Osteogenic Potential and Bone Matrix Maturity: Comparison of Demineralized Bone Matrix and P15 Polypeptide iFactor® in an In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
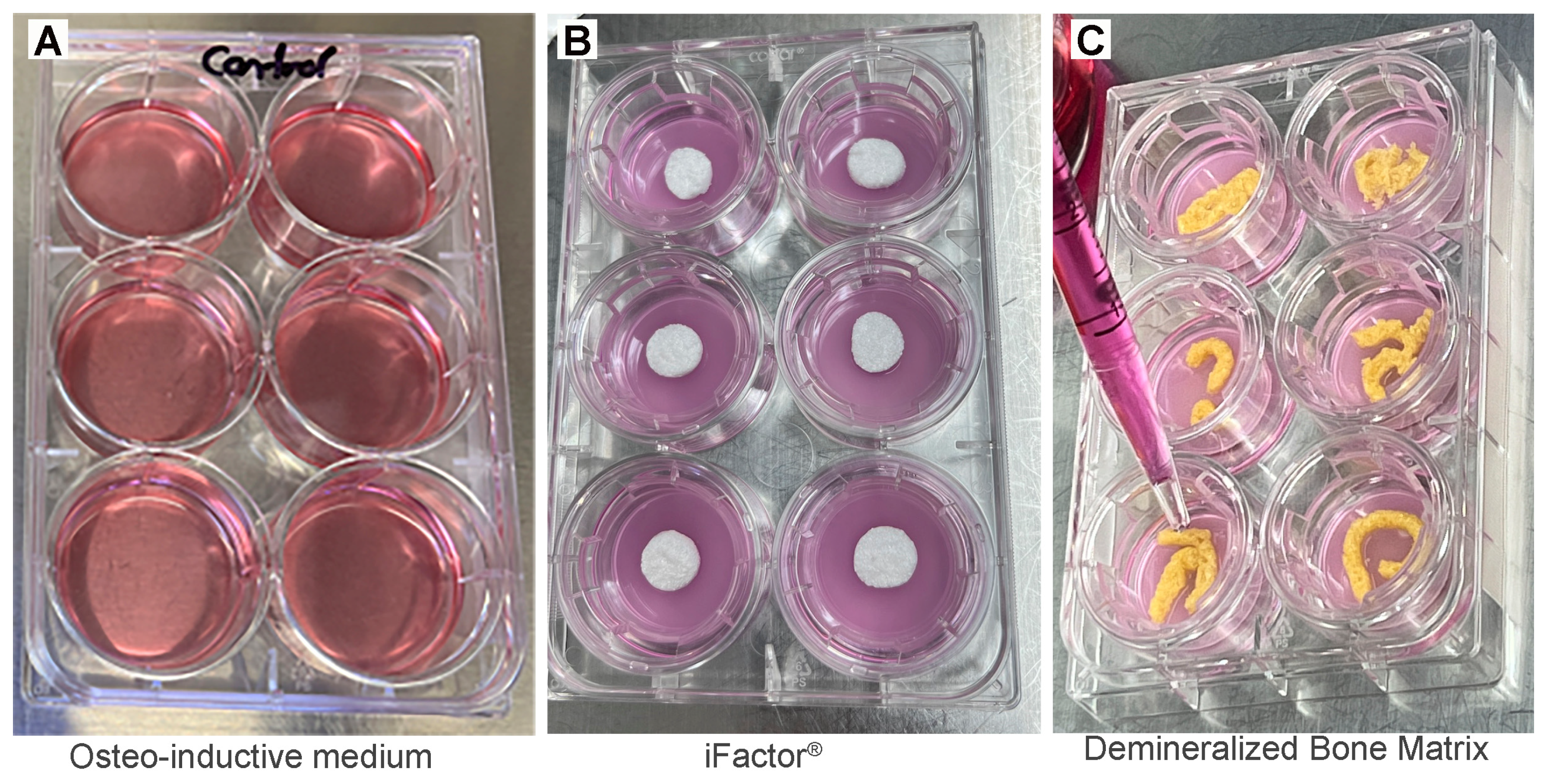
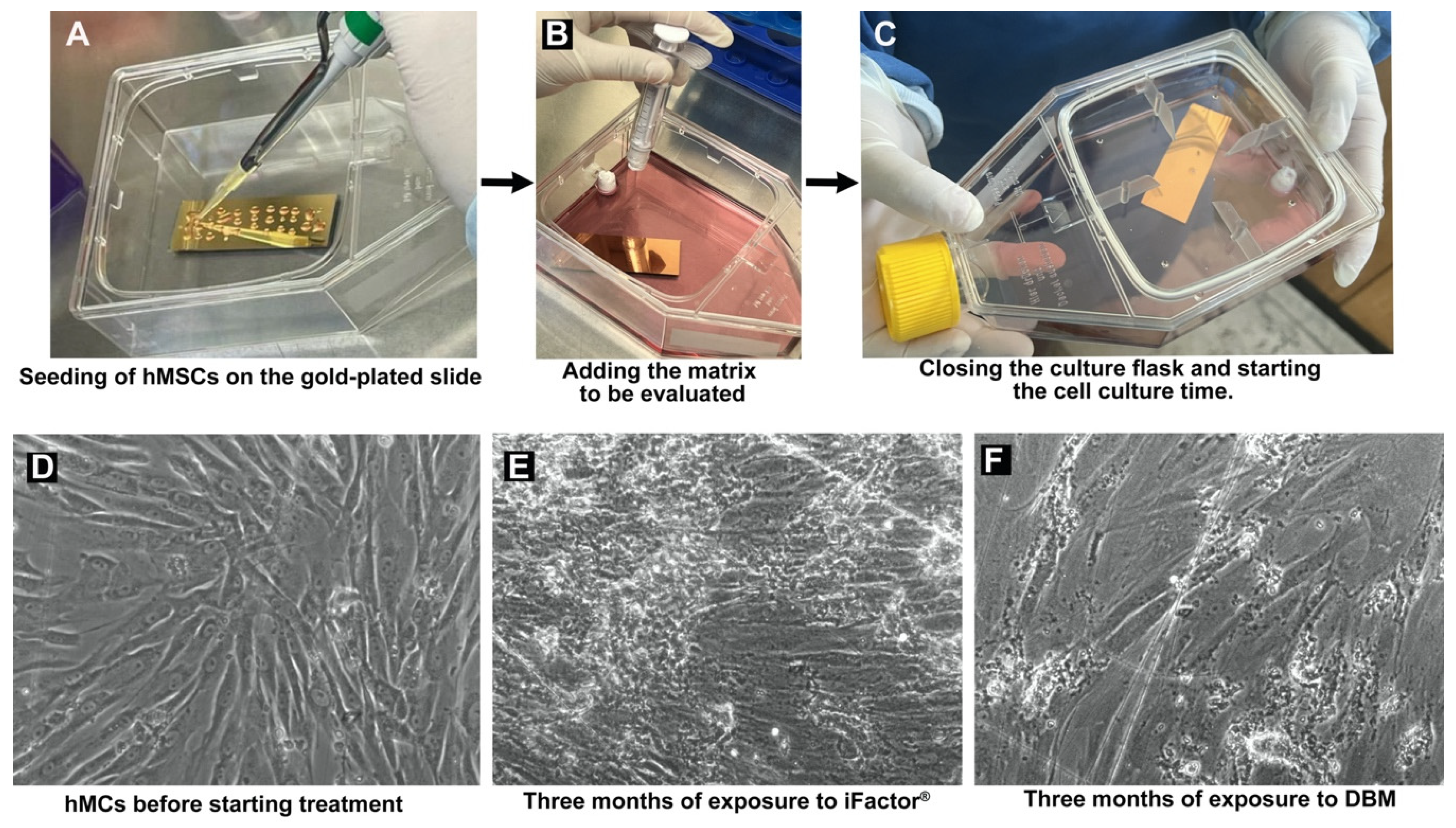
2.1. Handling of Human Mesenchymal Stem Cells
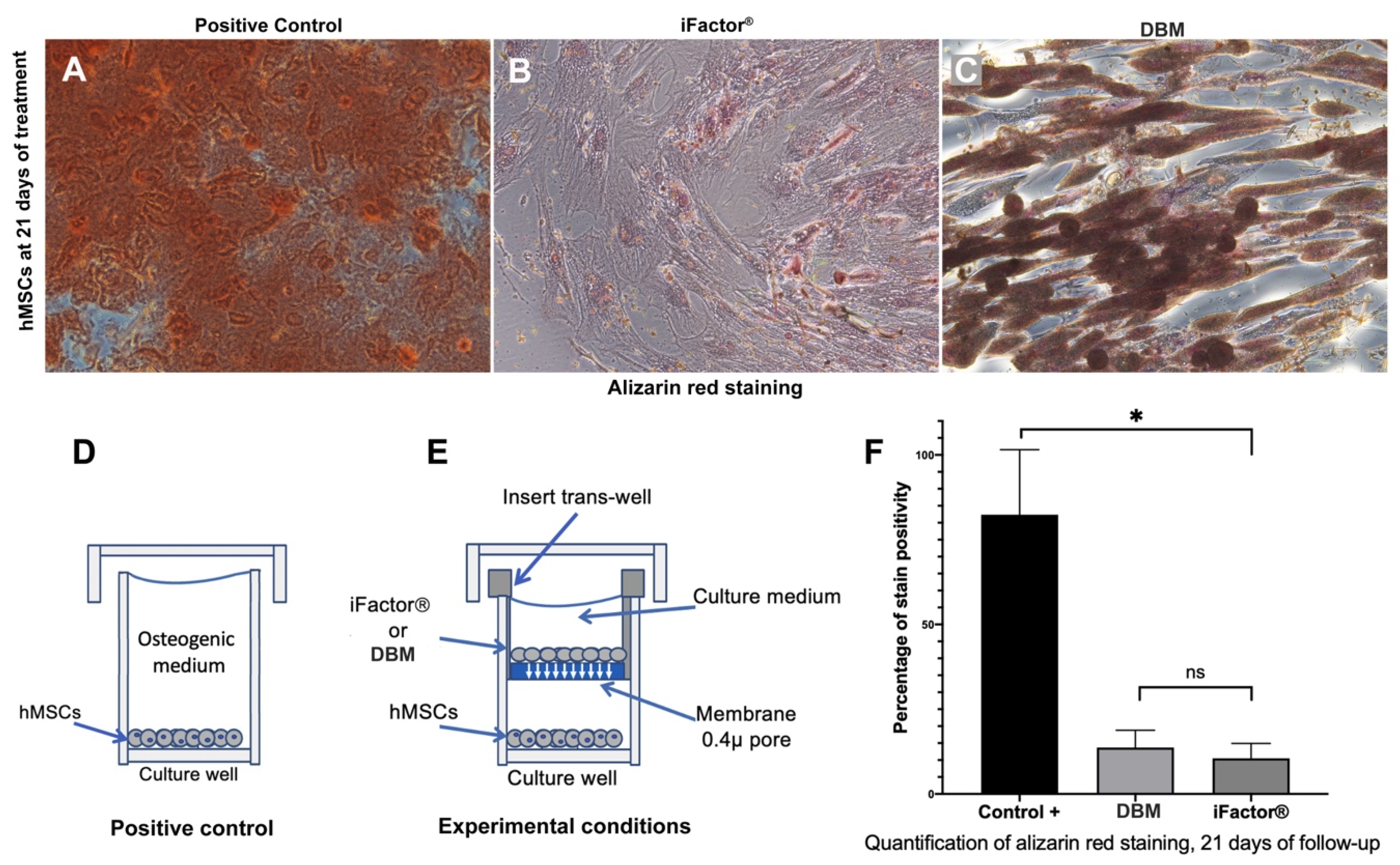
2.2. Evaluation of Calcium Nodules (Ca2+) Formation Capacity
2.3. Fourier Transform Infrared Micro-Spectroscopy Analysis (FTIR): 3-Month Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Calcium Nodules (Ca2+) Formation Capacity
3.2. Fourier Transform Infrared Spectroscopy Analysis
3.3. Comparative Analysis of FTIR Results
3.4. Main Trends Established Through FTIR Spectra Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DBM | demineralized bone matrix |
| FTIR | Fourier transform infrared spectroscopy |
| hMSCs | human mesenchymal stem cells |
| AATB | American Association of Tissue Banks |
| ABM | anorganic bone mineral |
| APS | acid phosphate replacement |
| CI | crystallinity index |
| HU | Hounsfield unit |
References
- Ren, J.; Li, Z.; Liu, W.; Fan, Y.; Qi, L.; Li, S.; Kong, C.; Zou, H.; Liu, Z. Demineralized bone matrix for repair and regeneration of maxillofacial defects: A narrative review. J. Dent. 2024, 143, 104899. [Google Scholar] [CrossRef]
- Schwartz, Z.; Hyzy, S.L.; Moore, M.A.; Hunter, S.A.; Ronholdt, C.J.; Sunwoo, M.; Boyan, B.D. Osteoinductivity of demineralized bone matrix is independent of donor bisphosphonate use. J. Bone Jt. Surg. Am. 2011, 93, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Ziran, B.; Cheung, S.; Smith, W.; Westerheide, K. Comparative efficacy of 2 different demineralized bone matrix allografts in treating long-bone nonunions in heavy tobacco smokers. Am. J. Orthop. 