Does MYO and ALA Supplementation Improve PCOS Outcomes?
Abstract
1. Introduction
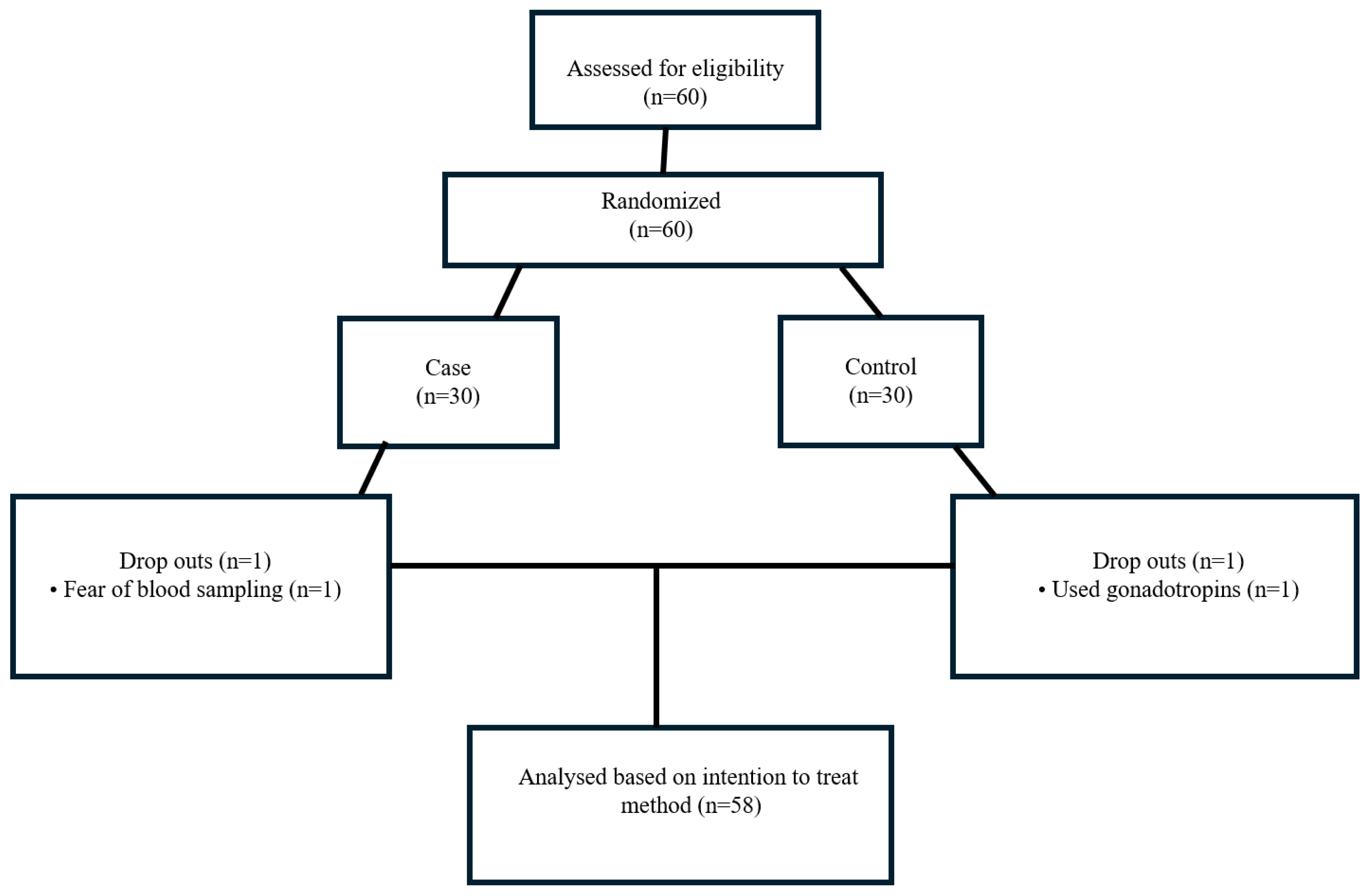
2. Material and Method
2.1. Subjects
2.2. 3 Days Dietary Records and Assessment of Hirsutism
2.3. Biochemical Measurements
2.4. Anthropometric Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, J.; Azziz, R.; Legro, R.; Dewailly, D.; Franks, S.; Tarlatzis, B.C.; The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270. [Google Scholar] [CrossRef]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2009, 25, 544–551. [Google Scholar] [CrossRef]
- Nardo, L.; Patchava, S.; Laing, I. Polycystic ovary syndrome: Pathophysiology, molecular aspects and clinical implications. Panminerva Med. 2008, 50, 267–278. [Google Scholar]
- Xita, N.; Tsatsoulis, A. Fetal programming of polycystic ovary syndrome by androgen excess: Evidence from experimental, clinical, and genetic association studies. J. Clin. Endocrinol. Metab. 2006, 91, 1660–1666. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perna, S.; Faliva, M.; Monteferrario, F.; Repaci, E.; Allieri, F. Focus on metabolic and nutritional correlates of polycystic ovary syndrome and update on nutritional management of these critical phenomena. Arch. Gynecol. Obstet. 2014, 290, 1079–1092. [Google Scholar] [CrossRef]
- Papavasiliou, K.; Papakonstantinou, E. Nutritional support and dietary interventions for women with polycystic ovary syndrome. Nutr. Diet. Suppl. 2017, 9, 63–85. [Google Scholar] [CrossRef]
- Alesi, S.; Ee, C.; Moran, L.J.; Rao, V.; Mousa, A. Nutritional supplements and complementary therapies in polycystic ovary syndrome. Adv. Nutr. 2022, 13, 1243–1266. [Google Scholar] [CrossRef]
- De Cicco, S.; Immediata, V.; Romualdi, D.; Policola, C.; Tropea, A.; Di Florio, C.; Tagliaferri, V.; Scarinci, E.; Della Casa, S.; Lanzone, A.; et al. Myoinositol combined with alpha-lipoic acid may improve the clinical and endocrine features of polycystic ovary syndrome through an insulin-independent action. Gynecol. Endocrinol. 2017, 33, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Di Tucci, C.; Di Feliciantonio, M.; Vena, F.; Capone, C.; Schiavi, M.C.; Pietrangeli, D.; Muzii, L.; Benedetti Panici, P. Alpha lipoic acid in obstetrics and gynecology. Gynecol. Endocrinol. 