Multimodal Prehabilitation in Major Abdominal Surgery—Rationale, Modalities, Results and Limitations
Abstract
1. Introduction
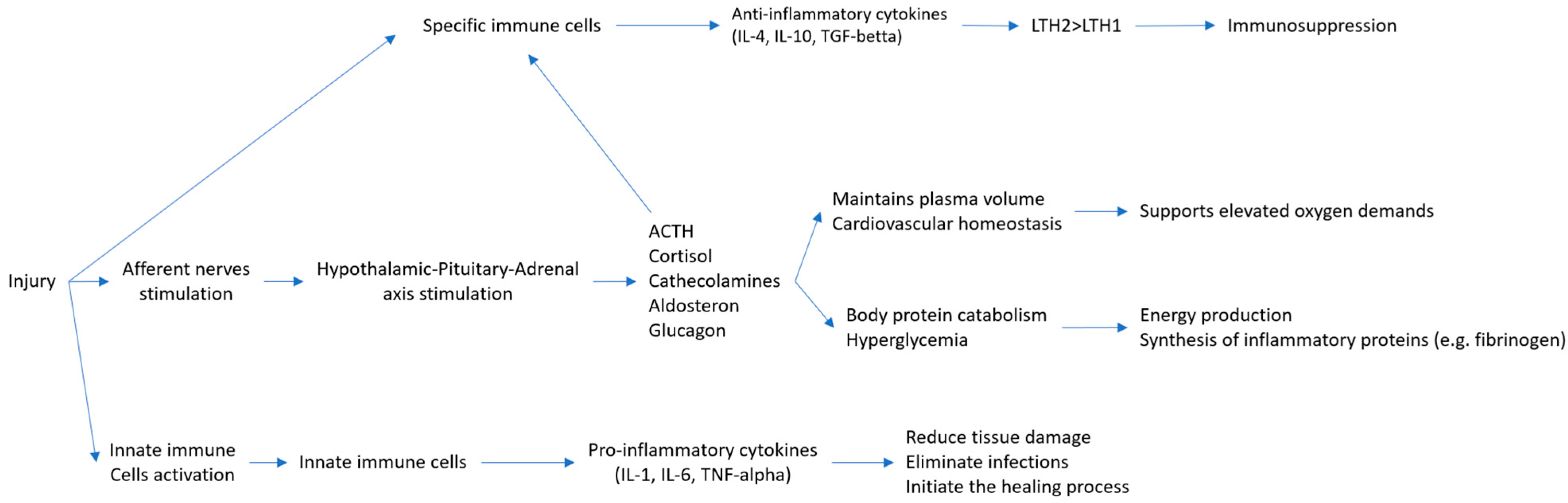
2. Mechanism of Surgical Stress
3. Functional Capacity and Physiological Reserve
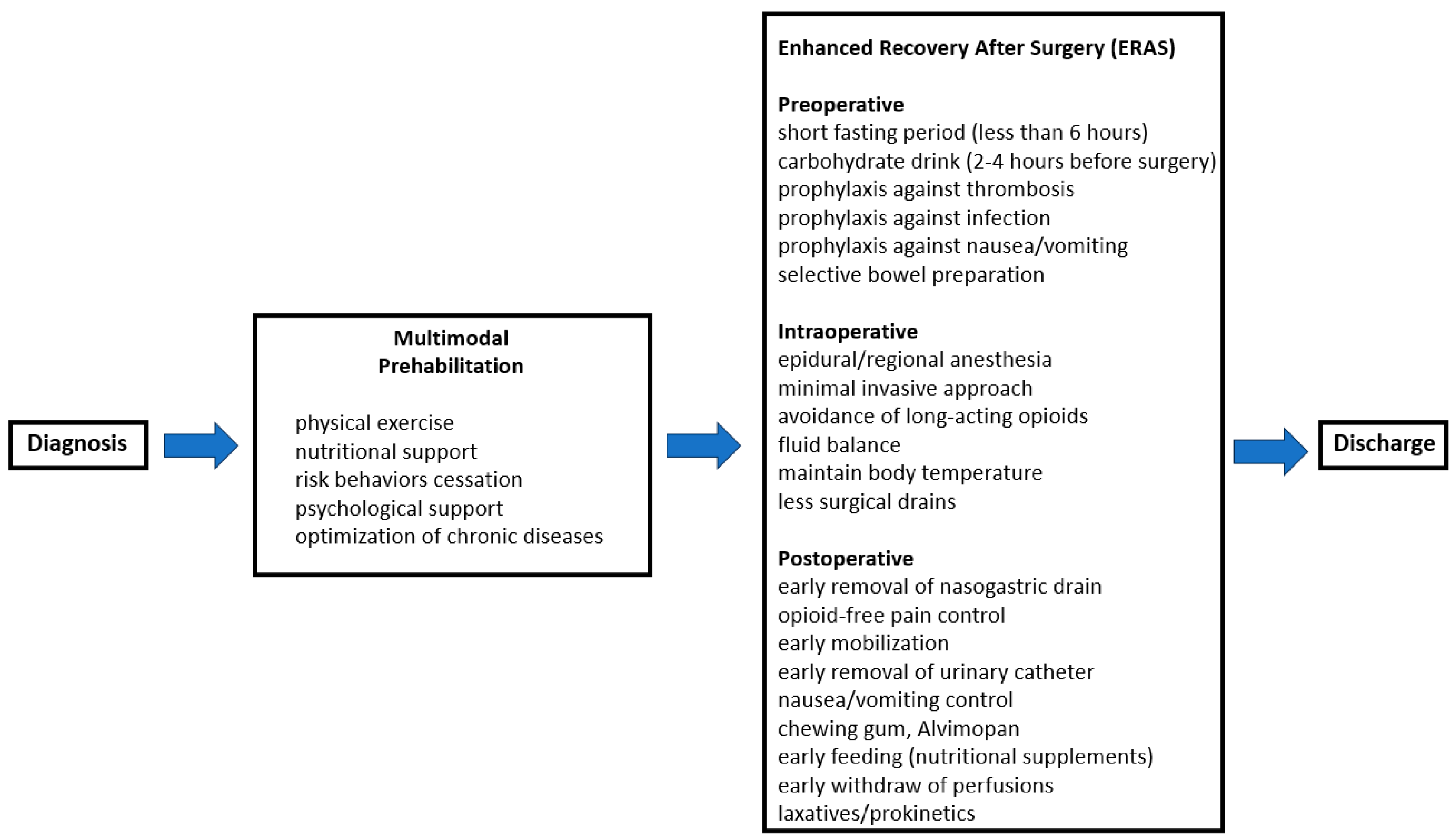
4. Enhanced Recovery After Surgery
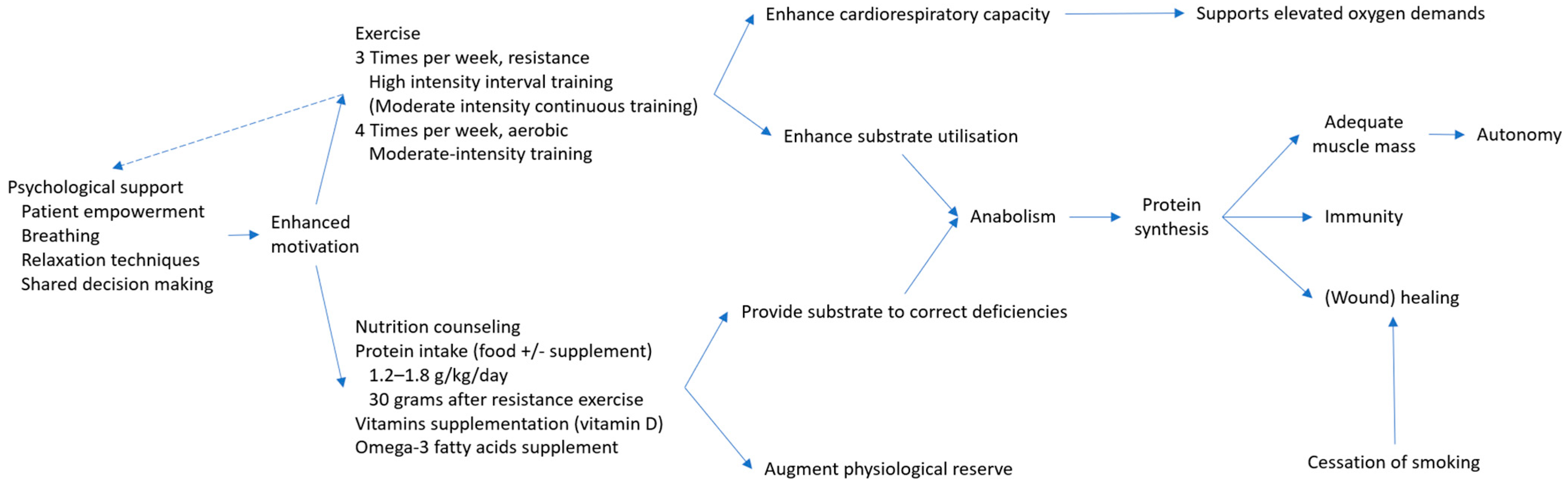
5. Principles of Prehabilitation
5.1. The Concept of Prehabilitation
5.2. Physical Activity
5.3. Nutritional Support and Correction of Comorbid Conditions
5.4. Harmful Behaviors Management
5.5. Psychological Support
6. Efficacy of Prehabilitation Interventions
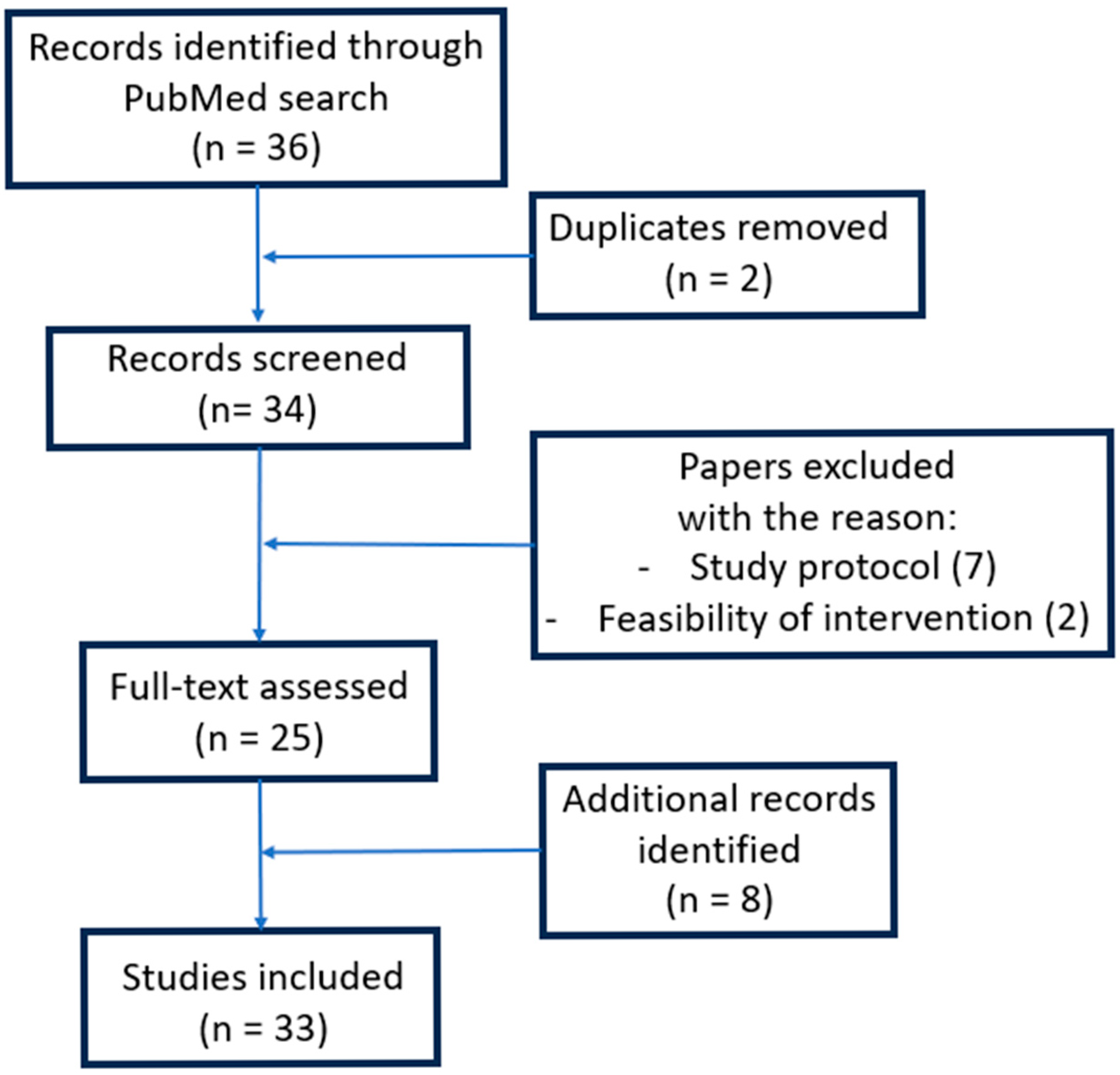
6.1. Methodology
6.2. Results
6.2.1. Functional Capacity
6.2.2. Length of Hospitalization
| Study | Pathology of Interest/Main Inclusion Criteria | Multimodal Prehabilitation | Number of Enrolled Patients | Length of Stay (Days) | ICU Days (Patients Admited in ICU) | Surgical Reintervention | Hospital Readmission | Mortality Rate | Rate of Postoperative Complications | Medical Complications | Surgical Complications | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A. Barberan-Garcia et al., Ann. Surg. 2018, 2019 [115] | Elective major abdominal surgery for cancer | Yes | 144 | 73 | 8 (8) | p = 0.078 | 3 (2) | p = 0.046 | 2 (3%) | p = 0.273 | 2 (3%) | p = 0.009 | 1 (2%) | p = 1 | 19 (31%) | p = 0.001 | 0.2 (0.6) * | p < 0.001 | 0.3 (0.7) * | p = 0.119 |
| No | 71 | 13 (20) | 12 (20) | 6 (10%) | 11 (18%) | 1 (2%) | 39 (62%) | 0.9 (1.2) * | 0.5 (0.6) * | ||||||||||||
| 2 | A. E. M. Berkel et al., Ann. Surg. 2022 [122] | Elective colorectal resection for cancer | Yes | 74 | 39 | 8.4 (7.4) | p = 0.14 | 2 (7%) | p > 0.99 | 4 (14%) | p > 0.99 | 1 (4%) | p = NS | 12 (43%) | p = 0.024 | 8 (29%) | p = 0.45 | 10 (36%) | p = 0.14 | ||
| No | 35 | 9.1 (7.0) | 2 (7%) | 5 (17%) | 0 | 21 (72%) | 11 (38%) | 16 (55%) | |||||||||||||
| 3 | C. J. L. Molenaar et al., JAMA Surg. 2023 [123] | Elective colorectal resection for cancer | Yes | 269 | 136 | 3 (3–5) | p = 0.20 | 4 (3.3%) | p = 0.26 | 39 (28%) | p = 0.039 | 19 (15.4%) | p = 0.02 | 26 (21.1%) | p = 0.25 | ||||||
| No | 133 | 3 (3–4) | 8 (6.3%) | 54 (40%) | 35 (27.3%) | 35 (27.3%) | |||||||||||||||
| 4 | A. Fulop et al., Anaesthesia 2021 [111] | Elective colorectal resection for cancer | Yes | 149 | 77 | 8 (7–10) | p = 0.712 | 1 (1.3%) | p = 0.483 | 17 (22%) | p = 0.569 | ||||||||||
| No | 72 | 8 (7–9) | 2 (2.7%) | 16 (22%) | |||||||||||||||||
| 5 | F. Carli et al., JAMA Surgery 2020 [124] | Elective colorectal resection for cancer | Yes | 110 | 55 | 4 (3–8) | p = 0.32 | 2 (3.6) | p = 0.18 | 25 (45%) | p = 0.9 | 0.56 * | p = NS | 14 (25%) | p = NS | ||||||
| No | 55 | 5 (3–9) | 5 (9.1) | 25 (45%) | 0.58 * | 14 (25%) | |||||||||||||||
| 6 | C. Gillis et al., Anesthesiology 2014 [1] | Elective colorectal resection for cancer | Yes | 77 | 38 | 4 (3–5) | p = 0.812 | 6 (15%) | p = 0.780 | 12 (32%) | p = 0.277 | 3 (9%) | p = 0.291 | 9 (24%) | p = 0.104 | ||||||
| No | 39 | 4 (3–7) | 5 (13%) | 17 (44%) | 1 (3%) | 16 (42%) | |||||||||||||||
| 7 | S. Gloor et al., Langenbecks Arch. Surg. 2022 [125] | Elective colorectal resection for cancer | Yes | 107 | 54 | 7 (3–18) | p = 0.874 | 2 (1–3) | p = 1 | 2 (4%) | p = 0.495 | 0 | 52 (97%) | p = 0.169 | |||||||
| No | 53 | 6 (2–20) | 2 (1–2) | 0 | 0 | 45 (85%) | |||||||||||||||
| 8 | A. Bausys et al. Br. J. Surg. 2023 [120] | Elective gastric resection for cancer | Yes | 122 | 61 | 11 (7) | p = 0.083 | 2 (3.4%) # | p = 0.163 | 1 (1.7%) # | p = NS | 14 (23%) # | p = 0.001 | ||||||||
| No | 61 | 13 (9) | 7 (11.9%) # | 3 (5.1%) # | 36 (59%) # | ||||||||||||||||
| 9 | G. Bousquet-Dion et al. Acta Oncologica 2018 [80] | Elective colorectal resection for cancer | Yes | 72 | 41 | 3 [3–7] | p = 0.057 | 5 (12.2%) | p = 0.415 | 0 | 14 (38%) | p = 0.562 | |||||||||
| No | 31 | 3 [2–4] | 2 (6.5%) | 0 | 8 (31%) | ||||||||||||||||
| 10 | F. Ausania et al. Rev Esp Enferm 2019 [126] | Pancreaticoduodenectomy | Yes | 40 | 18 | 11.4 (7–46) | p = 0.449 | 1 (5.6%) | p = 0.673 | 0 | 6 (33.3%) | p = 0.18 | |||||||||
| No | 22 | 13.2 (7–60) | 2 (9.6%) | 0 | 12 (54.5%) | ||||||||||||||||
| 11 | Triguero-Canovas et al. Supportive Care in Cancer 2023 [112] | Elective colorectal resection for cancer | Yes | 44 | 23 | 5.74 (3.54) | p = 0.30 | 4 (17.4%) | p = 0.22 | ||||||||||||
| No | 21 | 6.67 (3.49) | 7 (33.3%) | ||||||||||||||||||
| 12 | A. Onerup et al. Ann Surg 2022 [127] | Elective colorectal resection for cancer | Yes | 668 | 317 | 7 (1–91) | p = 0.13 | 58 (18%) | p = 0.04 | 73 (23%) | p = 0.67 | 3 (1%) | p = NS | 237 (75%) | p = 0.153 | ||||||
| No | 351 | 6 (1–78) | 44 (13%) | 76 (22%) | 1 (0.3%) | 245 (70%) | |||||||||||||||
| 13 | A. Pesce et al. Surg Endosc 2024 [128] | Elective colorectal resection for cancer | Yes | 71 | 35 | 5.45 (4.61) | p = 0.426 | 0.4 (2.04) | p = 0.246 | 1 (2.8%) | p = 0.572 | 0 | p = 0.321 | 0 | p = 0.321 | 7 (20%) | p = 0.565 | ||||
| No | 36 | 4.8 (1.53) | 0.02 (0.16) | 2 (5.5%) | 1 (2.8%) | 1 (2.8%) | 8 (22.2%) | ||||||||||||||
| 14 | A. Hamad BMC Sports Science, Medicine and Rehabilitation 2025 [78] | Gastro-intestinal surgery | Yes | 51 | 17 | 7.0 (4–7) | p = 0.446 | 2 (12%) | p = 0.703 | 14 (82%) | p > 0.999 | ||||||||||
| No | 34 | 5.5 (4–8) | 6 (18%) | 28 (82%) | |||||||||||||||||
| 15 | S. Liang Surgery 2025 [129] | Liver cancers | Yes | 205 | 104 | 8.51 (3.06) | p = 0.765 | 14 (13.9%) | p = 908 | 94 (91.4%) | p = 0.493 | ||||||||||