2005, 34, 329–332. [Google Scholar] [PubMed]
- Zhang, H.; Yang, L.; Yang, X.G.; Wang, F.; Feng, J.T.; Hua, K.C.; Li, Q.; Hu, Y.C. Demineralized Bone Matrix Carriers and their Clinical Applications: An Overview. Orthop. Surg. 2019, 11, 725–737. [Google Scholar] [CrossRef]
- Groessner-Schreiber, B.; Krukowski, M.; Lyons, C.; Osdoby, P. Osteoclast recruitment in response to human bone matrix is age related. Mech. Ageing Dev. 1992, 62, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42 (Suppl. S2), S16–S21. [Google Scholar] [CrossRef]
- Tatum, H., Jr. Maxillary and sinus implant reconstructions. Dent. Clin. North Am. 1986, 30, 207–229. [Google Scholar] [CrossRef]
- Zhang, M.; Powers, R.M., Jr.; Wolfinbarger, L., Jr. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J. Periodontol. 1997, 68, 1085–1092. [Google Scholar] [CrossRef]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M.W. Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J. Craniofac. Surg. 2005, 16, 981–989. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Manawar, S.; Myrick, E.; Awad, P.; Hung, V.; Hinton, C.; Kenter, K.; Bovid, K.; Li, Y. Use of allograft bone matrix in clinical orthopedics. Regen. Med. 2024, 19, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Su, C.Y.; Lai, C.C.; Tsou, Y.S.; Zheng, Y.; Fang, H.W. Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials. J. Funct. Biomater. 