Thermography in Stroke—A Systematic Review
Abstract
1. Introduction
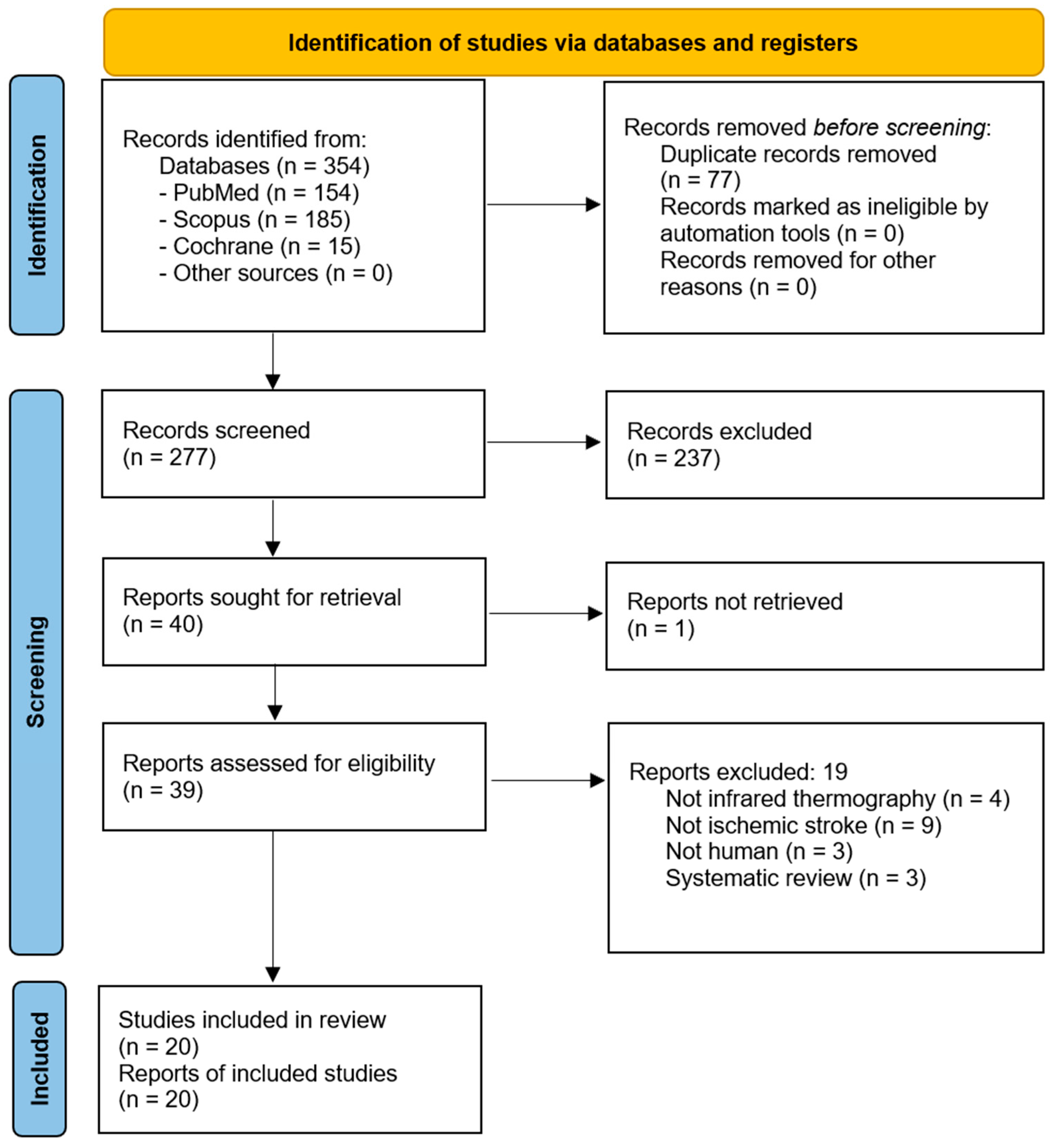
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Eligibility Criteria and PICO Framework
2.4. Data Extraction and Management
2.5. Statistical Analysis
2.6. Data Availability Statement
3. Results
3.1. Study Design and Settings
3.2. Instructions Prior to Imaging
3.3. Room Settings
3.4. Visualized Body Parts
3.5. Intended Use
3.6. Limitations of Thermography
3.7. Risk of Bias
4. Discussion
4.1. Thermography in Medicine
4.2. Acute Versus Chronic Stroke
4.3. Thermography Cameras and Systems
4.4. Strengths and Limitations of the Included Studies
4.5. Comparison with Established Imaging Modalities
4.6. Limitations
4.7. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROBINS-I | Risk Of Bias In Non-Randomized Studies—of Interventions |
| CVA | Cerebrovascular Accident |
| BST | Body Surface Temperature |
| LD | Laterality Difference |
| VAS | Visual Analog Scale |
| NCV | Noncentral Vertigo |
| IRIS | Infrared Imaging System |
| AI | Artificial Intelligence |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
References
- Sanches, I.; Brioschi, M.; Colman, D.; Adrat, E.; Andreoli, V.; Vargas, J. Identification of Altered Physiological Stress Response Using Infrared Thermography Monitoring. Thermol. Int. 2011, 21, 145. [Google Scholar]
- Brioschi, M.; Silva, F.; Matias, J.; Dias, F.; Vargas, J. Infrared Imaging for Emergency Medical Services (EMS): Using an IR Camera to Identify Life-Threatening Emergencies. Inframation 2008. Available online: https://infraredsolutions.co.nz/wp-content/uploads/2020/05/Thermal-imaging-use-by-ambulance-services..pdf (accessed on 16 April 2025).
