Genicular Arteries Embolization for Patients with Osteoarthritis, Their Selection, and Follow-Up Based on MRI Findings
Abstract
1. Introduction
2. Understanding GAE and Its Mechanism of Action
3. GAE Efficiency and Risk
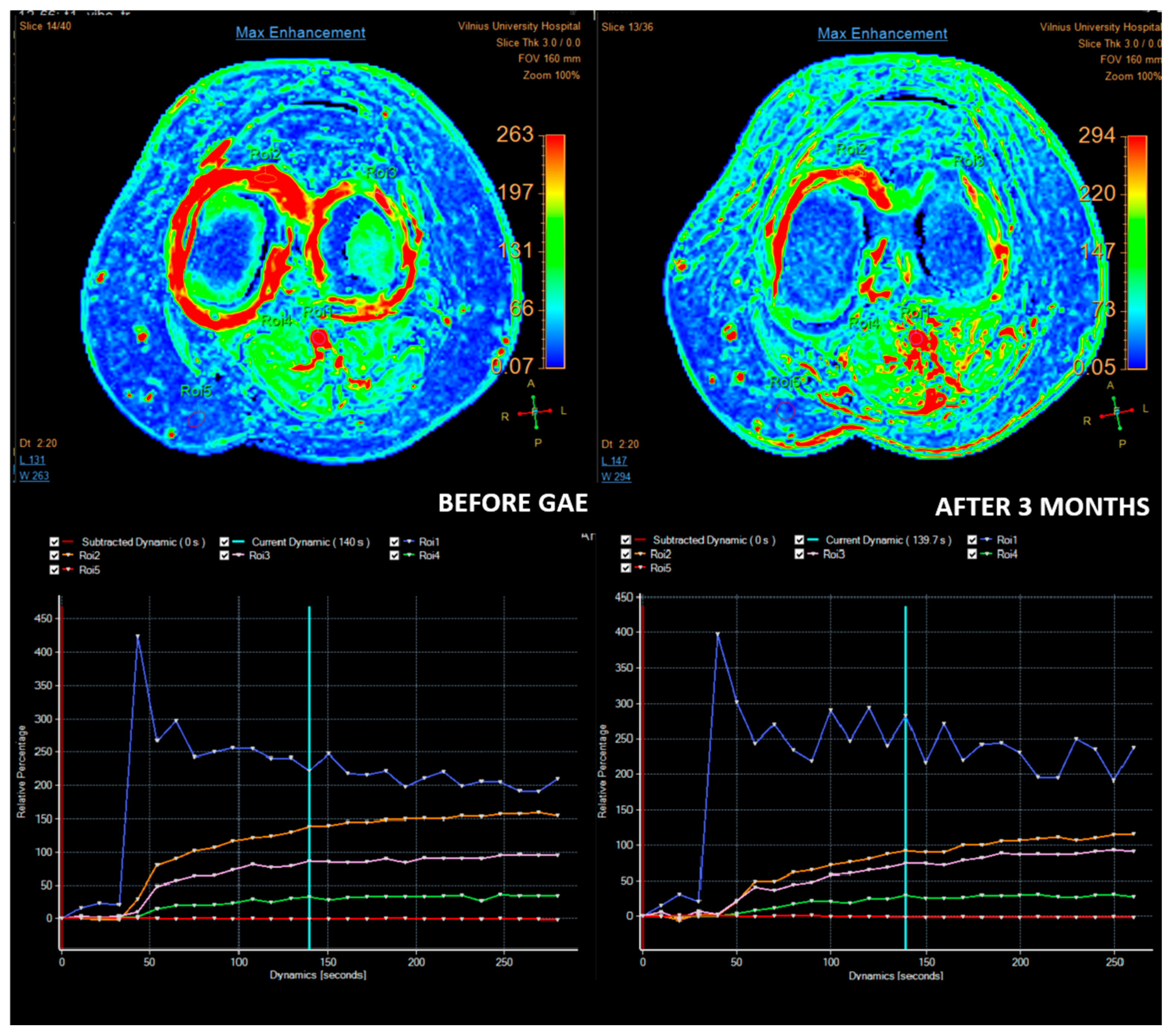
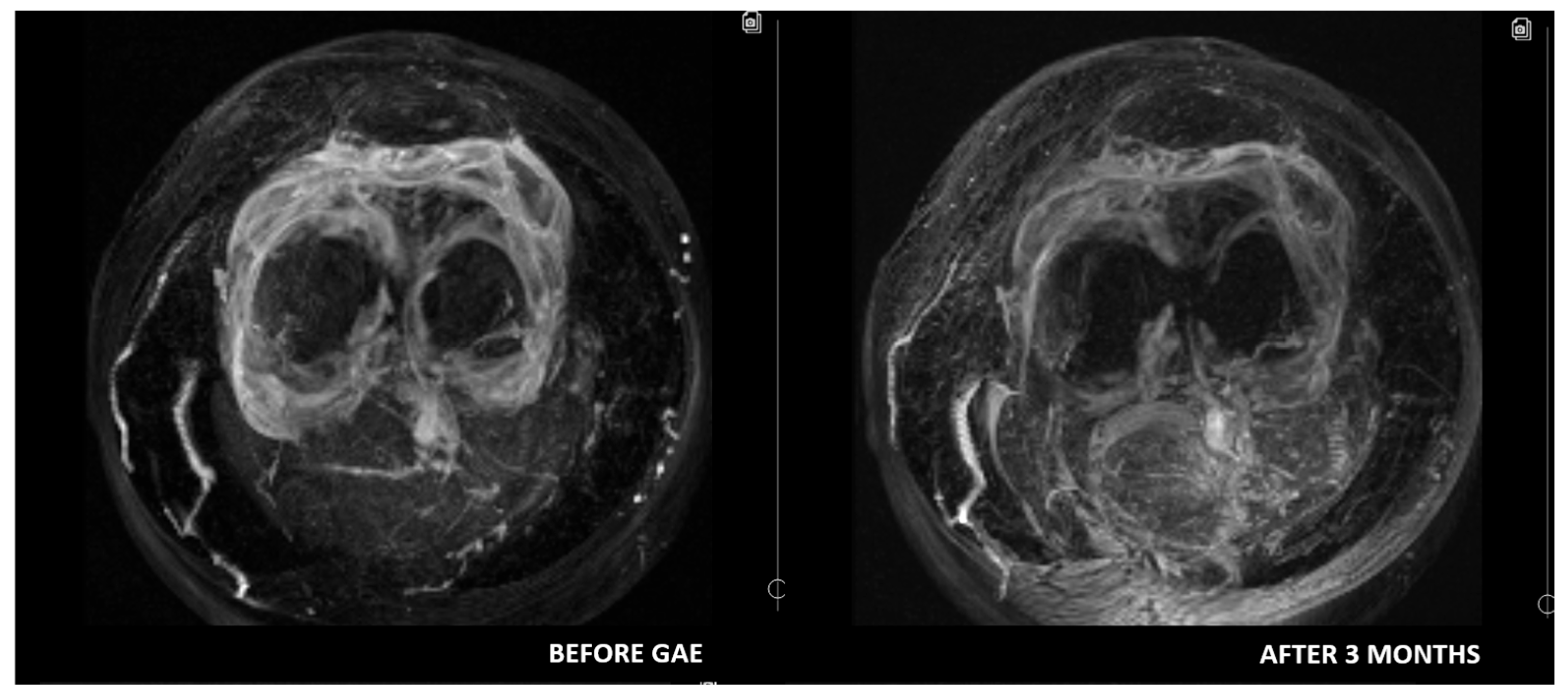
4. Role of Imaging and Most Important MRI Parameters
5. Patient Selection for GAE
6. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Driban, J.B.; Harkey, M.S.; Liu, S.H.; Salzler, M.; McAlindon, T.E. Osteoarthritis and Aging: Young Adults with Osteoarthritis. Curr. Epidemiol. Rep. 2020, 7, 9–15. [Google Scholar] [CrossRef]
- Zhao, X.; Shah, D.; Gandhi, K.; Wei, W.; Dwibedi, N.; Webster, L.; Sambamoorthi, U. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthr. Cartil. 2019, 27, 1618–1626. [Google Scholar] [CrossRef]
- Georgiev, T.; Angelov, A.K. Modifiable risk factors in knee osteoarthritis: Treatment implications. Rheumatol. Int. 2019, 39, 1145–1157. [Google Scholar] [CrossRef]
- Jüni, P.; Reichenbach, S.; Trelle, S.; Tschannen, B.; Wandel, S.; Jordi, B.; Züllig, M.; Guetg, R.; Häuselmann, H.J.; Schwarz, H.; et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: A randomized controlled trial. Arthritis Rheum. 2007, 56, 3610–3619. [Google Scholar] [CrossRef]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-articular Treatment of Knee Osteoarthritis: From Anti-inflammatories to Products of Regenerative Medicine. Physician Sportsmed. 2016, 44, 101–108. [Google Scholar] [CrossRef]
- Laupattarakasem, W.; Laopaiboon, M.; Laupattarakasem, P.; Sumananont, C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst. Rev. 2008, CD005118. [Google Scholar] [CrossRef]
- Talaie, R.; Torkian, P.; Clayton, A.; Wallace, S.; Cheung, H.; Chalian, M.; Golzarian, J. Emerging Targets for the Treatment of Osteoarthritis: New Investigational Methods to Identify Neo-Vessels as Possible Targets for Embolization. Diagnostics 2022, 12, 1403. [Google Scholar] [CrossRef]
- Okuno, Y.; Korchi, A.M.; Shinjo, T.; Kato, S. Transcatheter Arterial Embolization as a Treatment for Medial Knee Pain in Patients with Mild to Moderate Osteoarthritis. Cardiovasc. Interv. Radiol. 2015, 38, 336–343. [Google Scholar] [CrossRef]