| No | 101 | 8.37 (3.75) | 15 (14.4%) | 89 (88.1%) | |||||||||||||||||
| 16 | J. Chen BMC Gastroenterology 2024 [130] | Elective gastric resection for cancer | Yes | 115 | 57 | 8 (6–10) | p =0.493 | 2 (1–3) | p = 0.224 | 3 (5.3%) | p = 0.983 | 1 (1.8%) | p = 0.311 | 6 (10.5%) ‡ | p = 0.033 | 7 (12.3%) | p = 0.025 | 8 (14%) | p = 0.636 | ||
| No | 58 | 8 (7–11) | 2 (1–4) | 3 (5.2%) | 0 | 15 (25.9%) ‡ | 17 (29.3) | 10 (17.2) | |||||||||||||
| 17 | J. Woodfield Scand J Med Sci Sports 2022 [72] | Major abdominal surgery | Yes | 63 | 28 | 4 (2.8) | p = 0.313 | 0.64 (±0.95) * | p = 0.072 | ||||||||||||
| No | 35 | 5 (6) | 1.16 (±1.11) * | ||||||||||||||||||
| 18 | C. Griffiths J Surg Oncol. 2024 [95] | Major abdominal cancer surgery | Yes | 63 | 33 | 8 (24.2%) ¶ | p = 1 | ||||||||||||||
| No | 30 | 7 (23.3%) ¶ | |||||||||||||||||||
| 19 | López-Rodríguez-Arias Supportive Care in Cancer 2021 [119] | Elective colorectal resection for cancer | Yes | 20 | 10 | 4.8 (1) | p = 0.052 | 2 (20%) | p = 0.16 | ||||||||||||
| No | 10 | 7.2 (3.2) | 5 (50%) | ||||||||||||||||||
6.2.3. Postoperative Complications
Colorectal Cancer
Gastric/Esophago-Gastric Cancer
Hepato-Pancreato-Biliary Surgery
Major Abdominal Surgery
Elderly/Frail Patients
6.2.4. Cost-Effectiveness
6.3. Discussion
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPET | Cardio-pulmonary exercise test |
| RCT | Randomized clinical trial |
| 6MWT | 6 min walking test |
| ERAS | Enhanced recovery after surgery |
References
- Gillis, C.; Carli, F. Promoting Perioperative Metabolic and Nutritional Care. Anesthesiology 2015, 123, 1455–1472. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Ljungqvist, O.; Carli, F. Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br. J. Anaesth. 2022, 128, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Grimble, R.F. Dangers, and benefits of the cytokine mediated response to injury and infection. Clin. Nutr. 2009, 28, 583–596. [Google Scholar] [CrossRef] [PubMed]
- English, K.L.; Paddon-Jones, D. Protecting muscle mass and function in older adults during bed rest. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 34–39. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- de Araújo Martins-Romeo, D.; Domínguez, A.R. Complications after abdominal surgery. Radiologia 2023, 65 (Suppl. S1), S99–S108. [Google Scholar] [CrossRef]
- Kehlet, H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef]
- Weissman, C. The metabolic response to stress: An overview and update. Anesthesiology 1990, 73, 308–327. [Google Scholar] [CrossRef]
- Dobson, G.P. Addressing the Global Burden of Trauma in Major Surgery. Front. Surg. 2015, 2, 43. [Google Scholar] [CrossRef]
- Cusack, B.; Buggy, D.J. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321–328. [Google Scholar] [CrossRef]
- Bye, A.; Sjøblom, B.; Wentzel-Larsen, T.; Grønberg, B.H.; Baracos, V.E.; Hjermstad, M.J.; Aass, N.; Bremnes, R.M.; Fløtten, Ø.; Jordhøy, M. Muscle mass and association to quality of life in non-small cell lung cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. Edinb. Scotl. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.A.; Hill, G.L. Weight loss with physiologic impairment. A basic indicator of surgical risk. Ann. Surg. 1988, 207, 290–296. [Google Scholar] [CrossRef]
- Baba, H.; Tokai, R.; Hirano, K.; Watanabe, T.; Shibuya, K.; Hashimoto, I.; Hojo, S.; Yoshioka, I.; Okumura, T.; Nagata, T.; et al. Risk factors for postoperative pneumonia after general and digestive surgery: A retrospective single-center study. Surg. Today 2020, 50, 460–468. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Schricker, T.; Meterissian, S.; Lattermann, R.; Adegoke, O.A.J.; Marliss, E.B.; Mazza, L.; Eberhart, L.; Carli, F.; Nitschman, E.; Wykes, L. Anticatabolic effects of avoiding preoperative fasting by intravenous hypocaloric nutrition: A randomized clinical trial. Ann. Surg. 2008, 248, 1051–1059. [Google Scholar] [CrossRef]
- Smith, I.; Kranke, P.; Murat, I.; Smith, A.; O’Sullivan, G.; Søreide, E.; Spies, C.; in’t Veld, B.; European Society of Anaesthesiology. Perioperative fasting in adults and children: Guidelines from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 2011, 28, 556–569. [Google Scholar] [CrossRef]
- Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology 2017, 126, 376–393. [CrossRef]
- Abola, R.E.; Gan, T.J. Preoperative Fasting Guidelines: Why Are We Not Following Them?: The Time to Act Is NOW. Anesth. Analg. 2017, 124, 1041–1043. [Google Scholar] [CrossRef]
- Whitson, H.E.; Cohen, H.J.; Schmader, K.E.; Morey, M.C.; Kuchel, G.; Colon-Emeric, C.S. Physical Resilience: Not Simply the Opposite of Frailty. J. Am. Geriatr. Soc. 2018, 66, 1459–1461. [Google Scholar] [CrossRef]
- Arnett, S.W.; Laity, J.H.; Agrawal, S.K.; Cress, M.E. Aerobic reserve and physical functional performance in older adults. Age Ageing 2008, 37, 384–389. [Google Scholar] [CrossRef]
- Schwartz, R.S. Sarcopenia and physical performance in old age: Introduction. Muscle Nerve. 1997, 5, S10–S12. [Google Scholar] [CrossRef]
- Ince, C. Personalized physiological medicine. Crit. Care Lond. Engl. 2017, 21 (Suppl. S3), 308. [Google Scholar] [CrossRef]
- Older, P.; Smith, R.; Courtney, P.; Hone, R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest 1993, 104, 701–704. [Google Scholar] [CrossRef]