- Uematsu, S.; Edwin, D.H.; Jankel, W.R.; Kozikowski, J.; Trattner, M. Quantification of Thermal Asymmetry. Part 1: Normal Values and Reproducibility. J. Neurosurg. 1988, 69, 552–555. [Google Scholar] [CrossRef]
- Lubkowska, A.; Chudecka, M. Thermal Characteristics of Breast Surface Temperature in Healthy Women. Int. J. Environ. Res. Public Health 2021, 18, 1097. [Google Scholar] [CrossRef]
- García Becerra, A.; Olguín Tiznado, J.E.; García Alcaraz, J.L.; Camargo Wilson, C.; López Barreras, J.A.; Cano Gutiérrez, J.C.; Garcia-Rivera, R.B. Temperature Asymmetry Analysis between Left and Right Wrist with Sensory and Infrared Thermography. Int. J. Environ. Res. Public Health 2022, 19, 10240. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Zhao, M.; Guan, L.; Wang, Y. The Association of Autonomic Nervous System Function with Ischemic Stroke, and Treatment Strategies. Front. Neurol. 2020, 10, 1411. [Google Scholar] [CrossRef]
- Mankoo, A.; Roy, S.; Davies, A.; Panerai, R.B.; Robinson, T.G.; Brassard, P.; Beishon, L.C.; Minhas, J.S. The Role of the Autonomic Nervous System in Cerebral Blood Flow Regulation in Stroke: A Review. Auton. Neurosci. 2023, 246, 103082. [Google Scholar] [CrossRef]
- Korpelainen, J.T.; Sotaniemi, K.A.; Myllylä, V.V. Asymmetrical Skin Temperature in Ischemic Stroke. Stroke 1995, 26, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Ng, E.Y.K.; Canchi, T.; Tee, J.J.; Ng, L.K. Relation Between Neck Skin Temperature Measurement and Carotid Artery Stenosis: In-Vitro Evaluation. J. Biomech. Eng. 2020, 142, 114501. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, S.; Petrov, I.; Stankov, Z.; Janevska, L.; Adam, G. Infrared Thermography Imaging as a Diagnostic Tool in the Case of Acute Lower Limb Ischemia. Bulg. Cardiol. 2022, 28, 106–110. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Podlasek, A. Thermography in Stroke—A Systematic Review Protocol. Open Sci. Found. 2024. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Branco, J.H.L.; Branco, R.L.L.; Siqueira, T.C.; de Souza, L.C.; Dalago, K.M.S.; Andrade, A. Clinical Applicability of Infrared Thermography in Rheumatic Diseases: A Systematic Review. J. Therm. Biol. 2022, 104, 103172. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, G.; Capone, G.; Frize, M.; Zaffagnini, S.; Candrian, C.; Filardo, G. Infrared Thermography for the Evaluation of Inflammatory and Degenerative Joint Diseases: A Systematic Review. Cartilage 2021, 13, 1790S–1801S. [Google Scholar] [CrossRef]
- Dias de Lacerda, A.P.; Rodrigues de Andrade, P.; Kamonseki, D.H.; Parizotto, N.A.; Alves da Silva, A.S.; Bernardo de Medeiros, L.; de Almeida Ferreira, J.J. Accuracy of Infrared Thermography in Detecting Tendinopathy: A Systematic Review with Meta-Analysis. Phys. Ther. Sport. 2022, 58, 117–125. [Google Scholar] [CrossRef]
- Jensen, R.O.; Lo, R.S.L.; Kellett, J.G.; Graham, C.A.; Brabrand, M. Evaluation of Systemic Vasoconstriction and Prognosis Using Thermography: A Systematic Review. Acute Med. 2021, 20, 131–139. [Google Scholar] [CrossRef]
- Stanley, S.A.; Divall, P.; Thompson, J.P.; Charlton, M. Uses of Infrared Thermography in Acute Illness: A Systematic Review. Front. Med. 2024, 11, 1412854. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.; Berentson-Shaw, J. Thermography as a Screening and Diagnostic Tool: A Systematic Review. N. Z. Med. J. 2012, 125, 80–91. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Chapter 3: Defining the Criteria for Including Studies and How They Will Be Grouped for the Synthesis. Available online: https://training.cochrane.org/handbook/current/chapter-03 (accessed on 16 April 2025).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis|Request PDF. Available online: https://www.researchgate.net/publication/261773681_The_Newcastle-Ottawa_Scale_NOS_for_Assessing_the_Quality_of_Non-Randomized_Studies_in_Meta-Analysis (accessed on 11 April 2025).
