Prevalence of Genetic Variants Associated with Atrial Fibrillation Risk in the Asymptomatic Young Adult Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. DNA Isolation
2.3. Genotyping via Real-Time PCR
2.4. Hardy–Weinberg Equilibrium Analysis
2.5. Variant Allele Frequency Analysis
2.6. Risk Allele Categorization
2.7. Association Between Risk Allele Categories and Lifestyle Factors
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Young Adult Population
3.2. Distribution of Genotypic Frequency in the Young Adult Population
3.3. Distribution of Risk Alleles in the Young Adult Population
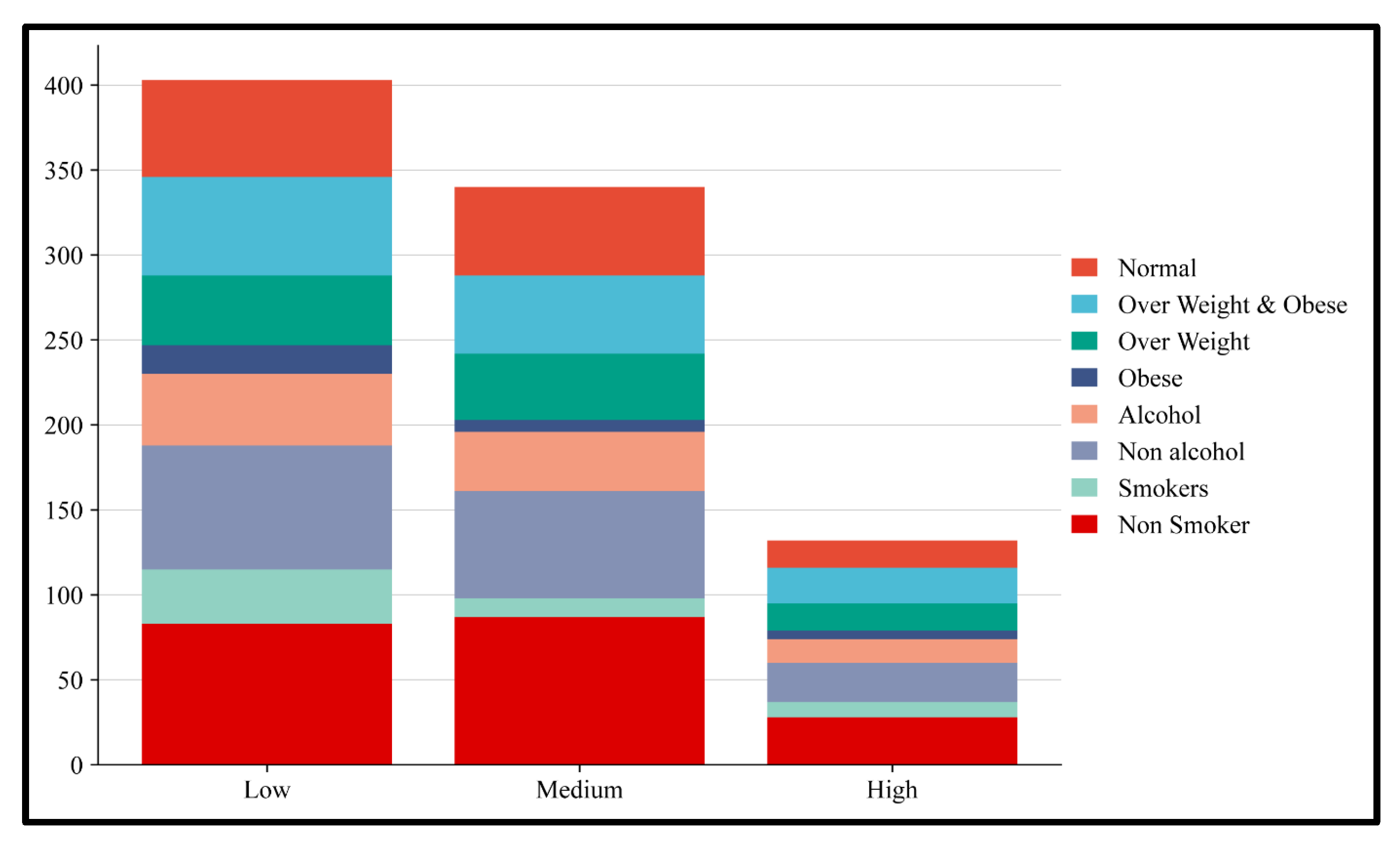
3.4. Risk Allele Association with Obesity and Alcohol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| SNP | Single-nucleotide polymorphism |
| BMI | Body mass index |
| PCR | Polymerase Chain Reaction |
| PITX2 | Paired-Like Homeodomain 2 |
| TBX5 | T-Box Transcription Factor 5 |
| PRRX1 | Paired Related Homeobox 1 |
| ZFHX3 | Zinc Finger Homeobox 3 |
| HAND2 | Heart and Neural Crest Derivatives Expressed 2 |
| OR | Odds ratio |
| CI | Confidence interval |
| SPSS | Statistical Package for the Social Sciences |
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Qiu, X.B.; Wang, J.; Guo, Y.H.; Yang, C.X.; Li, L.; Gao, R.F.; Ke, Z.P.; Di, R.M.; Sun, Y.M.; et al. PRRX1 Loss-of-Function Mutations Underlying Familial Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e023517. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.P.; Seward, J.B.; Tsang, T.S. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006, 114, 119–125. [Google Scholar] [CrossRef]
- Saggu, D.K.; Rangaswamy, V.V.; Yalagudri, S.; Sundar, G.; Reddy, N.K.; Shah, V.; Kotti, K.; Shankar, M.; Chennapragada, S.; Narasimhan, C. Prevalence, clinical profile, and stroke risk of atrial fibrillation in rural Andhra Pradesh, India (the AP-AF study). Indian. Heart J. 2022, 74, 86–90. [Google Scholar] [CrossRef]
- Mohanty, S.; Banerjee, A.; Kumar, A.; Deb, P.; Samantray, H.; Das, D. Non-Valvular Atrial Fibrillation in Young Adults in Eastern India: A Clinico-Aetiological Retrospective Analysis in a Tertiary Care Hospital. Cureus 2023, 15, e36918. [Google Scholar] [CrossRef]
- Abdul-Aziz, A.A.; Desikan, P.; Prabhakaran, D.; Schroeder, L.F. Tackling the Burden of Cardiovascular Diseases in India. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005195. [Google Scholar] [CrossRef]