- Wijeysundera, D.N.; Pearse, R.M.; Shulman, M.A.; Abbott, T.E.F.; Torres, E.; Ambosta, A.; Croal, B.L.; Granton, J.T.; Thorpe, K.E.; Grocott, M.P.W.; et al. Assessment of functional capacity before major non-cardiac surgery: An international, prospective cohort study. Lancet Lond. Engl. 2018, 391, 2631–2640. [Google Scholar] [CrossRef]
- Gillis, C.; Davies, S.J.; Carli, F.; Wischmeyer, P.E.; Wootton, S.A.; Jackson, A.A.; Riedel, B.; Marino, L.V.; Levett, D.Z.H.; West, M.A. Current Landscape of Nutrition Within Prehabilitation Oncology Research: A Scoping Review. Front. Nutr. 2021, 8, 644723. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Williams, M.A.; Gulati, M.; Kligfield, P.; Balady, G.J.; Collins, E.; Fletcher, G.; American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: A scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007, 116, 329–343. [Google Scholar] [CrossRef]
- Oliveira, M.R.M.; Fogaça, K.C.P.; Leandro-Merhi, V.A. Nutritional status and functional capacity of hospitalized elderly. Nutr. J. 2009, 8, 54. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.-J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Atamna, H.; Tenore, A.; Lui, F.; Dhahbi, J.M. Organ reserve, excess metabolic capacity, and aging. Biogerontology 2018, 19, 171–184. [Google Scholar] [CrossRef]
- Coen, P.M.; Musci, R.V.; Hinkley, J.M.; Miller, B.F. Mitochondria as a Target for Mitigating Sarcopenia. Front. Physiol. 2018, 9, 1883. [Google Scholar] [CrossRef] [PubMed]
- Consitt, L.A.; Dudley, C.; Saxena, G. Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults. Nutrients 2019, 11, 2636. [Google Scholar] [CrossRef]
- Tasch, M.D. The autonomic nervous system and geriatric anesthesia. Int. Anesthesiol. Clin. 1988, 26, 143–151. [Google Scholar] [CrossRef]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.H.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Lawrence, V.A.; Hazuda, H.P.; Cornell, J.E.; Pederson, T.; Bradshaw, P.T.; Mulrow, C.D.; Page, C.P. Functional independence after major abdominal surgery in the elderly. J. Am. Coll. Surg. 2004, 199, 762–772. [Google Scholar] [CrossRef]
- Sigalet, D.L.; Mackenzie, S.L.; Hameed, S.M. Enteral nutrition and mucosal immunity: Implications for feeding strategies in surgery and trauma. Can. J. Surg. J. Can. Chir. 2004, 47, 109–116. [Google Scholar]
- Jeejeebhoy, K.N.; Keller, H.; Gramlich, L.; Allard, J.P.; Laporte, M.; Duerksen, D.R.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B.; et al. Nutritional assessment: Comparison of clinical assessment and objective variables for the prediction of length of hospital stay and readmission. Am. J. Clin. Nutr. 2015, 101, 956–965. [Google Scholar] [CrossRef]
- Guerra, R.S.; Sousa, A.S.; Fonseca, I.; Pichel, F.; Restivo, M.T.; Ferreira, S.; Amaral, T.F. Comparative analysis of undernutrition screening and diagnostic tools as predictors of hospitalisation costs. J. Hum. Nutr. Diet. 2016, 29, 165–173. [Google Scholar] [CrossRef]
- Vaid, S.; Bell, T.; Grim, R.; Ahuja, V. Predicting risk of death in general surgery patients on the basis of preoperative variables using American College of Surgeons National Surgical Quality Improvement Program data. Perm. J. 2012, 16, 10–17. [Google Scholar] [CrossRef]
- Thiele, R.H.; Rea, K.M.; Turrentine, F.E.; Friel, C.M.; Hassinger, T.E.; McMurry, T.L.; Goudreau, B.J.; Umapathi, B.A.; Kron, I.L.; Sawyer, R.G.; et al. Standardization of care: Impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J. Am. Coll. Surg. 2015, 220, 430–443. [Google Scholar] [CrossRef]
- Varadhan, K.K.; Lobo, D.N.; Ljungqvist, O. Enhanced recovery after surgery: The future of improving surgical care. Crit. Care Clin. 2010, 26, 527–5472. [Google Scholar] [CrossRef] [PubMed]
- Sauro, K.M.; Smith, C.; Ibadin, S.; Thomas, A.; Ganshorn, H.; Bakunda, L.; Bajgain, B.; Bisch, S.P.; Nelson, G. Enhanced Recovery After Surgery Guidelines and Hospital Length of Stay, Readmission, Complications, and Mortality: A Meta-Analysis of Randomized Clinical Trials. JAMA Netw. Open 2024, 7, e2417310. [Google Scholar] [CrossRef] [PubMed]
- Moosburner, S.; Dahlke, P.M.; Neudecker, J.; Hillebrandt, K.H.; Koch, P.F.; Knitter, S.; Ludwig, K.; Kamali, C.; Gül-Klein, S.; Raschzok, N.; et al. From morbidity reduction to cost-effectiveness: Enhanced recovery after surgery (ERAS) society recommendations in minimal invasive liver surgery. Langenbecks Arch. Surg. 2024, 409, 137. [Google Scholar] [CrossRef]
- Nelson, G.; Kiyang, L.N.; Crumley, E.T.; Chuck, A.; Nguyen, T.; Faris, P.; Wasylak, T.; Basualdo-Hammond, C.; McKay, S.; Ljungqvist, O.; et al. Implementation of Enhanced Recovery After Surgery (ERAS) Across a Provincial Healthcare System: The ERAS Alberta Colorectal Surgery Experience. World J. Surg. 2016, 40, 1092–1103. [Google Scholar] [CrossRef]
- Noba, L.; Rodgers, S.; Chandler, C.; Balfour, A.; Hariharan, D.; Yip, V.S. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Improve Clinical Outcomes in Liver Surgery: A Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2020, 24, 918–932. [Google Scholar] [CrossRef]
- Greco, M.; Capretti, G.; Beretta, L.; Gemma, M.; Pecorelli, N.; Braga, M. Enhanced recovery program in colorectal surgery: A meta-analysis of randomized controlled trials. World J. Surg. 2014, 38, 1531–1541. [Google Scholar] [CrossRef]
- Pecorelli, N.; Hershorn, O.; Baldini, G.; Fiore, J.F.; Stein, B.L.; Liberman, A.S.; Charlebois, P.; Carli, F.; Feldman, L.S. Impact of adherence to care pathway interventions on recovery following bowel resection within an established enhanced recovery program. Surg. Endosc. 2017, 31, 1760–1771. [Google Scholar] [CrossRef]
- ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results from an International Registry. Ann. Surg. 2015, 261, 1153–1159. [Google Scholar] [CrossRef]
- Visioni, A.; Shah, R.; Gabriel, E.; Attwood, K.; Kukar, M.; Nurkin, S. Enhanced Recovery After Surgery for Noncolorectal Surgery?: A Systematic Review and Meta-analysis of Major Abdominal Surgery. Ann. Surg. 2018, 267, 57–65. [Google Scholar] [CrossRef]
- Martin, L.; Gillis, C.; Ljungqvist, O. Preoperative nutrition care in Enhanced Recovery After Surgery programs: Are we missing an opportunity? Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 453–463. [Google Scholar] [CrossRef]
- Irani, J.L.; Hedrick, T.L.; Miller, T.E.; Lee, L.; Steinhagen, E.; Shogan, B.D.; Goldberg, J.E.; Feingold, D.L.; Lightner, A.L.; Paquette, I.M. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons. Surg. Endosc. 2023, 37, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, M.; Casey, R.G.; Black, P.; Koupparis, A.J. Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can. Urol. Assoc. J. 2011, 5, 342–348. [Google Scholar] [CrossRef]
- Molenaar, C.J.L.; Papen-Botterhuis, N.E.; Herrle, F.; Slooter, G.D. Prehabilitation, making patients fit for surgery—A new frontier in perioperative care. Innov. Surg. Sci. 2019, 4, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the real polypill. Physiol. Bethesda Md. 2013, 28, 330–358. [Google Scholar] [CrossRef]
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet Lond. Engl. 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Sahakyan, M.A.; Tholfsen, T.; Kleive, D.; Yaqub, S.; Kazaryan, A.M.; Buanes, T.; Røsok, B.I.; Labori, K.J.; Edwin, B. Laparoscopic Distal Pancreatectomy Following Prior Upper Abdominal Surgery (Pancreatectomy and Prior Surgery). J. Gastrointest. Surg. 2021, 25, 1787–1794. [Google Scholar] [CrossRef]
- Carli, F.; Zavorsky, G.S. Optimizing functional exercise capacity in the elderly surgical population. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 23–32. [Google Scholar] [CrossRef]
- Carli, F.; Silver, J.K.; Feldman, L.S.; McKee, A.; Gilman, S.; Gillis, C.; Scheede-Bergdahl, C.; Gamsa, A.; Stout, N.; Hirsch, B. Surgical Prehabilitation in Patients with Cancer: State-of-the-Science and Recommendations for Future Research from a Panel of Subject Matter Experts. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 49–64. [Google Scholar] [CrossRef]
- Silver, J.K.; Baima, J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am. J. Phys. Med. Rehabil. 2013, 92, 715–727. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, T.; Pei, L.; Zhang, Y.; Xu, L.; Cui, Y.; Liang, N.; Li, S.; Chen, W.; Huang, Y. Two-Week Multimodal Prehabilitation Program Improves Perioperative Functional Capability in Patients Undergoing Thoracoscopic Lobectomy for Lung Cancer: A Randomized Controlled Trial. Anesth. Analg. 2020, 131, 840–849. [Google Scholar] [CrossRef]
- Minnella, E.M.; Bousquet-Dion, G.; Awasthi, R.; Scheede-Bergdahl, C.; Carli, F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: A five-year research experience. Acta Oncol. Stockh. Swed. 2017, 56, 295–300. [Google Scholar] [CrossRef]
- Scheede-Bergdahl, C.; Minnella, E.M.; Carli, F. Multi-modal prehabilitation: Addressing the why, when, what, how, who and where next? Anaesthesia 2019, 74 (Suppl. 1), 20–26. [Google Scholar] [CrossRef]
- Gillis, C.; Wischmeyer, P.E. Pre-operative nutrition and the elective surgical patient: Why, how and what? Anaesthesia 2019, 74 (Suppl. 1), 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pierre, S.; Rivera, C.; Le Maître, B.; Ruppert, A.-M.; Bouaziz, H.; Wirth, N.; Saboye, J.; Sautet, A.; Masquelet, A.C.; Tournier, J.-J.; et al. Guidelines on smoking management during the perioperative period. Anaesth. Crit. Care Pain. Med. 2017, 36, 195–200. [Google Scholar] [CrossRef]
- Al-Naime, K.; Al-Anbuky, A.; Mawston, G. Remote Monitoring Model for the Preoperative Prehabilitation Program of Patients Requiring Abdominal Surgery. Futur. Internet 2021, 13, 104. [Google Scholar] [CrossRef]
- Heldens, A.F.J.M.; Bongers, B.C.; de Vos-Geelen, J.; van Meeteren, N.L.U.; Lenssen, A.F. Feasibility and preliminary effectiveness of a physical exercise training program during neoadjuvant chemoradiotherapy in individual patients with rectal cancer prior to major elective surgery. Eur. J. Surg. Oncol. 2016, 42, 1322–1330. [Google Scholar] [CrossRef]
- Carli, F.; Scheede-Bergdahl, C. Prehabilitation to enhance perioperative care. Anesthesiol. Clin. 2015, 33, 17–33. [Google Scholar] [CrossRef]
- Rao, R.M.; Nagendra, H.R.; Raghuram, N.; Vinay, C.; Chandrashekara, S.; Gopinath, K.S.; Srinath, B.S. Influence of yoga on postoperative outcomes and wound healing in early operable breast cancer patients undergoing surgery. Int. J. Yoga 2008, 1, 33–41. [Google Scholar] [CrossRef]
- Valkenet, K.; Trappenburg, J.C.A.; Schippers, C.C.; Wanders, L.; Lemmens, L.; Backx, F.J.G.; van Hillegersberg, R. Feasibility of Exercise Training in Cancer Patients Scheduled for Elective Gastrointestinal Surgery. Dig. Surg. 2016, 33, 439–447. [Google Scholar] [CrossRef]
- Weggemans, R.M.; Backx, F.J.G.; Borghouts, L.; Chinapaw, M.; Hopman, M.T.E.; Koster, A.; Kremers, S.; van Loon, L.J.C.; May, A.; Mosterd, A.; et al. The 2017 Dutch Physical Activity Guidelines. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, J.C.; Clifford, K.; Wilson, G.A.; Munro, F.; Baldi, J.C. Short-term high-intensity interval training improves fitness before surgery: A randomized clinical trial. Scand. J. Med. Sci. Sports 2022, 32, 856–865. [Google Scholar] [CrossRef]