2018, 34, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.; Shefer, K.; Della Casa, D.; Prati, A.; Napolitano, A.; Manzo, A.; Despini, G.; Simoncini, T. Modulatory effects of alpha-lipoic acid (ALA) administration on insulin sensitivity in obese PCOS patients. J. Endocrinol. Investig. 2018, 41, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Pundir, J.; Psaroudakis, D.; Savnur, P.; Bhide, P.; Sabatini, L.; Teede, H.; Coomarasamy, A.; Thangaratinam, S. Inositol treatment of anovulation in women with polycystic ovary syndrome: A meta-analysis of randomised trials. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 299–308. [Google Scholar] [CrossRef]
- Coldebella, D.; Buzzaccarini, G.; Ferrari, J.; Sleiman, Z.; D’alterio, M.; Della Corte, L.; Cucinella, G.; Gullo, G. Inositols administration: Further insights on their biological role. Ital. J. Gynaecol. Obstet. 2023, 35, 30–36. [Google Scholar] [CrossRef]
- Gullo, G.; Carlomagno, G.; Unfer, V.; D’Anna, R. Myo-inositol: From induction of ovulation to menopausal disorder management. Minerva Ginecol. 2015, 67, 485–486. [Google Scholar]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Unfer, V.; Carlomagno, G.; Dante, G.; Facchinetti, F. Effects of myo-inositol in women with PCOS: A systematic review of randomized controlled trials. Gynecol. Endocrinol. 2012, 28, 509–515. [Google Scholar] [CrossRef]
- Unfer, V.; Nestler, J.E.; Kamenov, Z.A.; Prapas, N.; Facchinetti, F. Effects of Inositol(s) in Women with PCOS: A Systematic Review of Randomized Controlled Trials. Int. J. Endocrinol. 2016, 2016, 1849162. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Ma, X.; He, P.; Sun, P.; Han, P. Lipoic acid: An immunomodulator that attenuates glycinin-induced anaphylactic reactions in a rat model. J. Agric. Food Chem. 2010, 58, 5086–5092. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Song, K.-H.; Koh, E.H.; Won, J.C.; Kim, H.S.; Park, H.-S.; Kim, M.S.; Kim, S.W.; Lee, K.U.; Park, J.Y. α-Lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.W.; Zhu, M.J.; Tong, J.; Ren, J.; Du, M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by α-lipoic acid in C2C12 myotubes. Am. J. Physiol.