- Ellinor, P.T.; Lunetta, K.L.; Albert, C.M.; Glazer, N.L.; Ritchie, M.D.; Smith, A.V.; Arking, D.E.; Muller-Nurasyid, M.; Krijthe, B.P.; Lubitz, S.A.; et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat. Genet. 2012, 44, 670–675. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Yin, X.; Lin, H.J.; Kolek, M.; Smith, J.G.; Trompet, S.; Rienstra, M.; Rost, N.S.; Teixeira, P.L.; Almgren, P.; et al. Genetic Risk Prediction of Atrial Fibrillation. Circulation 2017, 135, 1311–1320. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Arnar, D.O.; Helgadottir, A.; Gretarsdottir, S.; Holm, H.; Sigurdsson, A.; Jonasdottir, A.; Baker, A.; Thorleifsson, G.; Kristjansson, K.; et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007, 448, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, I.E.; Rienstra, M.; Roselli, C.; Yin, X.; Geelhoed, B.; Barnard, J.; Lin, H.; Arking, D.E.; Smith, A.V.; Albert, C.M.; et al. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat. Genet. 2017, 49, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, T.H.; Yang, P.S.; Lim, H.E.; Choi, E.K.; Shim, J.; Shin, E.; Uhm, J.S.; Kim, J.S.; Joung, B.; et al. Korean atrial fibrillation network genome-wide association study for early-onset atrial fibrillation identifies novel susceptibility loci. Eur. Heart J. 2017, 38, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- Shen Han, Y.; Ahmed, A.M.; Chao, Z.; Jun, W.; Xiaochen, Y. Association Between rs2200733 Polymorphism of PITX2 Gene and the Risk of Atrial Fibrillation. Anatol. J. Cardiol. 2023, 27, 160–166. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Xu, J.; Xu, G.; Liu, X. Chromosome 4q25 Variants rs2200733, rs10033464, and rs1906591 Contribute to Ischemic Stroke Risk. Mol. Neurobiol. 2016, 53, 3882–3890. [Google Scholar] [CrossRef]
- Herraiz-Martinez, A.; Llach, A.; Tarifa, C.; Gandia, J.; Jimenez-Sabado, V.; Lozano-Velasco, E.; Serra, S.A.; Vallmitjana, A.; Vazquez Ruiz de Castroviejo, E.; Benitez, R.; et al. The 4q25 variant rs13143308T links risk of atrial fibrillation to defective calcium homoeostasis. Cardiovasc. Res. 2019, 115, 578–589. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, X.; Gao, L.; Li, H.; Yin, X.; Dong, Y.; Yang, Y.; Xia, Y. Common Variants in the TBX5 Gene Associated with Atrial Fibrillation in a Chinese Han Population. PLoS ONE 2016, 11, e0160467. [Google Scholar] [CrossRef]
- Okubo, Y.; Nakano, Y.; Ochi, H.; Onohara, Y.; Tokuyama, T.; Motoda, C.; Amioka, M.; Hironobe, N.; Okamura, S.; Ikeuchi, Y.; et al. Predicting atrial fibrillation using a combination of genetic risk score and clinical risk factors. Heart Rhythm 2020, 17, 699–705. [Google Scholar] [CrossRef]
- Syeda, F.; Holmes, A.P.; Yu, T.Y.; Tull, S.; Kuhlmann, S.M.; Pavlovic, D.; Betney, D.; Riley, G.; Kucera, J.P.; Jousset, F.; et al. PITX2 Modulates Atrial Membrane Potential and the Antiarrhythmic Effects of Sodium-Channel Blockers. J. Am. Coll. Cardiol. 2016, 68, 1881–1894. [Google Scholar] [CrossRef]
- Nadadur, R.D.; Broman, M.T.; Boukens, B.; Mazurek, S.R.; Yang, X.; van den Boogaard, M.; Bekeny, J.; Gadek, M.; Ward, T.; Zhang, M.; et al. Pitx2 modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci. Transl. Med. 2016, 8, 354ra115. [Google Scholar] [CrossRef]