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Shepard, N.A.; Rush, A.J., 3rd; Scarborough, N.L.; Carter, A.J.; Phillips, F.M. Demineralized Bone Matrix in Spine Surgery: A Review of Current Applications and Future Trends. Int. J. Spine Surg. 2021, 15, 113–119. [Google Scholar] [CrossRef]
- Driscoll, J.A.; Lubbe, R.; Jakus, A.E.; Chang, K.; Haleem, M.; Yun, C.; Singh, G.; Schneider, A.D.; Katchko, K.M.; Soriano, C.; et al. 3D-Printed Ceramic-Demineralized Bone Matrix Hyperelastic Bone Composite Scaffolds for Spinal Fusion. Tissue Eng. Part A 2020, 26, 157–166. [Google Scholar] [CrossRef]
- Xi, Z.; Chou, D.; Mummaneni, P.V.; Burch, S. The Navigated Oblique Lumbar Interbody Fusion: Accuracy Rate, Effect on Surgical Time, and Complications. Neurospine 2020, 17, 260–267. [Google Scholar] [CrossRef]
- Zuo, J.; Wu, Z.; Li, Y.; Shen, Z.; Feng, X.; Zhang, M.; Ye, H. Mitochondrial ABC Transporter ATM3 Is Essential for Cytosolic Iron-Sulfur Cluster Assembly. Plant Physiol. 2017, 173, 2096–2109. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999, 5, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Sathe, A.; Lee, S.H.; Kim, S.J.; Eun, S.S.; Choi, Y.S.; Lee, S.M.; Seuk, J.W.; Lee, Y.S.; Shin, S.H.; Bae, J. Comparative Analysis of ABM/P-15, Bone Morphogenic Protein and Demineralized Bone Matrix after Instrumented Lumbar Interbody Fusion. J. Korean Neurosurg. Soc. 2022, 65, 825–833. [Google Scholar] [CrossRef]
- Chá, D.; Cantú, E.; Sofí, A. Implementing P-15/ABM Bone Graft as a Standardized Technique for Lumbar Fusion Approaches. Open J. Mod. Neurosurg. 2024, 14, 256–266. [Google Scholar]