-Cell Physiol. 2007, 293, C1395–C1403. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E. AMPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Guarano, A.; Capozzi, A.; Cristodoro, M.; Di Simone, N.; Lello, S. Alpha Lipoic Acid Efficacy in PCOS Treatment: What Is the Truth? Nutrients 2023, 15, 3209. [Google Scholar] [CrossRef]
- Laganà, A.S.; Monti, N.; Fedeli, V.; Gullo, G.; Bizzarri, M. Does Alpha-lipoic acid improve effects on polycystic ovary syndrome? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1241–1247. [Google Scholar]
- Genazzani, A.; Despini, G.; Santagni, S.; Prati, A.; Rattighieri, E.; Chierchia, E.; Simoncini, T. Effects of a combination of alpha lipoic acid and myo-inositol on insulin dynamics in overweight/obese patients with PCOS. Endocrinol. Metab. Synd. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Fidecicchi, T.; Benelli, E.; Baldari, F.; Tonacchera, M. Body mass index is a good predictor of metabolic abnormalities in polycystic ovary syndrome. J. Endocrinol. Investig. 2024, 47, 927–936. [Google Scholar] [CrossRef]
- Cianci, A.; Panella, M.; Fichera, M.; Falduzzi, C.; Bartolo, M.; Caruso, S. D-chiro-Inositol and alpha lipoic acid treatment of metabolic and menses disorders in women with PCOS. Gynecol. Endocrinol. 2015, 31, 483–486. [Google Scholar] [CrossRef]
- Schmid, M. Beslenme Bilgi Sistemi BEBİS 8.1 Paket Program; Entwickelt an der Universital Hohenheim: Struttgart, Germany, 2011. [Google Scholar]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Ferriman, D.; Gallwey, J. Clinical assessment of body hair growth in women. J. Clin. Endocrinol. Metab. 1961, 21, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Deurenberg, P.; Weststrate, J.A.; Seidell, J.C. Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–114. [Google Scholar] [CrossRef]
- Watson, P.E.; Watson, I.D. The body water compartments: The influence of body weight and height on body water content. Am. J. Clin. Nutr. 1980, 33, 27–29. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- Pekcan, G. Hastanın Beslenme Durumunun Saptanması. Diyet El Kitabı. 5; Hatiboğlu Yayınevi: Ankara, Turkey, 2008; pp. 67–143. [Google Scholar]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Ashwell, M.; Hsieh, S.D. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef]