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a Critical Appraisal Tool to Assess the Quality of Cross-Sectional Studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef]
- 7. Systematic Reviews of Etiology and Risk. JBI Manual for Evidence Synthesis. JBI Global Wiki. Available online: https://jbi-global-wiki.refined.site/space/MANUAL/355598596/7.+Systematic+reviews+of+etiology+and+risk (accessed on 11 April 2025).
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Podlasek, A. Thermography in Stroke—A Systematic Review. Open Sci. Found. 2024. [Google Scholar] [CrossRef]
- Park, S.; Song, J.; Woo, H.G.; Chang, D.; Kim, S.B.; Heo, S.H. Transient Asymmetric Skin Temperature during the Acute Phase of Lacunar Infarction in the Basal Ganglia: A Case Report. Int. J. Pain. 2022, 13, 103–107. [Google Scholar] [CrossRef]
- Piskorz, J.; Wójcik, G.; Iłżecka, J.; Bulikowski, W. The Use of Thermographic Camera in Stroke Diagnostics—Introductory Report. Health Probl. Civiliz. 2016, 10, 14–19. [Google Scholar] [CrossRef]
- Stokholm, J.; Ahmed, A.A.B.O.; Birkmose, L.K.H.; Csillag, C.; Kjær, T.W.; Christensen, T. Facial Skin Temperature in Acute Stroke Patients with Delirium—A Pilot Study. J. Neurol. Sci. 2021, 431, 120036. [Google Scholar] [CrossRef]
- Takahashi, M.; Shinya, A.; Ito, N.; Ebina, J.; Abe, K.; Inaba, A.; Orimo, S. Utility of Thermographic Measurements of Laterality of Body Surface Temperature to Prevent Misdiagnosis of Acute Wallenberg’s Syndrome. Brain Behav. 2018, 8, e01040. [Google Scholar] [CrossRef]
- Takahashi, M.; Nanatsue, K.; Itaya, S.; Abe, K.; Sakata, A.; Inaba, A. Usefulness of Thermography for Differentiating Wallenberg’s Syndrome from Noncentral Vertigo in the Acute Phase. Neurol. Res. 2024, 46, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, F.M.; Massaro, A.R.; Filippo, T.R.; Portes, L.A.; Battistella, L.R. Evaluation of Body Temperature in Individuals with Stroke. NeuroRehabilitation 2017, 40, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, F.M.; Dias, C.D.S.; Santos, A.C.A.D.; Battistella, L.R. Acute Effect of Robotic Therapy (G-EO SystemTM) on the Lower Limb Temperature Distribution of a Patient with Stroke Sequelae. Case Rep. Neurol. Med. 2019, 2019, 8408492. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, F.M.; Da Silva Dias, C.; Dos Santos, A.C.A.; Battistella, L.R. Comparison of Sensitivity and Plantar Cutaneous Temperature of Patients with Stroke and Diabetes Mellitus: A Pilot Case-Control Study. Technol. Health Care 2020, 28, 129–134. [Google Scholar] [CrossRef]
- Alfieri, F.M.; dos Santos, A.C.A.; da Silva Dias, C.; Battistella, L.R. The Concordance Study of the Portable Camera FLIR C5 for Detecting Asymmetry of Skin Temperature in Patients with Stroke Sequelae. Thermol. Int. 2023, 33, 5–12. [Google Scholar]
- da Silva Dias, C.; Alfieri, F.M.; dos Santos, A.C.A.; Battistella, L.R. Body Temperature and Esthesia in Individuals with Stroke. Sci. Rep. 2021, 11, 10106. [Google Scholar] [CrossRef]
- da Silva Dias, C.; Alfieri, F.M.; dos Santos, A.C.A.; Battistella, L.R. Whole-Body Thermographic Assessment of Patients with Stroke Sequelae Who Report Temperature Differences between the Sides. Thermol. Int. 2022, 32, 14–19. [Google Scholar]
- Gomes, G.G.C.; Palinkas, M.; da Silva, G.P.; Gonçalves, C.R.; Lopes, R.F.T.; Verri, E.D.; Fabrin, S.C.V.; Fioco, E.M.; Siéssere, S.; Regalo, S.C.H. Bite Force, Thickness, and Thermographic Patterns of Masticatory Muscles Post-Hemorrhagic Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106173. [Google Scholar] [CrossRef]