- Mapp, P.I.; Walsh, D.A.; Bowyer, J.; Maciewicz, R.A. Effects of a metalloproteinase inhibitor on osteochondral angiogenesis, chondropathy and pain behavior in a rat model of osteoarthritis. Osteoarthr. Cartil. 2010, 18, 593–600. [Google Scholar] [CrossRef]
- Cruz, R.; Ramírez, C.; Rojas, O.I.; Casas-Mejía, O.; Kouri, J.B.; Vega-López, M.A. Menisectomized miniature Vietnamese pigs develop articular cartilage pathology resembling osteoarthritis. Pathol.-Res. Pract. 2015, 211, 829–838. [Google Scholar] [CrossRef]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef]
- Okuno, Y.; Korchi, A.M.; Shinjo, T.; Kato, S.; Kaneko, T. Midterm Clinical Outcomes and MR Imaging Changes after Transcatheter Arterial Embolization as a Treatment for Mild to Moderate Radiographic Knee Osteoarthritis Resistant to Conservative Treatment. J. Vasc. Interv. Radiol. 2017, 28, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.; Charalampopoulos, G.; Mazioti, A.; Alexopoulou, E.; Vrachliotis, T.; Brountzos, E.; Kelekis, N.; Kelekis, A. Interventional radiology techniques for pain reduction and mobility improvement in patients with knee osteoarthritis. Diagn. Interv. Imaging 2019, 100, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Torkian, P.; Golzarian, J.; Chalian, M.; Clayton, A.; Rahimi-Dehgolan, S.; Tabibian, E.; Talaie, R. Osteoarthritis-Related Knee Pain Treated with Genicular Artery Embolization: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2021, 9, 23259671211021356. [Google Scholar] [CrossRef]
- Zhang, W.; Robertson, J.; Jones, A.C.; Dieppe, P.A.; Doherty, M. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2008, 67, 1716–1723. [Google Scholar] [CrossRef]
- Van Zadelhoff, T.A.; Bos, P.K.; Moelker, A.; Bierma-Zeinstra, S.M.A.; van der Heijden, R.A.; Oei, E.H.G. Genicular artery embolisation versus sham embolisation for symptomatic osteoarthritis of the knee: A randomised controlled trial. BMJ Open 2024, 14, e087047. [Google Scholar] [CrossRef]
- Bagla, S.; Piechowiak, R.; Sajan, A.; Orlando, J.; Hartman, T.; Isaacson, A. Multicenter Randomized Sham Controlled Study of Genicular Artery Embolization for Knee Pain Secondary to Osteoarthritis. J. Vasc. Interv. Radiol. 2022, 33, 2–10.e2. [Google Scholar] [CrossRef]