- Cappelli, V.; Di, A.S.; Musacchio, M.; De, V.L. Evaluation of a new association between insulin-sensitizers and α-lipoic acid in obese women affected by PCOS. Minerva Ginecol. 2013, 65, 425–433. [Google Scholar]
- Rago, R.; Marcucci, I.; Leto, G.; Caponecchia, L.; Salacone, P.; Bonanni, P.; Fiori, C.; Sorrenti, G.; Sebastianelli, A. Effect of myo-inositol and alpha-lipoic acid on oocyte quality in polycystic ovary syndrome non-obese women undergoing in vitro fertilization: A pilot study. J. Biol. Regul. Homeost. Agents 2015, 29, 913–923. [Google Scholar]
- Capasso, I.; Esposito, E.; Maurea, N.; Montella, M.; Crispo, A.; De Laurentiis, M.; D’Aiuto, M.; Frasci, G.; Botti, G.; Grimaldi, M.; et al. Combination of inositol and alpha lipoic acid in metabolic syndrome-affected women: A randomized placebo-controlled trial. Trials 2013, 14, 273. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Fidecicchi, T.; Palla, G.; Gambacciani, M. Long-term treatment with alpha-lipoic acid and myo-inositol positively affects clinical and metabolic features of polycystic ovary syndrome. Gynecol. Endocrinol. 2019, 36, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Fruzzetti, F.; Benelli, E.; Fidecicchi, T.; Tonacchera, M. Clinical and Metabolic Effects of Alpha-Lipoic Acid Associated with Two Different Doses of Myo-Inositol in Women with Polycystic Ovary Syndrome. Int. J. Endocrinol. 2020, 2020, 2901393. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Catellani, C.; Lazzeroni, P.; Sartori, C.; Tridenti, G.; Vezzani, C.; Fulghesu, A.M.; Madeddu, E.; Amarri, S.; Street, M.E. HMGB1 is increased in adolescents with polycystic ovary syndrome (PCOS) and decreases after treatment with myo-inositol (MYO) in combination with alpha-lipoic acid (ALA). Gynecol. Endocrinol. 2020, 36, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Despini, G.; Marini, G.; Prati, A.; Simoncini, T. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol. Endocrinol. 2014, 30, 438–443. [Google Scholar] [CrossRef]