- Hegedűs, B. The Potential Role of Thermography in Determining the Efficacy of Stroke Rehabilitation. J. Stroke Cerebrovasc. Dis. 2018, 27, 309–314. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, D.-I.; Kim, K.-S.; Kim, D.-O.; Choi, Y.-K.; Choi, D.-Y.; Lim, S.; Lee, J.-D.; Lee, Y.-H. Correlations between the Changes of Pain and Temperature by Infrared Thermography Central Poststroke Pain. Key Eng. Mater. 2006, 321–323, 831–834. [Google Scholar] [CrossRef]
- Nowak, I.; Mraz, M.; Mraz, M. Thermography Assessment of Spastic Lower Limb in Patients after Cerebral Stroke Undergoing Rehabilitation. J. Therm. Anal. Calor. 2020, 140, 755–762. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.-L.; Ruescas-Nicolau, M.-A.; Carrasco, J.J.; Espí-López, G.-V.; Pérez-Alenda, S. Cross-Sectional Study of Quadriceps Properties and Postural Stability in Patients with Chronic Stroke and Limited vs. Non-Limited Community Ambulation. Top. Stroke Rehabil. 2019, 26, 503–510. [Google Scholar] [CrossRef]
- Satoh, M.; Terada, S.; Onouchi, K.; Takeda, K.; Kuzuhara, S. Somatosensory and Skin Temperature Disturbances Caused by Infarction of the Postcentral Gyrus: A Case Report. J. Neurol. 2002, 249, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Wanklyn, P.; Ilsley, D.W.; Greenstein, D.; Hampton, I.F.; Roper, T.A.; Kester, R.C.; Mulley, G.P. The Cold Hemiplegic Arm. Stroke 1994, 25, 1765–1770. [Google Scholar] [CrossRef]
- Zanona, A.D.F.; De Souza, R.F.; Aidar, F.J.; De Matos, D.G.; Santos, K.M.B.; Paixão, M.D.C.; Sampaio, P.Y.S.; Almeida Junior, H.; Monte-Silva, K.K. Use of Virtual Rehabilitation to Improve the Symmetry of Body Temperature, Balance, and Functionality of Patients with Stroke Sequelae. Ann. Neurosci. 2019, 25, 166–173. [Google Scholar] [CrossRef]
- Hegedus, B.; Hegedus, S. Treating Poststroke Limb Pain. Lasers Med. Sci. 2017, 32, 1707. [Google Scholar] [CrossRef]
- Takahashi, M.; Shinya, A.; Choh, Y.; Itaya, S.; Inaba, A.; Orimo, S. Portable Thermographic Screening for Detection of Acute Wallenberg’s Syndrome. J. Vis. Exp. 2019, 151, e59330. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Wang, W.; Jin, S.; Piao, H.; Jiang, Y.; Li, N.; Yao, H. Infrared Thermography in Clinical Practice: A Literature Review. Eur. J. Med. Res. 2025, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.C.; Gorbach, A.M.; Pluta, R.M.; Rak, R.; Heiss, J.D.; Oldfield, E.H. Real-Time Detection of Vascular Occlusion and Reperfusion of the Brain during Surgery by Using Infrared Imaging. J. Neurosurg. 2002, 96, 918–923. [Google Scholar] [CrossRef]
- Wilson, A.N.; Gupta, K.A.; Koduru, B.H.; Kumar, A.; Jha, A.; Cenkeramaddi, L.R. Recent Advances in Thermal Imaging and Its Applications Using Machine Learning: A Review. IEEE Sens. J. 2023, 23, 3395–3407. [Google Scholar] [CrossRef]
- Mikulska, D. Contemporary applications of infrared imaging in medical diagnostics. Ann. Acad. Med. Stetin. 2006, 52, 35–39. [Google Scholar] [PubMed]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical Applications of Infrared Thermography: A Review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef]
- Applications of Infrared Thermography in Ophthalmology—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36983878/ (accessed on 18 January 2025).