- Tucker, N.R.; Clauss, S.; Ellinor, P.T. Common variation in atrial fibrillation: Navigating the path from genetic association to mechanism. Cardiovasc. Res. 2016, 109, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.B.; Karki, R.; Hattoum, A.; Sharma, U.C. Arrhythmias in Patients >/=80 Years of Age: Pathophysiology, Management, and Outcomes. J. Am. Coll. Cardiol. 2018, 71, 2041–2057. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Gukathasan, N.; Sartori, S.; Baber, U. Chronic Kidney Disease and Atrial Fibrillation: A Contemporary Overview. J. Atr. Fibrillation 2012, 5, 448. [Google Scholar] [PubMed]
- Bano, A.; Rodondi, N.; Beer, J.H.; Moschovitis, G.; Kobza, R.; Aeschbacher, S.; Baretella, O.; Muka, T.; Stettler, C.; Franco, O.H.; et al. Association of Diabetes With Atrial Fibrillation Phenotype and Cardiac and Neurological Comorbidities: Insights From the Swiss-AF Study. J. Am. Heart Assoc. 2021, 10, e021800. [Google Scholar] [CrossRef]
- Aune, D.; Mahamat-Saleh, Y.; Kobeissi, E.; Feng, T.; Heath, A.K.; Janszky, I. Blood pressure, hypertension and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 2023, 38, 145–178. [Google Scholar] [CrossRef]
- Goudis, C.A.; Korantzopoulos, P.; Ntalas, I.V.; Kallergis, E.M.; Ketikoglou, D.G. Obesity and atrial fibrillation: A comprehensive review of the pathophysiological mechanisms and links. J. Cardiol. 2015, 66, 361–369. [Google Scholar] [CrossRef]
- Tsagkaris, C.; Zacharopoulou, L. Thyroid Disease (TD), Chronic Obstructive Pulmonary Disease (COPD) and Valvular Heart Disease (VHD) as modifiable risk factors of Atrial Fibrillation. Rom. J. Intern. Med. 2020, 58, 3–4. [Google Scholar] [CrossRef]
- Kany, S.; Reissmann, B.; Metzner, A.; Kirchhof, P.; Darbar, D.; Schnabel, R.B. Genetics of atrial fibrillation-practical applications for clinical management: If not now, when and how? Cardiovasc. Res. 2021, 117, 1718–1731. [Google Scholar] [CrossRef]
- Andersen, J.H.; Andreasen, L.; Olesen, M.S. Atrial fibrillation-a complex polygenetic disease. Eur. J. Hum. Genet. 2021, 29, 1051–1060. [Google Scholar] [CrossRef]
- Holmgren, A.; Giang, K.W.; Fedchenko, M.; Eriksson, P.; Dellborg, M.; Mandalenakis, Z. Ischemic Stroke in Patients With Congenital Heart Disease and Atrial Fibrillation. J. Am. Heart Assoc. 2024, 13, e032813. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Rosengren, A.; Lappas, G.; Eriksson, P.; Gilljam, T.; Hansson, P.O.; Skoglund, K.; Fedchenko, M.; Dellborg, M. Atrial Fibrillation Burden in Young Patients With Congenital Heart Disease. Circulation 2018, 137, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Mansour, M.C.; Ruskin, J.N.; Heist, E.K. Atrial Fibrillation-Mediated Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2019, 12, e007809. [Google Scholar] [CrossRef] [PubMed]