- Hindsø, L.; Hölmich, P.; Petersen, M.M.; Xu, J.J.; Heerwagen, S.; Nielsen, M.B.; Riis, R.G.C.; Hansen, A.E.; Terslev, L.; Taudorf, M.; et al. Reduction in Synovitis Following Genicular Artery Embolization in Knee Osteoarthritis: A Prospective Ultrasound and MRI Study. Diagnostics 2024, 14, 2564. [Google Scholar] [CrossRef]
- Taslakian, B.; Swilling, D.; Attur, M.; Alaia, E.F.; Kijowski, R.; Samuels, J.; Macaulay, W.; Ramos, D.; Liu, S.; Morris, E.M.; et al. Genicular Artery Embolization for Treatment of Knee Osteoarthritis: Interim Analysis of a Prospective Pilot Trial Including Effect on Serum Osteoarthritis-Associated Biomarkers. J. Vasc. Interv. Radiol. 2023, 34, 2180–2189.e3. [Google Scholar] [CrossRef]
- Davies, E.; Isaacson, A. 3:27 PM Abstract No. 204 Cost analysis of geniculate artery embolization versus conservative therapy for pain secondary to knee osteoarthritis. J. Vasc. Interv. Radiol. 2018, 29, S89. [Google Scholar] [CrossRef]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. In Classic Papers in Rheumatology; CRC Press: London, UK, 2001. [Google Scholar]
- Crema, M.D.; Nevitt, M.C.; Guermazi, A.; Felson, D.T.; Wang, K.; Lynch, J.A.; Marra, M.D.; Torner, J.; Lewis, C.E.; Roemer, F.W. Progression of cartilage damage and meniscal pathology over 30 months is associated with an increase in radiographic tibiofemoral joint space narrowing in persons with knee OA—The MOST study. Osteoarthr. Cartil. 2014, 22, 1743–1747. [Google Scholar] [CrossRef][Green Version]
- Ehmig, J.; Engel, G.; Lotz, J.; Lehmann, W.; Taheri, S.; Schilling, A.F.; Seif Amir Hosseini, A.; Panahi, B. MR-Imaging in Osteoarthritis: Current Standard of Practice and Future Outlook. Diagnostics 2023, 13, 2586. [Google Scholar] [CrossRef]
- Luo, P.; Lu, L.; Xu, R.; Jiang, L.; Li, G. Gaining Insight into Updated MR Imaging for Quantitative Assessment of Cartilage Injury in Knee Osteoarthritis. Curr. Rheumatol. Rep. 2024, 26, 311–320. [Google Scholar] [CrossRef]
- Guermazi, A.; Roemer, F.W.; Hayashi, D.; Crema, M.D.; Niu, J.; Zhang, Y.; Marra, M.D.; Katur, A.; Lynch, J.A.; El-Khoury, G.Y.; et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: The MOST study. Ann. Rheum. Dis 2011, 70, 805–811. [Google Scholar] [CrossRef]