- Wong, B.W.; Meredith, A.; Lin, D.; McManus, B.M. The biological role of inflammation in atherosclerosis. Can. J. Cardiol. 2012, 28, 631–641. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; González, F. Circulating inflammatory markers in polycystic ovary syndrome: A systematic review and metaanalysis. Fertil. Steril. 2011, 95, 1048–1058.e1042. [Google Scholar] [CrossRef]
- Barrea, L.; Marzullo, P.; Muscogiuri, G.; Di Somma, C.; Scacchi, M.; Orio, F.; Aimaretti, G.; Colao, A.; Savastano, S. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr. Res. Rev. 2018, 31, 291–301. [Google Scholar] [CrossRef]
| Case Group (n = 29) | Control Group (n = 29) | p *# | |||||
|---|---|---|---|---|---|---|---|
| Age | 29.65 ± 4.20 | 29.31 ± 4.78 | p = 0.772 | ||||
| Baseline | 3 months | p 1*# | Baseline | 3 months | p 2*# | p 3**# | |
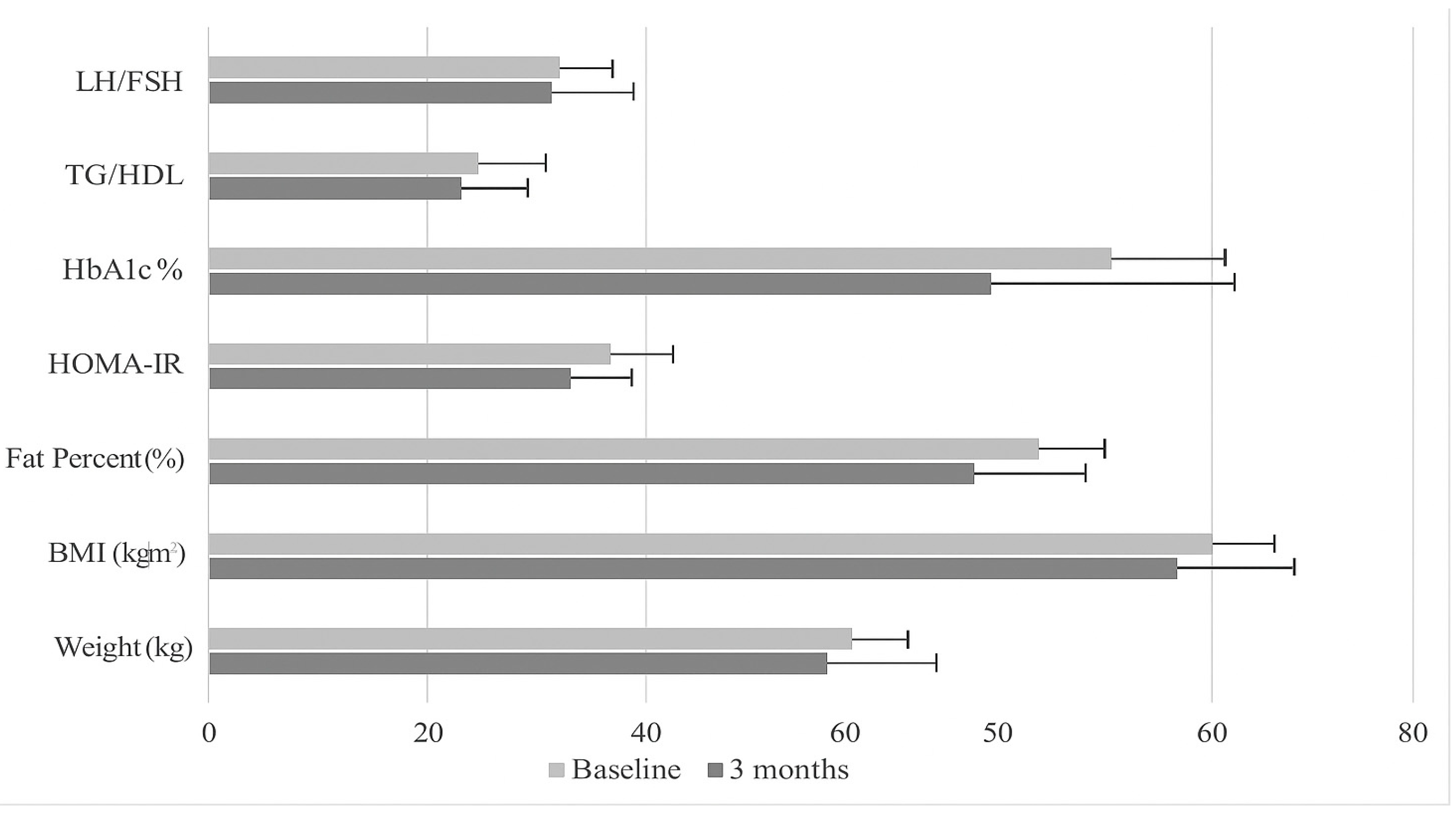
| Weight (kg) | 69.14 ± 10.68 | 68.37 ± 11.24 | p = 0.484 | 68.36 ± 14.22 | 70.31 ± 15.78 | p = 0.029 | p = 0.592 |
| BMI (kg/m2) | 26.62 ± 4.38 | 26.2 ± 4.45 | p = 0.405 | 25.50 ± 4.63 | 26.20 ± 5.04 | p = 0.026 | p = 0.998 |
| Fat Percent (%) | 32.40 ± 6.48 | 31.77 ± 6.58 | p = 0.406 | 30.74 ± 6.86 | 31.78 ± 7.46 | p = 0.026 | p = 0.998 |
| BMR (kcal) | 1475.98 ± 112.14 | 1468.47 ± 115.87 | p = 0.475 | 1473.04 ± 139.67 | 1492.53 ± 152.46 | p = 0.024 | p = 0.501 |
| Case Group (n = 29) | Control Group (n = 29) | |||||
|---|---|---|---|---|---|---|
| Variable | BMI < 25 kg/m2 (n = 11) | BMI ≥ 25 kg/m2 (n = 18) | p # | BMI < 25 kg/m2 (n = 11) | BMI ≥ 25 kg/m2 (n = 18) | p # |
| HbA1c (%) | 5.51 ± 0.17 | 5.76 ± 0.32 | p = 0.013 | 5.43 ± 0.21 | 5.86 ± 0.39 | p = 0.172 |
| HOMA-IR | 2.82 ± 1.56 | 3.21 ± 1.61 | p * = 0.528 | 2.25 ± 1.01 | 3.37 ± 2.31 | p = 0.400 |
| TG/HDL | 2.05 ± 0.56 | 2.64 ± 1.48 | p * = 0.719 | 1.39 ± 0.82 | 3.59 ± 2.44 | p * = 0.002 |
| WC < 80 cm (n = 13) | WC ≥ 80 cm (n = 16) | WC < 80 cm (n = 14) | WC ≥ 80 cm (n = 15) | |||
| HbA1c (%) | 5.49 ± 0.16 | 5.80 ± 0.31 | p = 0.002 | 5.44 ± 0.23 | 5.91 ± 0.38 | p < 0.001 |
| HOMA-IR | 2.62 ± 1.51 | 3.43 ± 1.57 | p = 0.168 | 2.22 ± 0.99 | 3.54 ± 2.39 | p * = 0.239 |
| TG/HDL | 1.94 ± 0.59 | 2.81 ± 1.49 | p = 0.044 | 1.39 ± 0.77 | 3.88 ± 2.45 | p * < 0.001 |
