Exploring the Potential of a P2X3 Receptor Antagonist: Gefapixant in the Management of Persistent Cough Associated with Interstitial Lung Disease
Abstract
1. Introduction
2. Methods
2.1. Study Design and Settings/Participants
2.2. Primary and Secondary Endpoints
2.3. LCQ Score, Cough VAS, and Cough Frequency
2.4. Statistical Analysis
2.5. Ethics Approval and Consent to Participate
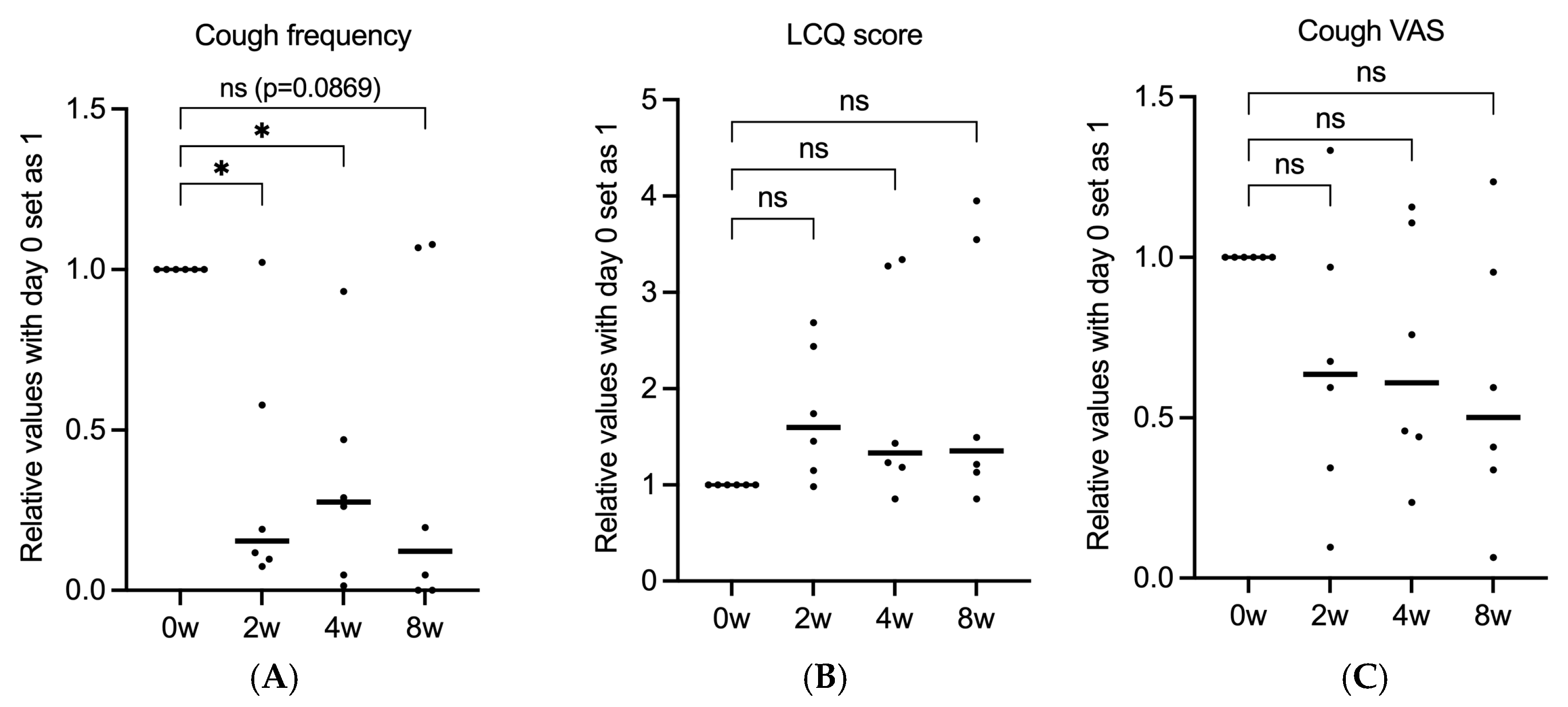
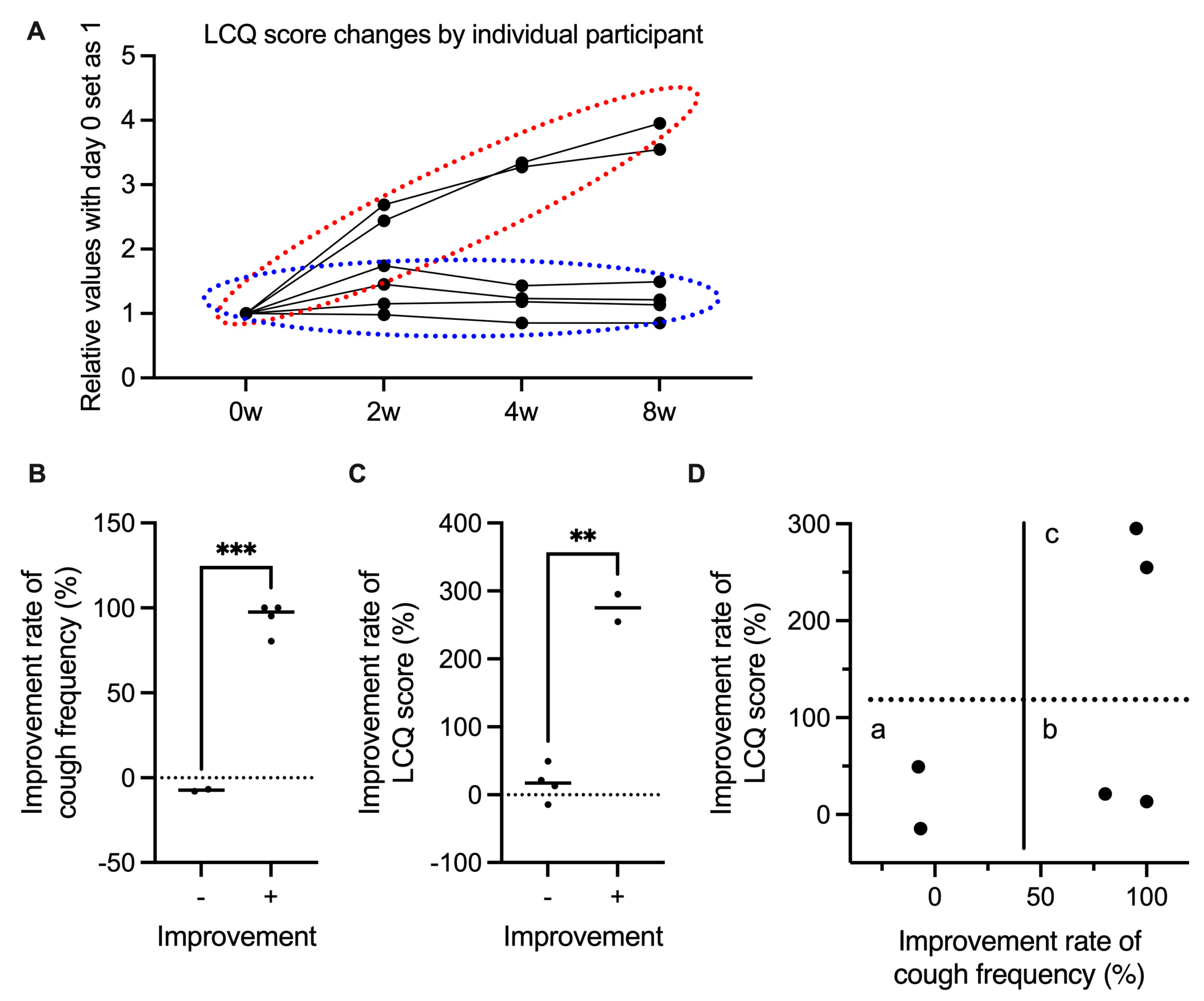
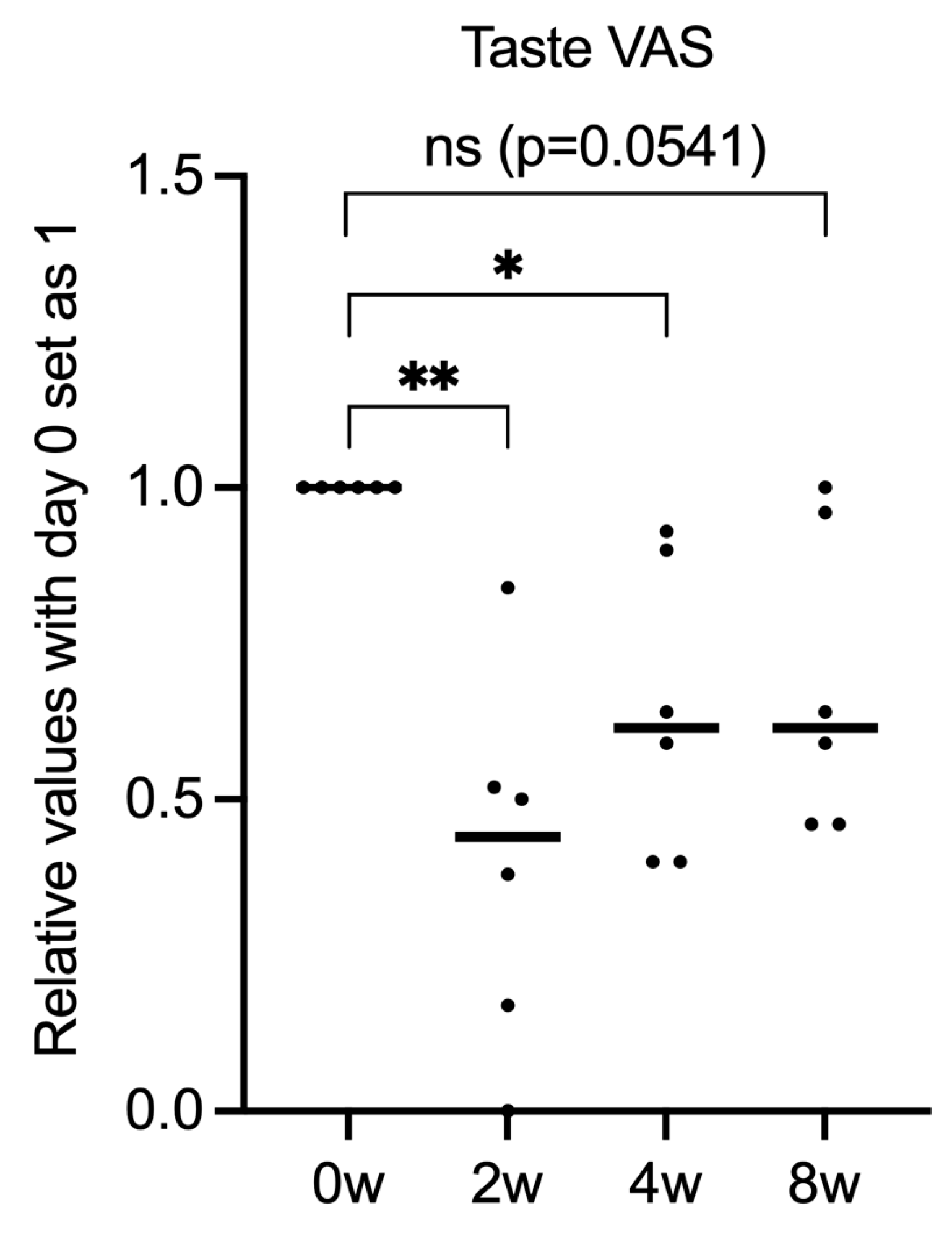
3. Results
3.1. Patient Characteristics
3.2. Gefapixant Tends to Suppress ILD-Associated Chronic Cough
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial lung diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.; George, P.M.; Renzoni, E. Cough in interstitial lung disease. Pulm. Pharmacol. Ther. 2015, 35, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Wakwaya, Y.; Ramdurai, D.; Swigris, J.J. Managing Cough in Idiopathic Pulmonary Fibrosis. Chest 2021, 160, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Hilldrup, S.; Hope-Gill, B.D.; Eccles, R.; Harrison, N.K. Mechanical induction of cough in Idiopathic Pulmonary Fibrosis. Cough 2011, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Riteau, N.; Gasse, P.; Fauconnier, L.; Gombault, A.; Couegnat, M.; Fick, L.; Kanellopoulos, J.; Quesniaux, V.F.; Marchand-Adam, S.; Crestani, B.; et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Morice, A.H.; Kitt, M.M.; Ford, A.P.; Tershakovec, A.M.; Wu, W.C.; Brindle, K.; Thompson, R.; Thackray-Nocera, S.; Wright, C. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: A randomised placebo-controlled study. Eur. Respir. J. 2019, 54, 1900439. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.; Kollarik, M.; Nassenstein, C.; Ru, F.; Undem, B.J. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L858–L865. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Afzal, A.S.; Smith, J.A.; Ford, A.P.; Li, J.J.; Li, Y.; Kitt, M.M.; on behalf of the Chronic Cough in IPF Study Group. Treatment of Persistent Cough in Subjects with Idiopathic Pulmonary Fibrosis (IPF) with Gefapixant, a P2X3 Antagonist, in a Randomized, Placebo-Controlled Clinical Trial. Pulm. Ther. 2021, 7, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Morice, A.H.; Millqvist, E.; Bieksiene, K.; Birring, S.S.; Dicpinigaitis, P.; Domingo Ribas, C.; Hilton Boon, M.; Kantar, A.; Lai, K.; McGarvey, L.; et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur. Respir. J. 2020, 55, 1901136. [Google Scholar] [CrossRef] [PubMed]
- Mukae, H.; Kaneko, T.; Obase, Y.; Shinkai, M.; Katsunuma, T.; Takeyama, K.; Terada, J.; Niimi, A.; Matsuse, H.; Yatera, K.; et al. The Japanese respiratory society guidelines for the management of cough and sputum (digest edition). Respir. Investig. 