- Bae, H.W.; Zhao, L.; Kanim, L.E.; Wong, P.; Delamarter, R.B.; Dawson, E.G. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine 2006, 31, 1299–1306; discussion 307-8. [Google Scholar] [CrossRef]
- Drosos, G.I.; Kazakos, K.I.; Kouzoumpasis, P.; Verettas, D.A. Safety and efficacy of commercially available demineralised bone matrix preparations: A critical review of clinical studies. Injury 2007, 38 (Suppl. S4), S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pujic, Z.; Xiao, Y.; Bartold, P.M. Identification of bone morphogenetic proteins 2 and 4 in commercial demineralized freeze-dried bone allograft preparations: Pilot study. Clin. Implant. Dent. Relat. Res. 2000, 2, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lan, L.; Miron, R.J.; Wei, L.; Zhang, M.; Zhang, Y. Variability in Particle Degradation of Four Commonly Employed Dental Bone Grafts. Clin. Implant. Dent. Relat. Res. 2015, 17, 996–1003. [Google Scholar] [CrossRef]
- Martínez-Flores, K.; Plata-Rodríguez, R.; Olivos-Meza, A.; López-Macay, A.; Fernández-Torres, J.; Landa-Solís, C.; Zamudio-Cuevas, Y. Osteogenic Potential of Monosodium Urate Crystals in Synovial Mesenchymal Stem Cells. Medicina 2022, 58, 1724. [Google Scholar] [CrossRef]
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif. Tissue Int. 2002, 71, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M. Three-dimensional macroporous calcium phosphate bioceramics with nested chitosan sponges for load-bearing bone implants. J. Biomed. Mater. Res. 2002, 61, 1–8. [Google Scholar] [CrossRef]