- Kesztyüs, D.; Brucher, S.; Kesztyüs, T. Use of Infrared Thermography in Medical Diagnostics: A Scoping Review Protocol. BMJ Open 2022, 12, e059833. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Ng, E.Y.K.; Lim, S.T. Infrared (IR) Thermography as a Potential Screening Modality for Carotid Artery Stenosis. Comput. Biol. Med. 2019, 113, 103419. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, Y.; Deng, X.; Zhou, S.; Liu, Y.; Zhang, J.; Zeng, Y.; Li, X.; Gao, X.; Xu, B.; et al. Application of intraoperative infrared thermography in bypass surgery for adult moyamoya syndrome: A preliminary study. Front. Neurol. 2023, 14, 1174072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papatheofanis, F.J. Health Technology Assessment. Q. J. Nucl. Med. 2000, 44, 105–111. [Google Scholar]
- Stefańczyk, L.; Woźniakowski, B.; Pietrzak, P.; Majos, A.; Grzelak, P. Comparison of Thermography and Doppler Sonography in the Evaluation of the Cold Immersion Test in Women with Excessive Vasospastic Reaction. Med. Sci. Monit. 2007, 13 (Suppl. S1), 121–128. [Google Scholar]
- Nahm, F.S. Infrared Thermography in Pain Medicine. Korean J. Pain. 2013, 26, 219–222. [Google Scholar] [CrossRef]
- Chapter 4: Searching for and Selecting Studies. Available online: https://training.cochrane.org/handbook/current/chapter-04 (accessed on 29 April 2024).
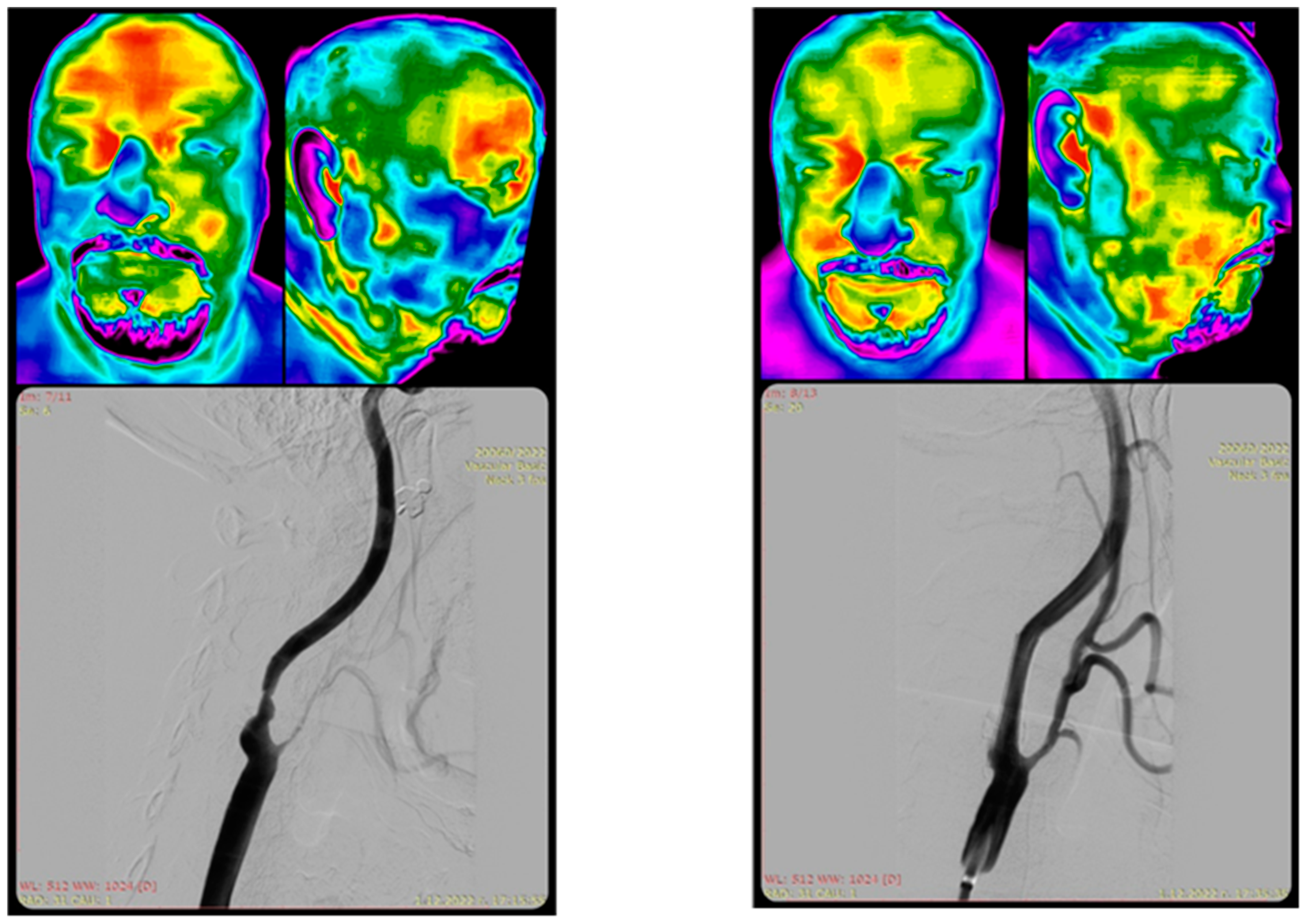
| Author, Year Country Citation | Study Design | Number of Participants | Thermography System; Software | Visualized Area of Body | Intended Use | Results | Conclusions |
|---|---|---|---|---|---|---|---|
| ACUTE | |||||||
| Korpelainen, 1995 Finland [9] | Prospective follow-up study | 63 | Digital thermometer (TC-1100, Line Seiki) | Whole body | To evaluate skin temperature asymmetry and its relation to autonomic dysfunction. | There was asymmetry in skin temperatures in patients with brain infarction, with the limbs contralateral to the infarction being markedly colder than the ipsilateral ones. | In hemispheric brain infarction, the coldness of the paretic limbs is associated with clinical signs of the pyramidal tract lesion. |
| Park, 2022 South Korea [30] | Case report | 1 | Therma CAMTM SC 500; FLIR tools | Upper and lower limb | To quantitatively evaluate differences in body temperature of limbs and the body to monitor stroke patients’ condition. | Lacunar infarction in the basal ganglia caused an anomaly in the white matter pathway between their patient’s brainstem and cerebral cortex, which caused body temperature to drop opposite to the side of the lesion (~2 °C difference). | People with acute lacunar infarction syndrome may experience issues with thermoregulation, along with cerebral hemisphere and brain stem infarction, and thermography may help with its detection. |
| Piskorz, 2016 Poland [31] | Cross-sectional study | 38 | FLIR E 30; FLIR tools | Head | To evaluate temperature differences in the brain as a diagnostic tool for strokes, and differentiate between different types of strokes (ischemic and hemorrhagic). | Temperature decreased in the stroke area compared to the healthy area. The difference was statistically significant (p < 0.05) both on the first and fourth day. The average temperature change was 0.49 °C on day 1 and 0.38 °C on day 4. No significant difference was found between ischemic and hemorrhagic stroke. | Thermography is a useful tool for stroke diagnosis, but it does not differentiate between hemorrhagic and ischemic strokes. Temperature change does not depend on the size of the focal area in CT and it does not correlate with the clinical status of patients. |