2021, 59, 270–290. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, Y.; Makino, S.; Ogura, T.; Suda, T.; Tomioka, H.; Amano, H.; Anraku, M.; Enomoto, N.; Fujii, T.; Fujisawa, T.; et al. 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease. Respir. Investig. 2021, 59, 709–740. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, L.P.; Birring, S.S.; Morice, A.H.; Dicpinigaitis, P.V.; Pavord, I.D.; Schelfhout, J.; Nguyen, A.M.; Li, Q.; Tzontcheva, A.; Iskold, B.; et al. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): Results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022, 399, 909–923. [Google Scholar] [CrossRef] [PubMed]
| N | % | |
|---|---|---|
| Sex | ||
| Male | 1 | 83.3 |
| Female | 5 | 16.7 |
| Age (years) | 65.3 ± 12.3 | |
| BMI | 26.4 ± 4.3 | |
| Interstitial lung disease | ||
| IPF | 2 | 33 |
| NSIP | 2 | 33 |
| CTD-ILD | 2 | 33 |
| Comorbidities | ||
| Allergic rhinitis | 1 | 16.7 |
| Bronchial asthma | 1 | 16.7 |
| Chronic obstructive pulmonary disease | 1 | 16.7 |
| Gastroesophageal reflux disease | 0 | 0 |
| Concomitant medications | ||
| Narcotic antitussive | 6 | 100 |
| Systemic corticosteroid | 3 | 50 |
| Drugs for obstructive airways disease | 2 | 33 |
| Antihistamine | 3 | 50 |
| Home oxygen therapy | 4 | 66 |
| Baseline values for efficacy endpoints | ||
| Cough frequency (30 min) | 88.5 ± 48.7 | |