- van Rooijen, S.; Carli, F.; Dalton, S.; Thomas, G.; Bojesen, R.; Le Guen, M.; Barizien, N.; Awasthi, R.; Minnella, E.; Beijer, S.; et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: The first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019, 19, 98. [Google Scholar] [CrossRef]
- Minnella, E.M.; Ferreira, V.; Awasthi, R.; Charlebois, P.; Stein, B.; Liberman, A.S.; Scheede-Bergdahl, C.; Morais, J.A.; Carli, F. Effect of two different pre-operative exercise training regimens before colorectal surgery on functional capacity: A randomised controlled trial. Eur. J. Anaesthesiol. 2020, 37, 969–978. [Google Scholar] [CrossRef]
- West, M.A.; Loughney, L.; Lythgoe, D.; Barben, C.P.; Sripadam, R.; Kemp, G.J.; Grocott, M.P.W.; Jack, S. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: A blinded interventional pilot study. Br. J. Anaesth. 2015, 114, 244–251. [Google Scholar] [CrossRef]
- Khosravi, N.; Stoner, L.; Farajivafa, V.; Hanson, E.D. Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta-analysis. Brain. Behav. Immun. 2019, 81, 92–104. [Google Scholar] [CrossRef]
- Flynn, M.G.; McFarlin, B.K.; Markofski, M.M. The Anti-Inflammatory Actions of Exercise Training. Am. J. Lifestyle Med. 2007, 1, 220–235. [Google Scholar] [CrossRef]
- Hamad, A.; Zhang, H.; Zhang, Y.; Shen, C.; Fa, P.; Huang, H.; Ejaz, A.; Tsung, A. Understanding the mechanism behind preoperative exercise therapy in patients with gastrointestinal cancers: A prospective randomized clinical trial. BMC Sports Sci. Med. Rehabil. 2025, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Gil-Andrés, D.; Cabañas-Alite, L. A Narrative Review Comparing Nutritional Screening Tools in Outpatient Management of Cancer Patients. Nutrients 2024, 16, 752. [Google Scholar] [CrossRef]
- Bousquet-Dion, G.; Awasthi, R.; Loiselle, S.-È.; Minnella, E.M.; Agnihotram, R.V.; Bergdahl, A.; Carli, F.; Scheede-Bergdahl, C. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: A randomized control trial. Acta Oncol. Stockh. Swed. 2018, 57, 849–859. [Google Scholar] [CrossRef]
- Campbell, W.W.; Leidy, H.J. Dietary protein and resistance training effects on muscle and body composition in older persons. J. Am. Coll. Nutr. 2007, 26, 696S–703S. [Google Scholar] [CrossRef]
- Thomas, M.N.; Kufeldt, J.; Kisser, U.; Hornung, H.-M.; Hoffmann, J.; Andraschko, M.; Werner, J.; Rittler, P. Effects of malnutrition on complication rates, length of hospital stay, and revenue in elective surgical patients in the G-DRG-system. Nutr. Burbank Los. Angel. Cty. Calif. 2016, 32, 249–254. [Google Scholar] [CrossRef]
- Simonsen, C.; de Heer, P.; Bjerre, E.D.; Suetta, C.; Hojman, P.; Pedersen, B.K.; Svendsen, L.B.; Christensen, J.F. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann. Surg. 2018, 268, 58–69. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. Edinb. Scotl. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Sadlonova, M.; Katz, N.B.; Jurayj, J.S.; Flores, L.; Celano, C.M.; von Arnim, C.A.F.; Silver, J.K. Surgical prehabilitation in older and frail individuals: A scoping review. Int. Anesthesiol. Clin. 2023, 61, 34–46. [Google Scholar] [CrossRef]
- Williams, G.R.; Al-Obaidi, M.; Dai, C.; Mir, N.; Challa, S.A.; Daniel, M.; Patel, H.; Barlow, B.; Young-Smith, C.; Gbolahan, O.; et al. Association of malnutrition with geriatric assessment impairments and health-related quality of life among older adults with gastrointestinal malignancies. Cancer 2020, 126, 5147–5155. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Paddon-Jones, D.; Hays, N.P.; Kortebein, P.; Ronsen, O.; Williams, R.H.; McComb, A.; Symons, T.B.; Wolfe, R.R.; Evans, W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin. Nutr. Edinb. Scotl. 2010, 29, 18–23. [Google Scholar] [CrossRef]
- Beasley, J.M.; LaCroix, A.Z.; Neuhouser, M.L.; Huang, Y.; Tinker, L.; Woods, N.; Michael, Y.; Curb, J.D.; Prentice, R.L. Protein intake and incident frailty in the Womens Health Initiative observational study. J. Am. Geriatr. Soc. 2010, 58, 1063–1071. [Google Scholar] [CrossRef]
- van Erven, C.; Ten Cate, D.; van Lieshout, R.; Beijer, S.; Dieleman, J.; Geertsema, S.; Jalink, M.; van der Meulen-Franken, J.; Rademakers, N.; Gillis, C.; et al. Changes in nutritional status and effectiveness of the dietary intervention of multimodal prehabilitation for patients with colorectal cancer: A secondary analysis of the PREHAB randomized clinical trial. Clin. Nutr. ESPEN 2025, 65, 469–477. [Google Scholar] [CrossRef]
- Bamgbade, O.A.; Rutter, T.W.; Nafiu, O.O.; Dorje, P. Postoperative complications in obese and nonobese patients. World J. Surg. 2007, 31, 556–560. [Google Scholar] [CrossRef]
- Ngo-Huang, A.; Herbert, A.; Fontillas, R.C.; Parker, N.H.; Asumbrado, R.; Garg, N.; Dibaj, S.; Liu, D.D.; Ng, A.H.; Guo, Y.; et al. Frequency of Sarcopenia, Sarcopenic Obesity, and Changes in Physical Function in Surgical Oncology Patients Referred for Prehabilitation. Integr. Cancer Ther. 2021, 20, 15347354211000118. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Cappellari, G.G. Double burden of malnutrition in persons with obesity. Rev. Endocr. Metab. Disord. 2020, 21, 307–313. [Google Scholar] [CrossRef]