| Waist/Hip < 0.85 (n = 24) | Waist/Hip ≥ 0.85 (n = 5) | Waist/Hip < 0.85 (n = 25) | Waist/Hip ≥ 0.85 (n = 4) | |||
| HbA1c (%) | 5.61 ± 0.28 | 5.92 ± 0.24 | p * = 0.020 | 5.64 ± 0.39 | 5.95 ± 0.33 | p = 0.149 |
| HOMA-IR | 3.25 ± 1.49 | 2.18 ± 1.81 | p = 0.172 | 2.65 ± 1.79 | 4.51 ± 2.51 | p * = 0.067 |
| TG/HDL | 2.29 ± 1.18 | 3.08 ± 1.44 | p * = 0.326 | 2.17 ± 1.63 | 5.88 ± 2.95 | p * = 0.010 |
| Waist/Height < 0.50 (n = 14) | Waist/Height ≥ 0.50 (n = 15) | Waist/Height < 0.50 (n = 13) | Waist/Height ≥ 0.50 (n = 16) | |||
| HbA1c (%) | 5.49 ± 0.15 | 5.82 ± 0.31 | p = 0.002 | 5.42 ± 0.22 | 5.91 ± 0.36 | p < 0.001 |
| HOMA-IR | 2.83 ± 1.66 | 3.28 ± 1.50 | p = 0.452 | 2.30 ± 0.98 | 3.39 ± 2.39 | p * = 0.511 |
| TG/HDL | 1.92 ± 0.56 | 2.89 ± 1.51 | p = 0.093 | 1.37 ± 0.79 | 3.72 ± 2.43 | p * < 0.001 |
| Variable | Case Group (n = 29) | p 1*# | Control Group (n = 29) | p 2*# | p 3**# | ||
|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | ||||
| Glucose (mg/dL) | 95.10 ± 10.10 | 93.44 ± 1.06 | p β = 0.437 | 95.72 ± 10.74 | 91.41 ± 10.47 | p = 0.045 | p = 0.477 |
| HbA1c (%) | 5.66 ± 0.42 | 5.67 ± 0.29 | p = 0.892 | 5.66 ± 0.40 | 5.69 ± 0.39 | p = 0.656 | p β = 0.950 |
| Insulin (μU/mL) | 13.57 ± 8.56 | 13.24 ± 6.97 | p = 0.885 | 10.47 ± 5.36 | 12.81 ± 8.31 | p = 0.136 | p = 0.570 |
| HOMA-IR | 3.28 ± 2.42 | 3.06 ± 1.57 | p = 0.854 | 2.48 ± 1.29 | 2.91 ± 1.94 | p = 0.265 | p = 0.441 |
| HDL (mg/dL) | 49.91 ± 6.29 | 53.05 ± 9.00 | p = 0.071 | 52.95 ± 11.89 | 52.96 ± 15.09 | p = 0.957 | p = 0.624 |
| LDL (mg/dL) | 128.02 ± 22.22 | 120.08 ± 25.16 | p = 0.024 | 126.65 ± 30.32 | 119.14 ± 29.78 | p = 0.090 | p β = 0.87 |
| Cholesterol (mg/dL) | 190.85 ± 26.27 | 185.45 ± 30.51 | p = 0.191 | 191.65 ± 34.01 | 186.31 ± 33.24 | p = 0.294 | p β = 0.630 |
| TG (mg/dL) | 119.97 ± 54.82 | 123.52 ± 60.19 | p = 0.619 | 102.03 ± 49.51 | 120.14 ± 71.86 | p = 0.116 | p = 0.343 |
| TG/HDL | 2.45 ± 1.11 | 2.42 ± 1.23 | p = 0.534 | 2.12 ± 1.34 | 2.68 ± 2.21 | p = 0.127 | p = 0.509 |
| TSH (uIU/mL) | 2.33 ± 1.10 | 2.90 ± 4.30 | p = 0.370 | 1.89 ± 1.12 | 1.86 ± 1.14 | p = 0.729 | p = 0.152 |
| Progesterone (ng/mL) | 0.97 ± 2.40 | 0.59 ± 1.27 | p = 0.013 | 0.65 ± 0.86 | 0.51 ± 1.34 | p = 0.006 | p = 0.276 |