| ICQ score (baseline total score) | 8.3 | 3.1 |
| Cough VAS (mm) | 75.8 ± 16.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, T.; Saito, A.; Yorozuya, T.; Nishikiori, H.; Kuronuma, K.; Chiba, H. Exploring the Potential of a P2X3 Receptor Antagonist: Gefapixant in the Management of Persistent Cough Associated with Interstitial Lung Disease. Medicina 2025, 61, 892. https://doi.org/10.3390/medicina61050892
Takahashi T, Saito A, Yorozuya T, Nishikiori H, Kuronuma K, Chiba H. Exploring the Potential of a P2X3 Receptor Antagonist: Gefapixant in the Management of Persistent Cough Associated with Interstitial Lung Disease. Medicina. 2025; 61(5):892. https://doi.org/10.3390/medicina61050892
Chicago/Turabian StyleTakahashi, Tomoyuki, Atsushi Saito, Takafumi Yorozuya, Hirotaka Nishikiori, Koji Kuronuma, and Hirofumi Chiba. 2025. "Exploring the Potential of a P2X3 Receptor Antagonist: Gefapixant in the Management of Persistent Cough Associated with Interstitial Lung Disease" Medicina 61, no. 5: 892. https://doi.org/10.3390/medicina61050892
APA StyleTakahashi, T., Saito, A., Yorozuya, T., Nishikiori, H., Kuronuma, K., & Chiba, H. (2025). Exploring the Potential of a P2X3 Receptor Antagonist: Gefapixant in the Management of Persistent Cough Associated with Interstitial Lung Disease. Medicina, 61(5), 892. https://doi.org/10.3390/medicina61050892