- Kusano, K.F.; Taniyama, M.; Nakamura, K.; Miura, D.; Banba, K.; Nagase, S.; Morita, H.; Nishii, N.; Watanabe, A.; Tada, T.; et al. Atrial fibrillation in patients with Brugada syndrome relationships of gene mutation, electrophysiology, and clinical backgrounds. J. Am. Coll. Cardiol. 2008, 51, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; McNitt, S.; Polonsky, B.; Rosero, S.Z.; Zareba, W. Atrial Fibrillation in Long QT Syndrome by Genotype. Circ. Arrhythm. Electrophysiol. 2019, 12, e007213. [Google Scholar] [CrossRef]
- Knoche, J.W.; Orland, K.M.; January, C.T.; Maginot, K.R. Atrial Fibrillation and Long QT Syndrome Presenting in a 12-Year-Old Girl. Case Rep. Pediatr. 2012, 2012, 124838. [Google Scholar] [CrossRef]
- Yang, L.; Chung, M.K. Lifestyle changes in atrial fibrillation management and intervention. J. Cardiovasc. Electrophysiol. 2023, 34, 2163–2178. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Kirkwood, G.; Dibb, K.; Garratt, C.J. Comparison of Atrial Fibrillation in the Young versus That in the Elderly: A Review. Cardiol. Res. Pract. 2013, 2013, 976976. [Google Scholar] [CrossRef]
- Wang, N.; Yu, Y.; Sun, Y.; Zhang, H.; Wang, Y.; Chen, C.; Tan, X.; Wang, B.; Lu, Y. Acquired risk factors and incident atrial fibrillation according to age and genetic predisposition. Eur. Heart J. 2023, 44, 4982–4993. [Google Scholar] [CrossRef]
- Ebana, Y.; Sun, Y.; Yang, X.; Watanabe, T.; Makita, S.; Ozaki, K.; Tanaka, T.; Arai, H.; Furukawa, T. Pathway analysis with genome-wide association study (GWAS) data detected the association of atrial fibrillation with the mTOR signaling pathway. Int. J. Cardiol. Heart Vasc. 2019, 24, 100383. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Lunetta, K.L.; Lin, H.; Arking, D.E.; Trompet, S.; Li, G.; Krijthe, B.P.; Chasman, D.I.; Barnard, J.; Kleber, M.E.; et al. Novel genetic markers associate with atrial fibrillation risk in Europeans and Japanese. J. Am. Coll. Cardiol. 2014, 63, 1200–1210. [Google Scholar] [CrossRef]
- Wang, J.; Klysik, E.; Sood, S.; Johnson, R.L.; Wehrens, X.H.; Martin, J.F. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc. Natl. Acad. Sci. USA 2010, 107, 9753–9758. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, L.; Lin, C.; Xie, Y.; Bao, Y.; Luo, Q.; Zhang, N. Association between the rs2106261 polymorphism in the zinc finger homeobox 3 gene and risk of atrial fibrillation: Evidence from a PRISMA-compliant meta-analysis. Medicine 2021, 100, e27749. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chu, M.; Zhuang, W. Association between ZFHX3 and PRRX1 Polymorphisms and Atrial Fibrillation Susceptibility from Meta-Analysis. Int. J. Hypertens. 2021, 2021, 9423576. [Google Scholar] [CrossRef] [PubMed]