| Stokholm, 2021, Denmark [32] | Prospective, longitudinal, observational study | 64 | FLIR T430sc; FLIR tools | Face | To measure facial temperature in acute stroke patients to investigate its potential as a marker for delirium. | There was no difference between facial temperature measurements while delirium was present and measurements obtained while delirium was absent except in medial palpebral commissure (MPC) region where skin temperature was significantly lower during delirium (difference of 0.40 °C, 95% CI (0.72 to 0.08), p value = 0.014). | Since skin is under autonomic control and delirium can cause autonomic dysregulation, they think temperature can show the occurrence of delirium. The only facial region to show signs of delirium is the medial palpebral commissure. Biological sex and body temperature both have “significant association” with facial temperature, so they should be considered when using facial thermography. |
| Takahashi, 2018 Japan [33] | Observational study | 18 | FLIR E5; FLIR tools | Whole body | To measure body surface temperature and its laterality in patients with acute Wallenberg’s syndrome (WS) compared to pontine infarction patients, to aid in diagnosis. | A total of 89% of WS patients showed a laterality of body surface temperature (BST) at multiple sites, some with whole-body laterality, in contrast to pontine infarction patients, and the BST laterality decreased over time. | Thermography can help prevent misdiagnosis of acute Wallenberg’s syndrome. |
| Takahashi, 2024 Japan [34] | Observational study | 48 | FLIR E5; FLIR tools | Whole body | To measure body surface temperature (BST) to differentiate Wallenberg’s syndrome from noncentral vertigo in the acute phase. | BST findings observed only in patients with WS included (i) LD ≥ 1.0 °C in the abdomen, (ii) LD ≥1.5 °C in the upper limbs, (iii) LD ≥ 2.5 °C, and (iv) LD ≥ 0.5 °C in the four ipsilateral locations. However, the sensitivity in WS was low at (i) 33%, (ii) 22%, (iii) 56%, and (iv) 22%, respectively. None of these findings were observed in patients with NCV. | Thermography may be useful for detecting temperature differences in Wallenberg’s syndrome compared to noncentral vertigo. |
| CHRONIC | |||||||
| Alfieri, 2016 Brazil [35] | Cross-sectional study | 100 | ThermaCAM SC 500; FLIR tools | Upper and lower limb | To measure cutaneous temperature in hemiplegic patients, in comparison with healthy subjects, to investigate thermal sensitivity after stroke. | Individuals with stroke sequelae present lower temperature in the paretic side, especially on their feet. | Thermography reveals temperature asymmetry in stroke patients, with the paretic side being cooler. |
| Alfieri, 2019 Brazil [36] | Case report | 1 | FLIR T650SC; FLIR Tools | Lower limbs, feet | To evaluate the cutaneous temperature distribution before, during, and after robotic therapy for gait training in a stroke patient. | Temperature values increased immediately after robotic gait training, followed by a thermoregulation 30 min after rest. For example, temperature differences in the thigh region changed from 1.1 °C at baseline to 2.0 °C immediately after the training, then to 0.3 °C after 30 min. | Thermography can track temperature changes in stroke patients undergoing robotic therapy. Difference between the sides normalizes after training. |
