N.; Seanseeha, S.; Saenboonsiri, J.; Kraitree, P.; Piyapromdee, J.; Silsirivanit, A. Misleading HbA1c measurement in diabetic patients with hemoglobin variants. Med. Sci. 2021, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Böyük, B.; Okuturlar, Y.; Uludağ, E.; Atalay, H.; Güzel, S.; Çelebi, A. The relationship between c-peptide and microalbuminuria in patients with type 2 diabetes mellitus. Cukurova Med. J. 2018, 43, 56–61. [Google Scholar] [CrossRef]
- Park, S.Y.; Gautier, J.F.; Chon, S. Assessment of insulin secretion and insulin resistance in human. Diabetes Metab. J. 2021, 45, 641–654. [Google Scholar] [CrossRef]
- Serhan, M.; Jackemeyer, D.; Long, M.; Sprowls, M.; Perez, I.D.; Maret, W.; Forzani, E. Total iron measurement in human serum with a novel smartphone-based assay. IEEE J. Transl. Eng. Health Med. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Kurt, D. Adaptability and stability models in promising genotype selection for hybrid breeding of sun cured tobacco. S Afr J Bot. 2023, 154, 190–202. [Google Scholar] [CrossRef]
- Orhan, E. Physical Activity at Every Age. Koç Cm. In Holistic Health and Exercise, 1st ed.; Efe Academy Publishing: Istanbul, Turkey, 2021; pp. 8–20. [Google Scholar]
- König, D.; Hörmann, J.; Predel, H.G.; Berg, A.A. 12-month lifestyle intervention program improves body composition and reduces the prevalence of prediabetes in obese patients. Obes. Facts 2018, 11, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Lu, Z.; Yang, J.; Zhang, L.; Li, Y.; Zhang, X. Drug delivery system in the treatment of diabetes mellitus. Front. Bioeng. Biotechnol. 2020, 8, 880. [Google Scholar] [CrossRef]
- Gaitán, J.M.; Eichner, N.; Gilbertson, N.M.; Heiston, E.M.; Weltman, A.; Malin, S.K. Two weeks of interval training enhances fat oxidation during exercise in obese adults with prediabetes. J. Sports Sci. Med. 2019, 18, 636. [Google Scholar]
- Bulguroğlu, M.A.; Bulguroğlu, H.İ.; Karaduman, A.A. the effects of pilates exercise training on health-related physical fitness parameters in individuals with type 2 diabetes: A randomized controlled trial. Smyrna Med. J. 2020, 5, 1–8. [Google Scholar]
- Fex, A.; Leduc-Gaudet, J.P.; Filion, M.E.; Karelis, A.D.; Aubertin-Leheudre, M. Effect of elliptical high intensity interval training on metabolic risk factor in pre-and type 2 diabetes patients: A pilot study. J. Phys. Act. Health 2015, 12, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.Ö. Evaluation of the effects of aerobic exercises on body composition components and anthropometric measurements in middle-aged women. J. Phys. Educ. Sport 2016, 18, 9–20. [Google Scholar]
- Rogers, K.; Gibson, A. Eight-week traditional mat pilates training-program effects on adult fitness characteristics. Res. Q. Exerc. Sport 2009, 80, 569–574. [Google Scholar] [CrossRef]
- Al-Shreef, F.M.; Al-Jiffri, O.H.; Abd El-Kader, S.M. Bone metabolism and hand grip strength response to aerobic versus resistance exercise training in non-insulin dependent diabetic patients. Afr. Health Sci. 2015, 15, 896–901. [Google Scholar] [CrossRef][Green Version]
- Sekendiz, B.; Cug, M.; Korkusuz, F. Effects of swiss-ball core strength training on strength, endurance, flexibility, and balance in sedentary women. J. Strength. Cond. Res. 2010, 24, 3032–3040. [Google Scholar] [CrossRef]
- Cosio-Lima, L.M.; Reynolds, K.L.; Winter, C.; Paolone, V.; Jones, M.T. Effects of physioball and conventional floor exercises on early phase adaptations in back and abdominal core stability and balance in women. J. Strength. Cond. Res. 2003, 17, 721–725. [Google Scholar]
- Hsieh, P.L.; Tseng, C.H.; Tseng, Y.J.; Yang, W.S. Resistance training improves muscle function and cardiometabolic risks but not quality of life in older people with type 2 diabetes mellitus: A randomized controlled trial. J. Geriatr. Phys. Ther. 2018, 41, 65–76. [Google Scholar] [CrossRef]
- Aka, H.; İbiş, S.; Arıcı, R. The effects of 8-week reformer pilates exercises on body composition and some physical fitness parameters in women. Gaziantep Univ. J. Sports Sci. 2020, 5, 573–589. [Google Scholar]
- Hwang, H.; Koo, J. Effects of stretching exercises and core muscle exercises on flexibility and balance ability. J. Int. Acad. Phys. Ther. Res. 2019, 10, 1717–1724. [Google Scholar] [CrossRef]
- Kirwan, M.; Chiu, C.L.; Laing, T.; Chowdhury, N.; Gwynne, K. A web-delivered, clinician-led group exercise intervention for older adults with type 2 diabetes: Single-arm pre-post intervention. J. Med. Internet Res. 2022, 24, e39800. [Google Scholar] [CrossRef]