- Kokura, Y.; Ueshima, J.; Saino, Y.; Maeda, K. Enhanced protein intake on maintaining muscle mass, strength, and physical function in adults with overweight/obesity: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2024, 63, 417–426. [Google Scholar] [CrossRef]
- García-Delgado, Y.; López-Madrazo-Hernández, M.J.; Alvarado-Martel, D.; Miranda-Calderín, G.; Ugarte-Lopetegui, A.; González-Medina, R.A.; Hernández-Lázaro, A.; Zamora, G.; Pérez-Martín, N.; Sánchez-Hernández, R.M.; et al. Prehabilitation for Bariatric Surgery: A Randomized, Controlled Trial Protocol and Pilot Study. Nutrients 2021, 13, 2903. [Google Scholar] [CrossRef]
- Griffiths, C.D.; DSouza, D.; Rodriguez, F.; Park, L.J.; Serrano, P.E. Quality of life following perioperative optimization with nutritional supplements in patients undergoing gastrointestinal surgery for cancer: A randomized, placebo-controlled feasibility clinical trial. J. Surg. Oncol. 2024, 129, 1289–1294. [Google Scholar] [CrossRef]
- Mao, R.-M.D.; Franco-Mesa, C.; Samreen, S. Prehabilitation in metabolic and bariatric surgery: A narrative review. Ann. Laparosc. Endosc. Surg. 2023, 8, 3. [Google Scholar] [CrossRef]
- Aills, L.; Blankenship, J.; Buffington, C.; Furtado, M.; Parrott, J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg. Obes. Relat. Dis. 2008, 4, S73–S108. [Google Scholar] [CrossRef]
- Wilson, R.J.T.; Davies, S.; Yates, D.; Redman, J.; Stone, M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br. J. Anaesth. 2010, 105, 297–303. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, S.J.; Huisman, D.; Stuijvenberg, M.; Stens, J.; Roumen, R.M.H.; Daams, F.; Slooter, G.D. Intraoperative modifiable risk factors of colorectal anastomotic leakage: Why surgeons and anesthesiologists should act together. Int. J. Surg. Lond. Engl. 2016, 36, 183–200. [Google Scholar] [CrossRef]
- Schmid, M.; Sood, A.; Campbell, L.; Kapoor, V.; Dalela, D.; Klett, D.E.; Chun, F.K.-H.; Kibel, A.S.; Sammon, J.D.; Menon, M.; et al. Impact of smoking on perioperative outcomes after major surgery. Am. J. Surg. 2015, 210, 221–229. [Google Scholar] [CrossRef]
- Thomsen, T.; Villebro, N.; Møller, A.M. Interventions for preoperative smoking cessation. Cochrane Database Syst. Rev. 2014, 2014, CD002294. [Google Scholar] [CrossRef] [PubMed]
- Furtunescu, F.; Minca, D.; Vasile, A.; Domnariu, C. Alcohol consumption impact on premature mortality in Romania. Rom. J. Leg. Med. 2009, 17, 296–302. [Google Scholar] [CrossRef]
- Eliasen, M.; Grønkjær, M.; Skov-Ettrup, L.S.; Mikkelsen, S.S.; Becker, U.; Tolstrup, J.S.; Flensborg-Madsen, T. Preoperative alcohol consumption and postoperative complications: A systematic review and meta-analysis. Ann. Surg. 2013, 258, 930–942. [Google Scholar] [CrossRef]
- Oppedal, K.; Møller, A.M.; Pedersen, B.; Tønnesen, H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst. Rev. 2012, 11, CD008343. [Google Scholar] [CrossRef]
- Rosenberger, P.H.; Jokl, P.; Ickovics, J. Psychosocial factors and surgical outcomes: An evidence-based literature review. J. Am. Acad. Orthop. Surg. 2006, 14, 397–405. [Google Scholar] [CrossRef]
- Levett, D.Z.H.; Grimmett, C. Psychological factors, prehabilitation and surgical outcomes: Evidence and future directions. Anaesthesia 2019, 74, 36–42. [Google Scholar] [CrossRef]
- Greenwood, B.N. The role of dopamine in overcoming aversion with exercise. Brain Res. 2019, 1713, 102–108. [Google Scholar] [CrossRef]
- Ip, H.Y.V.; Abrishami, A.; Peng, P.W.H.; Wong, J.; Chung, F. Predictors of postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology 2009, 111, 657–677. [Google Scholar] [CrossRef]
- Foster, C.; Haviland, J.; Winter, J.; Grimmett, C.; Chivers Seymour, K.; Batehup, L.; Calman, L.; Corner, J.; Din, A.; Fenlon, D.; et al. Pre-Surgery Depression and Confidence to Manage Problems Predict Recovery Trajectories of Health and Wellbeing in the First Two Years following Colorectal Cancer: Results from the CREW Cohort Study. PLoS ONE 2016, 11, e0155434. [Google Scholar] [CrossRef]
- Powell, R.; Davies, A.; Rowlinson-Groves, K.; French, D.P.; Moore, J.; Merchant, Z. Impact of a prehabilitation and recovery programme on emotional well-being in individuals undergoing cancer surgery: A multi-perspective qualitative study. BMC Cancer 2023, 23, 1232. [Google Scholar] [CrossRef]
- Fulop, A.; Lakatos, L.; Susztak, N.; Szijarto, A.; Banky, B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: A randomised clinical trial. Anaesthesia 2021, 76, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Triguero-Cánovas, D.; López-Rodríguez-Arias, F.; Gómez-Martínez, M.; Sánchez-Guillén, L.; Peris-Castelló, F.; Alcaide-Quirós, M.J.; Morillas-Blasco, P.; Arroyo, A.; Ramírez, J.M. Home-based prehabilitation improves physical conditions measured by ergospirometry and 6MWT in colorectal cancer patients: A randomized controlled pilot study. Support. Care Cancer 2023, 31, 673. [Google Scholar] [CrossRef]
- Ten Cate, D.W.G.; Molenaar, C.J.L.; Garcia, R.S.; Bojesen, R.D.; Tahasildar, B.L.R.; Jansen, L.; López-Baamonde, M.; Feo, C.V.; Martínez-Palli, G.; Gögenur, I.; et al. Multimodal prehabilitation in elective oncological colorectal surgery enhances postoperative functional recovery: A secondary analysis of the PREHAB randomized clinical trial. Eur. J. Surg. Oncol. 2024, 50, 108270. [Google Scholar] [CrossRef]
- Gillis, C.; Fenton, T.R.; Sajobi, T.T.; Minnella, E.M.; Awasthi, R.; Loiselle, S.-È.; Liberman, A.S.; Stein, B.; Charlebois, P.; Carli, F. Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: A pooled analysis of randomized controlled trials. Clin. Nutr. Edinb. Scotl. 2019, 38, 1053–1060. [Google Scholar] [CrossRef]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef]