| Alfieri, 2020 Brazil [37] | Case-control, cross sectional observational study | 16 | FLIR T650SC; FLIR Tools | Plantar region of both feet | To assess plantar cutaneous temperature in patients with stroke, and in those with stroke and diabetes mellitus. | There were no significant differences in temperature between the stroke with DM and stroke only groups. | Diabetes was not shown to cause additional difference in temperature of plegic/non-plegic limb among patient with stroke. |
| Alfieri, 2023 Brazil [38] | Cross-sectional study | 14 | FLIR T650SC; FLIR Tools | Whole body | To detect thermal asymmetry in patients with stroke sequelae using a portable, low-cost camera, and to assess its agreement with a high-resolution device. | The FLIR C5 showed adequate-to-excellent general concordance with the FLIR T650sc. Temperature differences were found between the plegic and contralateral side with the values being lower in the plegic side by 0.5 °C in the hand (anterior view), 1.4 °C in the leg (anterior view), 0.8 °C in the hand (posterior view), and 1.5 °C in the leg (posterior view). | A portable thermographic camera can be used reliably to measure thermal asymmetry in stroke patients. |
| Da Silva Dias, 2021, Brazil [39] | Cross-sectional study | 86 | FLIR T650SC; FLIR Tools | Upper and lower limb, feet | To quantify skin temperature of each limb to examine the association between temperature, tactile sensibility, and sensorimotor recovery after stroke. | There was an association between temperature differences and reports of sensation. Among those reporting thermal alterations, a higher temperature difference was associated with increased tactile sensibility difference. The opposite was found in those not reporting thermal alterations. | Thermography can help to evaluate the association between temperature differences and tactile sensation in stroke patients |
| Da Silva Dias, 2022 America [40] | Cross-sectional study | 43 | FLIR T650SC; FLIR tools | Upper and lower limb | To assess whole-body temperature distribution in stroke patients who report temperature differences between sides of the body. | The plegic limb had significantly lower temperatures than the contralateral side in all segments evaluated. The overall mean temperature difference was 0.7 °C, with the most significant differences in the dorsal forearm (0.8 °C), ventral leg (0.7 °C), and dorsal hand (0.6 °C). | Thermography can detect temperature differences in stroke patients who report feeling cold in their plegic limb. |
| Gomes, 2022 Brazil [41] | Cross-sectional study | 24 | FLIR 72,001; FLIR Tools | Face | To record the thermographic patterns of the masseter and temporalis muscles in patients after hemorrhagic stroke, and compare with healthy controls. | No significant differences in skin temperature were found in the masseter and temporal muscles between the post-hemorrhagic stroke and control groups. A significant difference (p < 0.05) was noted in the thickness of the left temporal muscle at rest (p = 0.01). | Thermography may not detect temperature differences in the masticatory muscles after hemorrhagic stroke, but other changes may be detectable using other methods |
| Hegedus, 2017 Hungary [42] | Randomized controlled trial | 16 | Fluke Ti20, Fluke Corporation | Upper limbs | To monitor the effectiveness of stroke rehabilitation treatment by measuring changes in microcirculation and joint function. | Microcirculatory dysfunction was found in all affected extremities. Following treatment, temperature significantly increased (p ≥ 0.5 °C) on the affected side. A strong correlation was found between joint function and temperature change (p < 0.05). | Thermography is a reliable method for monitoring the effects of stroke rehabilitation treatment. |