- Fillebeen, C.; Lam, N.H.; Chow, S.; Botta, A.; Sweeney, G.; Pantopoulos, K. Regulatory connections between iron and glucose metabolism. Int. J. Mol. Sci. 2020, 21, 7773. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; López-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Han, J.; Gu, Q.; Cai, Y.; Li, J.; Wang, S.; Liu, X. Effect of Yijinjing combined with elastic band exercise on muscle mass and function in middle-aged and elderly patients with prediabetes: A randomized controlled trial. Front. Med. 2022, 9, 990100. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Nazarali, P. Alizadeh rpilates and trx training methods can improve insulin resistance in overweight women by increasing an exercise-hormone, irisin. J. Diabetes Metab. Disord. 2021, 20, 1455–1460. [Google Scholar] [CrossRef]
- Baker, C.; Hocking, S.L.; Wang, X.; Gerofi, J.; Colagiuri, S.; Sabag, A.; Twigg, S.M. Effect of low-volume exercise on hepatic steatosis in adults with obesity plus normal glucose, prediabetes or type 2 diabetes: A randomised controlled trial. BMJ Open Sport. Exerc. Med. 2024, 10, e001878. [Google Scholar] [CrossRef]
- Özdamar, M.; Erkek, Ö.K.; Tümkaya, S.; Özdamar, H.Ç.; Özdamar, A.; Pakyürek, H.; Küçükatay, Z.M.B. Examination of the effectiveness of 12-week nordic walking exercise in prediabetic individuals. Postgrad. Med. J. 2022, 15, 285–301. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, W.; Chen, Q.; Zhang, Y.; Kuo, C.H.; Korivi, M. Resistance exercise intensity is correlated with attenuation of HbA1c and insulin in patients with type 2 diabetes: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 40. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Zierath, J.R. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the american college of sports medicine. Med. Sci. Sports Exerc. 2022, 54, 353. [Google Scholar] [CrossRef]
- Wan, Y.; Su, Z. The impact of resistance exercise training on glycemic control among adults with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Biol. Res. Nurs. 2024, 26, 597–623. [Google Scholar] [CrossRef]
- Yasul, Y.; Akçınar, F.; Çınar, V.; Akbulut, T.; Aydemir, İ.; Yalçın, M.H.; Avcu, E.Ç.; Aydın, S.; Aydın, S. Moderate/high-intensity exercise and coenzyme q10 supplementation may reduce tumstatin and improve the lipid dynamics and body mass in rats. Appl. Sci. 2025, 15, 2618. [Google Scholar] [CrossRef]
- Cicek, G.; Imamoglu, O.; Gullu, A.; Celik, O.; Ozcan, O.; Gullu, E.; Yamaner, F. The effect of exercises on left ventricular systolic and diastolic heart function in sedentary women: Step-aerobic vs core exercises. J. Exerc. Sci. Fit. 2017, 15, 70–75. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, A.T.; Tolves, T.; Lenzi, T.L.; Signori, L.U.; da Silva, A.M.V. High-intensity interval training versus continuous training on physiological and metabolic variables in prediabetes and type 2 diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2018, 137, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Aggarwala, J.; Sharma, S.; Saroochi, J.A.; Sarkar, A. Effects of aerobic exercise on blood glucose levels and lipid profile in Diabetes Mellitus type 2 subjects. Al Ameen J. Med. Sci. 2016, 9, 65–69. [Google Scholar]
- Magalhães, J.P.; Santos, D.A.; Correia, I.R.; Hetherington-Rauth, M.; Ribeiro, R.; Raposo, J.F.; Sardinha, L.B. Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: A secondary analysis from a 1-year randomized controlled trial. Cardiovasc. Diabetol. 2020, 19, 169. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, S.; Wang, S.; Gu, Q.; Huang, Y.; Li, J.; Liu, X. Effects of Yijinjing combined with resistance training on body fat distribution and hepatic lipids in middle-aged and older people with prediabetes mellitus: A randomized controlled trial. Exp. Gerontol. 2023, 179, 112250. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yiğiter, N.; Akçınar, F.; Yasul, Y.; Çınar, V.; Akbulut, T.; Migliaccio, G.M. Core Exercise as Non-Pharmacological Strategy for Improving Metabolic Health in Prediabetic Women. Medicina 2025, 61, 942. https://doi.org/10.3390/medicina61050942
Yiğiter N, Akçınar F, Yasul Y, Çınar V, Akbulut T, Migliaccio GM. Core Exercise as Non-Pharmacological Strategy for Improving Metabolic Health in Prediabetic Women. Medicina. 2025; 61(5):942. https://doi.org/10.3390/medicina61050942
Chicago/Turabian StyleYiğiter, Nuray, Faruk Akçınar, Yavuz Yasul, Vedat Çınar, Taner Akbulut, and Gian Mario Migliaccio. 2025. "Core Exercise as Non-Pharmacological Strategy for Improving Metabolic Health in Prediabetic Women" Medicina 61, no. 5: 942. https://doi.org/10.3390/medicina61050942
APA StyleYiğiter, N., Akçınar, F., Yasul, Y., Çınar, V., Akbulut, T., & Migliaccio, G. M. (2025). Core Exercise as Non-Pharmacological Strategy for Improving Metabolic Health in Prediabetic Women. Medicina, 61(5), 942. https://doi.org/10.3390/medicina61050942