- Chabot, K.; Gillis, C.; Minnella, E.M.; Ferreira, V.; Awasthi, R.; Baldini, G.; Carli, F. Functional capacity of prediabetic patients: Effect of multimodal prehabilitation in patients undergoing colorectal cancer resection. Acta Oncol. Stockh. Swed. 2021, 60, 1025–1031. [Google Scholar] [CrossRef]
- Loughney, L.; Murphy, K.; Tully, R.; Robb, W.B.; McCaffrey, N.; Dowd, K.; Skelly, F. The effect of a pre- and post-operative exercise program versus standard care on physical activity and sedentary behavior of patients with esophageal and gastric cancer undergoing neoadjuvant treatment prior to surgery (the PERIOP-OG Trial): A randomized controlled trial. Dis. Esophagus 2024, 37, doae044. [Google Scholar] [CrossRef]
- Allen, S.K.; Brown, V.; White, D.; King, D.; Hunt, J.; Wainwright, J.; Emery, A.; Hodge, E.; Kehinde, A.; Prabhu, P.; et al. Multimodal Prehabilitation During Neoadjuvant Therapy Prior to Esophagogastric Cancer Resection: Effect on Cardiopulmonary Exercise Test Performance, Muscle Mass and Quality of Life-A Pilot Randomized Clinical Trial. Ann. Surg. Oncol. 2022, 29, 1839–1850. [Google Scholar] [CrossRef]
- López-Rodríguez-Arias, F.; Sánchez-Guillén, L.; Aranaz-Ostáriz, V.; Triguero-Cánovas, D.; Lario-Pérez, S.; Barber-Valles, X.; Lacueva, F.J.; Ramirez, J.M.; Arroyo, A. Effect of home-based prehabilitation in an enhanced recovery after surgery program for patients undergoing colorectal cancer surgery during the COVID-19 pandemic. Support. Care Cancer 2021, 29, 7785–7791. [Google Scholar] [CrossRef]
- Bausys, A.; Luksta, M.; Anglickiene, G.; Maneikiene, V.V.; Kryzauskas, M.; Rybakovas, A.; Dulskas, A.; Kuliavas, J.; Stratilatovas, E.; Macijauskiene, L.; et al. Effect of home-based prehabilitation on postoperative complications after surgery for gastric cancer: Randomized clinical trial. Br. J. Surg. 2023, 110, 1800–1807. [Google Scholar] [CrossRef]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, L.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of Nutritional Prehabilitation, With and Without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-analysis. Gastroenterology 2018, 155, 391–410.e4. [Google Scholar] [CrossRef] [PubMed]
- Berkel, A.E.M.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; de Jongh, F.H.C.; Eijsvogel, M.M.M.; Wymenga, M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; van Det, M.J.; et al. Effects of Community-based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery With High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann. Surg. 2022, 275, e299–e306. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; Ten Cate, D.W.G.; Regis, M.; Awasthi, R.; Martínez-Palli, G.; López-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 233–242. [Google Scholar] [CrossRef]
- Gloor, S.; Misirlic, M.; Frei-Lanter, C.; Herzog, P.; Müller, P.; Schäfli-Thurnherr, J.; Lamdark, T.; Schregel, D.; Wyss, R.; Unger, I.; et al. Prehabilitation in patients undergoing colorectal surgery fails to confer reduction in overall morbidity: Results of a single-center, blinded, randomized controlled trial. Langenbecks Arch. Surg. 2022, 407, 897–907. [Google Scholar] [CrossRef]
- Ausania, F.; Senra, P.; Meléndez, R.; Caballeiro, R.; Ouviña, R.; Casal-Núñez, E. Prehabilitation in patients undergoing pancreaticoduodenectomy: A randomized controlled trial. Rev. Esp. Enferm. Dig. 2019, 111, 603–608. [Google Scholar] [CrossRef]
- Onerup, A.; Andersson, J.; Angenete, E.; Bock, D.; Börjesson, M.; Ehrencrona, C.; Fagevik Olsén, M.; Larsson, P.-A.; de la Croix, H.; Wedin, A.; et al. Effect of Short-term Homebased Pre- and Postoperative Exercise on Recovery After Colorectal Cancer Surgery (PHYSSURG-C): A Randomized Clinical Trial. Ann. Surg. 2022, 275, 448–455. [Google Scholar] [CrossRef]
- Pesce, A.; Fabbri, N.; Colombari, S.; Uccellatori, L.; Grazzi, G.; Lordi, R.; Anania, G.; Feo, C.V. A randomized controlled clinical trial on multimodal prehabilitation in colorectal cancer patients to improve functional capacity: Preliminary results. Surg. Endosc. 2024, 38, 7440–7450. [Google Scholar] [CrossRef]
- Liang, S.; Yuan, L.; Wang, A.; Li, S.; Wei, Y.; Wen, T.; Li, T.; Yang, X.; Ren, Q.; Zhu, C.; et al. Effect of short-term exercise-based prehabilitation program for patients undergoing liver cancer surgery: A randomized controlled trial. Surgery 2025, 180, 109115. [Google Scholar] [CrossRef]
- Chen, J.; Hong, C.; Chen, R.; Zhou, M.; Lin, S. Prognostic impact of a 3-week multimodal prehabilitation program on frail elderly patients undergoing elective gastric cancer surgery: A randomized trial. BMC Gastroenterol. 2024, 24, 403. [Google Scholar] [CrossRef]