| E2 (pg/mL) | 42.81 ± 16.78 | 57.57 ± 47.72 | p = 0.165 | 62.81 ± 98.72 | 50.57 ± 59.05 | p = 0.785 | p = 0.015 |
| LH (mlU/mL) | 11.69 ± 8.68 | 11.18 ± 6.64 | p = 0.561 | 10.39 ± 12.12 | 9.20 ± 5.69 | p = 0.658 | p = 0.111 |
| FSH (mlU/mL) | 6.23 ± 1.41 | 5.78 ± 1.40 | p β = 0.190 | 7.59 ± 1.93 | 7.23 ± 4.21 | p = 0.005 | p = 0.078 |
| LH/FSH | 1.87 ± 0.35 | 1.49 ± 0.80 | p = 0.325 | 1.34 ± 1.04 | 1.79 ± 0.92 | p = 0.010 | p = 0.199 |
| CRP (mg/L) | 1.45 ± 1.63 | 3.64 ± 6.58 | p = 0.041 | 2.13 ± 2.48 | 3.21 ± 3.25 | p = 0.048 | p = 0.756 |
| Correlation | Ferriman−Gallwey Score | |||||
|---|---|---|---|---|---|---|
| Case Group (n = 29) | Control Group (n = 29) | Total (n = 58) | ||||
| r | p # | r | p # | r | p # | |
| HbA1c (%) | 0.193 | 0.316 | −0.270 | 0.156 | −0.039 | 0.772 |
| Insulin (μU/mL) | −0.061 | 0.755 | −0.334 | 0.077 | −0.172 | 0.196 |
| HOMA−IR | −0.056 | 0.775 | −0.324 | 0.086 | −0.152 | 0.256 |
| DHEAS (ug/dl) | 0.388 | 0.037 | −0.135 | 0.486 | 0.208 | 0.117 |
| Progesterone (ng/mL) | −0.080 | 0.678 | 0.042 | 0.829 | 0.018 | 0.891 |
| E2 (pg/mL) | 0.001 | 0.997 | 0.037 | 0.849 | 0.071 | 0.595 |
| Androstenodione (ng/mL) | 0.388 | 0.038 | −0.074 | 0.702 | 0.262 | 0.047 |
| LH (mlU/mL) | 0.108 | 0.578 | 0.099 | 0.609 | 0.172 | 0.198 |
| FSH (mlU/mL) | −0.010 | 0.961 | 0.035 | 0.859 | −0.037 | 0.783 |
| LH/FSH | −0.360 | 0.055 | 0.020 | 0.920 | −0.226 | 0.087 |
| CRP (mg/L) | −0.345 | 0.066 | −0.123 | 0.524 | −0.239 | 0.070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firat, S.; Elter, K.; Ateş, S.; Fisunoğlu, M. Does MYO and ALA Supplementation Improve PCOS Outcomes? Medicina 2025, 61, 885. https://doi.org/10.3390/medicina61050885
Firat S, Elter K, Ateş S, Fisunoğlu M. Does MYO and ALA Supplementation Improve PCOS Outcomes? Medicina. 2025; 61(5):885. https://doi.org/10.3390/medicina61050885
Chicago/Turabian StyleFirat, Selma, Koray Elter, Sinan Ateş, and Mehmet Fisunoğlu. 2025. "Does MYO and ALA Supplementation Improve PCOS Outcomes?" Medicina 61, no. 5: 885. https://doi.org/10.3390/medicina61050885
APA StyleFirat, S., Elter, K., Ateş, S., & Fisunoğlu, M. (2025). Does MYO and ALA Supplementation Improve PCOS Outcomes? Medicina, 61(5), 885. https://doi.org/10.3390/medicina61050885