- Lewis, C.S.; Supronowicz, P.R.; Zhukauskas, R.M.; Gill, E.; Cobb, R.R. Local antibiotic delivery with demineralized bone matrix. Cell Tissue Bank. 2012, 13, 119–127. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivos-Meza, A.; Mata-Miranda, M.M.; Robles-Rodríguez, M.; Vázquez-Zapién, G.J.; Guerrero-Ruiz, M.; Landa-Solís, C. Osteogenic Potential and Bone Matrix Maturity: Comparison of Demineralized Bone Matrix and P15 Polypeptide iFactor® in an In Vitro Study. Medicina 2025, 61, 914. https://doi.org/10.3390/medicina61050914
Olivos-Meza A, Mata-Miranda MM, Robles-Rodríguez M, Vázquez-Zapién GJ, Guerrero-Ruiz M, Landa-Solís C. Osteogenic Potential and Bone Matrix Maturity: Comparison of Demineralized Bone Matrix and P15 Polypeptide iFactor® in an In Vitro Study. Medicina. 2025; 61(5):914. https://doi.org/10.3390/medicina61050914
Chicago/Turabian StyleOlivos-Meza, Anell, Monica Maribel Mata-Miranda, Marcelo Robles-Rodríguez, Gustavo Jesús Vázquez-Zapién, Melissa Guerrero-Ruiz, and Carlos Landa-Solís. 2025. "Osteogenic Potential and Bone Matrix Maturity: Comparison of Demineralized Bone Matrix and P15 Polypeptide iFactor® in an In Vitro Study" Medicina 61, no. 5: 914. https://doi.org/10.3390/medicina61050914
APA StyleOlivos-Meza, A., Mata-Miranda, M. M., Robles-Rodríguez, M., Vázquez-Zapién, G. J., Guerrero-Ruiz, M., & Landa-Solís, C. (2025). Osteogenic Potential and Bone Matrix Maturity: Comparison of Demineralized Bone Matrix and P15 Polypeptide iFactor® in an In Vitro Study. Medicina, 61(5), 914. https://doi.org/10.3390/medicina61050914