- Wijma, A.G.; Hoogwater, F.J.H.; Nijkamp, M.W.; Klaase, J.M. Personalized multimodal prehabilitation reduces cardiopulmonary complications after pancreatoduodenectomy: Results of a propensity score matching analysis. HPB 2023, 25, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Garcia, A.; Ubre, M.; Pascual-Argente, N.; Risco, R.; Faner, J.; Balust, J.; Lacy, A.M.; Puig-Junoy, J.; Roca, J.; Martinez-Palli, G. Post-discharge impact and cost-consequence analysis of prehabilitation in high-risk patients undergoing major abdominal surgery: Secondary results from a randomised controlled trial. Br. J. Anaesth. 2019, 123, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Fenton, T.R.; Gramlich, L.; Sajobi, T.T.; Culos-Reed, S.N.; Bousquet-Dion, G.; Elsherbini, N.; Fiore, J.F.; Minnella, E.M.; Awasthi, R.; et al. Older frail prehabilitated patients who cannot attain a 400 m 6-min walking distance before colorectal surgery suffer more postoperative complications. Eur. J. Surg. Oncol. 2021, 47, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Bojesen, R.D.; Dalton, S.O.; Skou, S.T.; Jørgensen, L.B.; Walker, L.R.; Eriksen, J.R.; Grube, C.; Justesen, T.F.; Johansen, C.; Slooter, G.; et al. Preoperative multimodal prehabilitation before elective colorectal cancer surgery in patients with WHO performance status I or II: Randomized clinical trial. BJS Open 2023, 7, zrad134. [Google Scholar] [CrossRef]
- Awasthi, R.; Minnella, E.M.; Ferreira, V.; Ramanakumar, A.V.; Scheede-Bergdahl, C.; Carli, F. Supervised exercise training with multimodal pre-habilitation leads to earlier functional recovery following colorectal cancer resection. Acta Anaesthesiol. Scand. 2019, 63, 461–467. [Google Scholar] [CrossRef]
- Hijazi, Y.; Gondal, U.; Aziz, O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int. J. Surg. Lond. Engl. 2017, 39, 156–162. [Google Scholar] [CrossRef]
- Onerup, A.; Li, Y.; Afshari, K.; Angenete, E.; de la Croix, H.; Ehrencrona, C.; Wedin, A.; Haglind, E. Long-term results of a short-term home-based pre- and postoperative exercise intervention on physical recovery after colorectal cancer surgery (PHYSSURG-C): A randomized clinical trial. Colorectal Dis. 2024, 26, 545–553. [Google Scholar] [CrossRef]
- Waller, E.; Sutton, P.; Rahman, S.; Allen, J.; Saxton, J.; Aziz, O. Prehabilitation with wearables versus standard of care before major abdominal cancer surgery: A randomised controlled pilot study (trial registration: NCT04047524). Surg. Endosc. 2022, 36, 1008–1017. [Google Scholar] [CrossRef]
- Zhang, B.; Najarali, Z.; Ruo, L.; Alhusaini, A.; Solis, N.; Valencia, M.; Sanchez, M.I.P.; Serrano, P.E. Effect of Perioperative Nutritional Supplementation on Postoperative Complications-Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2019, 23, 1682–1693. [Google Scholar] [CrossRef]
- Moran, J.; Guinan, E.; McCormick, P.; Larkin, J.; Mockler, D.; Hussey, J.; Moriarty, J.; Wilson, F. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: A systematic review and meta-analysis. Surgery 2016, 160, 1189–1201. [Google Scholar] [CrossRef]
- Taha, A.; Taha-Mehlitz, S.; Staartjes, V.E.; Lunger, F.; Gloor, S.; Unger, I.; Mungo, G.; Tschuor, C.; Breitenstein, S.; Gingert, C. Association of a prehabilitation program with anxiety and depression before colorectal surgery: A post hoc analysis of the pERACS randomized controlled trial. Langenbecks Arch. Surg. 2021, 406, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Atoui, S.; Carli, F.; Bernard, P.; Lee, L.; Stein, B.; Charlebois, P.; Liberman, A.S. Does a multimodal prehabilitation program improve sleep quality and duration in patients undergoing colorectal resection for cancer? Pilot randomized control trial. J. Behav. Med. 2024, 47, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Talbot, A.; Jebb, S.A.; Foster, C.; Realpe, A.X.; Wheatstone, P.; Buczacki, S.; Koutoukidis, D.A. Participants perspectives of being recruited into a randomised trial of a weight loss intervention before colorectal cancer surgery: A qualitative interview study. BMC Cancer 2024, 24, 802. [Google Scholar] [CrossRef]
- Keller, D.S.; Curtis, N.; Burt, H.A.; Ammirati, C.A.; Collings, A.T.; Polk, H.C.; Carrano, F.M.; Antoniou, S.A.; Hanna, N.; Piotet, L.-M.; et al. EAES/SAGES evidence-based recommendations and expert consensus on optimization of perioperative care in older adults. Surg. Endosc. 2024, 38, 4104–4126. [Google Scholar] [CrossRef]
- Berkel, A.E.M.; Bongers, B.C.; van Kamp, M.-J.S.; Kotte, H.; Weltevreden, P.; de Jongh, F.H.C.; Eijsvogel, M.M.M.; Wymenga, A.N.M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; et al. The effects of prehabilitation versus usual care to reduce postoperative complications in high-risk patients with colorectal cancer or dysplasia scheduled for elective colorectal resection: Study protocol of a randomized controlled trial. BMC Gastroenterol. 2018, 18, 29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, G.A.; Minca, D.G.; Jafal, N.M.; Toma, C.V.; Alexandrescu, S.T.; Costea, R.V.; Vasilescu, C. Multimodal Prehabilitation in Major Abdominal Surgery—Rationale, Modalities, Results and Limitations. Medicina 2025, 61, 908. https://doi.org/10.3390/medicina61050908
Popescu GA, Minca DG, Jafal NM, Toma CV, Alexandrescu ST, Costea RV, Vasilescu C. Multimodal Prehabilitation in Major Abdominal Surgery—Rationale, Modalities, Results and Limitations. Medicina. 2025; 61(5):908. https://doi.org/10.3390/medicina61050908
Chicago/Turabian StylePopescu, George Andrei, Dana Galieta Minca, Nader Mugurel Jafal, Cristian Valentin Toma, Sorin Tiberiu Alexandrescu, Radu Virgil Costea, and Catalin Vasilescu. 2025. "Multimodal Prehabilitation in Major Abdominal Surgery—Rationale, Modalities, Results and Limitations" Medicina 61, no. 5: 908. https://doi.org/10.3390/medicina61050908
APA StylePopescu, G. A., Minca, D. G., Jafal, N. M., Toma, C. V., Alexandrescu, S. T., Costea, R. V., & Vasilescu, C. (2025). Multimodal Prehabilitation in Major Abdominal Surgery—Rationale, Modalities, Results and Limitations. Medicina, 61(5), 908. https://doi.org/10.3390/medicina61050908