- Shakoor, D.; Demehri, S.; Roemer, F.W.; Loeuille, D.; Felson, D.T.; Guermazi, A. Are contrast-enhanced and non-contrast MRI findings reflecting synovial inflammation in knee osteoarthritis: A meta-analysis of observational studies. Osteoarthr. Cartil. 2020, 28, 126–136. [Google Scholar] [CrossRef]
- MacKay, J.W.; Nezhad, F.S.; Rifai, T.; Kaggie, J.D.; Naish, J.H.; Roberts, C.; Graves, M.J.; Waterton, J.C.; Janiczek, R.L.; Roberts, A.R.; et al. Dynamic contrast-enhanced MRI of synovitis in knee osteoarthritis: Repeatability, discrimination and sensitivity to change in a prospective experimental study. Eur. Radiol. 2021, 31, 5746–5758. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.H.; Kim, D.H.; So, Y.H.; Park, J.; Cho, S.B.; Kim, J.-E.; Kim, Y.J.; Hur, S.; Jae, H.J. Clinical Outcomes of Transcatheter Arterial Embolisation for Chronic Knee Pain: Mild-to-Moderate Versus Severe Knee Osteoarthritis. Cardiovasc. Intervent. Radiol. 2019, 42, 1530–1536. [Google Scholar] [CrossRef]
- van Zadelhoff, T.A.; Okuno, Y.; Bos, P.K.; Bierma-Zeinstra, S.M.A.; Krestin, G.P.; Moelker, A.; Oei, E.H.G. Association between Baseline Osteoarthritic Features on MR Imaging and Clinical Outcome after Genicular Artery Embolization for Knee Osteoarthritis. J. Vasc. Interv. Radiol. 2021, 32, 497–503. [Google Scholar] [CrossRef]
- Little, M.W.; O’Grady, A.; Briggs, J.; Gibson, M.; Speirs, A.; Al-Rekabi, A.; Yoong, P.; Ariyanayagam, T.; Davies, N.; Tayton, E.; et al. Genicular Artery embolisation in Patients with Osteoarthritis of the Knee (GENESIS) Using Permanent Microspheres: Long-Term Results. Cardiovasc. Intervent. Radiol. 2024, 47, 1750–1762. [Google Scholar] [CrossRef]
- Bagla, S.; Piechowiak, R.; Hartman, T.; Orlando, J.; Del Gaizo, D.; Isaacson, A. Genicular Artery Embolization for the Treatment of Knee Pain Secondary to Osteoarthritis. J. Vasc. Interv. Radiol. 2020, 31, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Hindsø, L.; Hölmich, P.; Petersen, M.M.; Nielsen, M.B.; Heerwagen, S.; Taudorf, M.; Lönn, L. Transarterial Embolization of Geniculate Arteries Reduces Pain and Improves Physical Function in Knee Osteoarthritis—A Prospective Cohort Study. Diagnostics 2024, 14, 1627. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Awasthi, R.; Agarwal, V.; Agrawal, V.; Rathore, R.K.S.; Sharma, K.; Pandey, C.M.; Gupta, R.K. Diffusion Tensor and Dynamic Contrast-Enhanced Magnetic Resonance Imaging Correlate with Molecular Markers of Inflammation in the Synovium. Diagnostics 2022, 12, 3041. [Google Scholar] [CrossRef]
- Gait, A.D.; Hodgson, R.; Parkes, M.J.; Hutchinson, C.E.; O’Neill, T.W.; Maricar, N.; EMarjanovic, J.; Cootes, T.F.; Felson, D.T. Synovial volume vs synovial measurements from dynamic contrast enhanced MRI as measures of response in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1392–1398. [Google Scholar] [CrossRef]
- Riis, R.G.C.; Gudbergsen, H.; Henriksen, M.; Ballegaard, C.; Bandak, E.; Röttger, D.; Bliddal, H.; Hansen, B.B.; Hangaard, S.; Boesen, M. Synovitis assessed on static and dynamic contrast-enhanced magnetic resonance imaging and its association with pain in knee osteoarthritis: A cross-sectional study. Eur. J. Radiol. 2016, 85, 1099–1108. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domarkienė, A.; Kalytis, L.; Kanapienis, G.; Kurminas, M.; Tamošiūnas, A.E. Genicular Arteries Embolization for Patients with Osteoarthritis, Their Selection, and Follow-Up Based on MRI Findings. Medicina 2025, 61, 941. https://doi.org/10.3390/medicina61050941
Domarkienė A, Kalytis L, Kanapienis G, Kurminas M, Tamošiūnas AE. Genicular Arteries Embolization for Patients with Osteoarthritis, Their Selection, and Follow-Up Based on MRI Findings. Medicina. 2025; 61(5):941. https://doi.org/10.3390/medicina61050941
Chicago/Turabian StyleDomarkienė, Aurelija, Lukas Kalytis, Gytis Kanapienis, Marius Kurminas, and Algirdas Edvardas Tamošiūnas. 2025. "Genicular Arteries Embolization for Patients with Osteoarthritis, Their Selection, and Follow-Up Based on MRI Findings" Medicina 61, no. 5: 941. https://doi.org/10.3390/medicina61050941
APA StyleDomarkienė, A., Kalytis, L., Kanapienis, G., Kurminas, M., & Tamošiūnas, A. E. (2025). Genicular Arteries Embolization for Patients with Osteoarthritis, Their Selection, and Follow-Up Based on MRI Findings. Medicina, 61(5), 941. https://doi.org/10.3390/medicina61050941