- Low, S.K.; Takahashi, A.; Ebana, Y.; Ozaki, K.; Christophersen, I.E.; Ellinor, P.T.; Consortium, A.F.; Ogishima, S.; Yamamoto, M.; Satoh, M.; et al. Identification of six new genetic loci associated with atrial fibrillation in the Japanese population. Nat. Genet. 2017, 49, 953–958. [Google Scholar] [CrossRef]
- Patel, K.K.; Venkatesan, C.; Abdelhalim, H.; Zeeshan, S.; Arima, Y.; Linna-Kuosmanen, S.; Ahmed, Z. Genomic approaches to identify and investigate genes associated with atrial fibrillation and heart failure susceptibility. Hum. Genom. 2023, 17, 47. [Google Scholar] [CrossRef]
- Wasmer, K.; Eckardt, L.; Breithardt, G. Predisposing factors for atrial fibrillation in the elderly. J. Geriatr. Cardiol. 2017, 14, 179–184. [Google Scholar] [CrossRef]

| Gene | SNP ID | Allele Change | SNP Type | Context Sequence | TaqMan Assay Id |
|---|---|---|---|---|---|
| PITX2 | rs2200733 | C/T | Intergenic | GTGGTACTTGGGTTTTGATTTTGAT[C/T]AGAGAAAATTAGAACAGGTAATATT | C_16158671_10 |
| PITX2 | rs10033464 | T/G | Intergenic | TTTTTACATTGTTAGAGTCAAGAAA[T/G]AAGTGCTTTCATCAAGCTCTGAGTT | C_30351088_30 |
| PITX2 | rs13143308 | T/G | Regulatory region | GATGGATAAACCAAATGCATAAATA[G/T]CTTTGCCTTATTTCTAACCTCTTTG | C_31214706_10 |
| TBX5 | rs883079 | C/T | 3′UTR variant | ATTGGGTGAAATGAAAAATCTTGTC[C/T]GTGGGGTTCTCTTGGCTACTGTCTC | C_7526955_10 |
| PRRX1 | rs3903239 | A/G | Intron | TTTCCTCTGCAATGTTTAATCTGCC[A/G]TTAATCCTGTCCATTGTATTTTTCA | C_8919547_10 |
| ZFHX3 | rs2106261 | C/T | Intron | AGATAGAGCTCGTCCAGAGAATTGT[C/T]CAACCATCCATTAAAATATCCAAGT | C_16097559_10 |
| HAND2 | rs7698692 | G/A | Intergenic | GGGACTTTTAATAAAACCACTTGGG[A/G]ATATTAATGTGATACGTAAGTGGCT | C_310074_10 |
| Demographic Characteristics | Young Adult Population, n = 250 (%) |
|---|---|
| Age (years) (mean ± SD) | 25.81 ± 6.19 |
| Male, n (%) | 173 (69) |
| Female, n (%) | 77 (31) |
| BMI (kg/m2) (mean ± SD) | 24.9 ± 4.2 |
| Normal, n (%) | 125 (50) |
| Overweight, n (%) | 96 (38) |
| Obese, n (%) | 29 (12) |
| SBP (mmHg) | 127.4 ± 15.3 |
| DBP (mmHg) | 84.8 ± 9.1 |
| Alcohol consumption, n (%) | 91(36) |
| Smoking, n (%) | 52 (21) |
| Family History of Obesity, n (%) | 42 (17) |
| Family History of Cardiovascular Disease, n (%) | 38 (15) |
| Family History of Diabetics, n (%) | 58 (23) |
| Gene | SNP ID | Genotype and Alleles | Study Population | 95% CI | ||
|---|---|---|---|---|---|---|
| N = 250 | Percentage (%) | Lower | Upper | |||
| PITX2 | rs2200733 | CC | 177 | 71 | 60 | 82 |
| CT | 65 | 26 | 20 | 33 | ||
| TT | 8 | 3 | 1 | 6 | ||
| C | 419 | 84 | 75 | 92 | ||
| T | 81 | 16 | 12 | 20 | ||
| rs10033464 | GG | 138 | 55 | 46 | 65 | |
| GT | 88 | 35 | 28 | 43 | ||
| TT | 24 | 10 | 6 | 14 | ||
| G | 364 | 73 | 65 | 80 | ||
| T | 136 | 27 | 22 | 32 | ||
| rs13143308 | GG | 117 | 47 | 38 | 56 | |
| TG | 107 | 43 | 35 | 51 | ||
| TT | 26 | 10 | 6 | 15 | ||
| G | 341 | 68 | 61 | 75 | ||
| T | 159 | 32 | 27 | 37 | ||
| TBX5 | rs883079 | CC | 71 | 28 | 22 | 35 |
| CT | 127 | 51 | 42 | 60 | ||
| TT | 52 | 21 | 15 | 27 | ||
| C | 269 | 54 | 47 | 60 | ||
| T | 231 | 46 | 40 | 52 | ||
| PRRX1 | rs3903239 | AA | 144 | 58 | 48 | 67 |
| AG | 88 | 35 | 28 | 43 | ||
| GG | 18 | 7 | 4 | 11 | ||
| A | 376 | 75 | 67 | 83 | ||
| G | 124 | 25 | 20 | 29 | ||
| ZFHX3 | rs2106261 | CC | 138 | 55 | 46 | 65 |
| CT | 94 | 38 | 30 | 46 | ||
| TT | 18 | 7 | 4 | 11 | ||
| C | 370 | 74 | 66 | 81 | ||
| T | 130 | 26 | 21 | 30 | ||
| HAND2 | rs7698692 | AA | 184 | 74 | 63 | 85 |
| AG | 63 | 25 | 19 | 32 | ||
| GG | 3 | 1 | 0 | 3 | ||
| A | 431 | 86 | 78 | 94 | ||
| G | 69 | 14 | 10 | 17 | ||
| Chr:Position: | rsID | Gene | Variant Allele | 1000 Genome | gnomAD | IndiGenome | In Our Study |
|---|---|---|---|---|---|---|---|
| chr4-110789013 | rs2200733 | PITX2 | T | 0.1309 | 0.1338 | 0.1320 | 0.16 |
| chr4-4110799605 | rs10033464 | PITX2 | G | 0.8006 | 0.7767 | 0.7706 | 0.74 |
| chr4-110793263 | rs13143308 | PITX2 | G | 0.6759 | 0.6527 | 0.6466 | 0.68 |
| chr12-114355435 | rs883079 | TBX5 | T | 0.5133 | 0.5198 | 0.5034 | 0.46 |
| chr1-170600176 | rs3903239 | PRRX1 | G | 0.2168 | 0.2232 | 0.2373 | 0.25 |
| chr16-73017721 | rs2106261 | ZFHX3 | T | 0.2393 | 0.2298 | 0.2627 | 0.26 |
| chr4-173682953 | rs7698692 | HAND2 | A | 0.8793 | 0.8775 | 0.8483 | 0.86 |
| Number of Risk Alleles | Study Population | 95% CI | ||
|---|---|---|---|---|
| N = 250 | Percentage (%) | Lower | Upper | |
| 0 | 7 | 3 | 1 | 5 |
| 1 | 23 | 9 | 5 | 13 |
| 2 | 37 | 15 | 10 | 20 |
| 3 | 48 | 19 | 14 | 25 |
| 4 | 50 | 20 | 14 | 26 |
| 5 | 48 | 19 | 14 | 25 |
| 6 | 18 | 7 | 4 | 11 |
| 7 | 11 | 4 | 2 | 7 |
| 8 | 8 | 3 | 1 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugan, M.; Ravikumar, S.; Ganesh, I.; Vetriselvan, Y.; Priyadharshini, A.; Ballambattu, V.B. Prevalence of Genetic Variants Associated with Atrial Fibrillation Risk in the Asymptomatic Young Adult Population. Medicina 2025, 61, 900. https://doi.org/10.3390/medicina61050900
Murugan M, Ravikumar S, Ganesh I, Vetriselvan Y, Priyadharshini A, Ballambattu VB. Prevalence of Genetic Variants Associated with Atrial Fibrillation Risk in the Asymptomatic Young Adult Population. Medicina. 2025; 61(5):900. https://doi.org/10.3390/medicina61050900
Chicago/Turabian StyleMurugan, Manoranjani, Sambandam Ravikumar, Irisappan Ganesh, Yogesh Vetriselvan, Arunagiri Priyadharshini, and Vishnu Bhat Ballambattu. 2025. "Prevalence of Genetic Variants Associated with Atrial Fibrillation Risk in the Asymptomatic Young Adult Population" Medicina 61, no. 5: 900. https://doi.org/10.3390/medicina61050900
APA StyleMurugan, M., Ravikumar, S., Ganesh, I., Vetriselvan, Y., Priyadharshini, A., & Ballambattu, V. B. (2025). Prevalence of Genetic Variants Associated with Atrial Fibrillation Risk in the Asymptomatic Young Adult Population. Medicina, 61(5), 900. https://doi.org/10.3390/medicina61050900