| Kim, 2006 South Korea [43] | Prospective cohort study | 70 | IRIS 5000, Medicore | Whole body | To correlate temperature changes with pain relief in central poststroke pain as measured by VAS scores and using infrared thermography. | The skin temperature of the pain site was significantly lower than the non-pain site before treatment and improved after treatment, in accordance with improvement of VAS pain scores, Significant correlation between the change of pain and temperature in CPSP (Central post-stroke pain) patients was found. | It is suggested that infrared thermography is very useful device for the evaluation of central post-stroke pain and its treatment. |
| Nowak, 2020 Poland [44] | Prospective, single-centre study | 40 | FLIR T335; FLIR tools | Lower limbs | To measure the effects of rehabilitation on spasticity in stroke patients, by assessing the surface temperature of the shank. | The temperature difference between the spastic and non-spastic shank was 0.78 °C at baseline and 0.50 °C after rehabilitation | Thermography can be used to measure the effects of rehabilitation on spasticity in stroke patients. |
| Sánchez-Sánchez, 2019, Spain [45] | Cross-sectional study | 44 | FLIR E60bx; FLIR tools | Lower limbs | To assess cutaneous temperature variation as a marker of muscle metabolism in stroke patients. | A temperature difference of −0.57 °C was found between the paretic and non-paretic thigh in the group with limited ambulation. | Paretic VI (vastus intermedius) muscle wasting may be an important factor to reach normal walking. |
| Satoh, 2002 Japan [46] | Case report | 1 | NA | Upper limbs | To monitor skin temperature decrease and somatosensory function disturbances following stroke. | Ischemic stroke of the postcentral gyrus was associated with somatosensory and skin temperature disturbances. After cooling, the affected hand still had a low skin temperature; it was lower on the ulnar side than on the radial side, while the skin temperature had recovered completely on the unaffected hand. | Thermography can help detect sensory disturbances after stroke. |
| Wanklyn, 1994 United Kingdom [47] | Observational study | 21 | Starsight Thermographic camera; Insight Vision Systems | Upper limbs | To objectively verify temperature differences between hemiplegic and normal arms. | During cold stress, the median temperature difference between non-hemiplegic and hemiplegic hands was 0.65 °C at pre-cooling, 0.1 °C at 0 min, 2.035 °C at 3 min, 0.47 °C at 5 min, and 0.65 °C at 10 min, and for patients not reporting cold, these values were −0.2 °C, 0 °C, 0 °C, −0.72 °C, and −1.4 °C respectively. | Thermography is useful for assessing the cold hemiplegic arm in stroke. |
| Zanona, 2018 Brazil [48] | Single group pre/post intervention study | 10 | C2 Camera, Flir Tools | Upper and lower limb | To analyze body asymmetry as an indicator of the effects of virtual reality rehabilitation on stroke patients. | Use of virtual reality-based rehabilitation resulted in improved symmetry of body temperature, with changes including the right forearm (+1.23 °C), previous direct hand (+1.56 °C), rear right hand (+1.28 °C), and left back hand (+0.9 °C). | VR can improve body temperature symmetry after stroke, as measured by thermography. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podlasek, A.; Petrov, I.; Stankov, Z.; Snyder, K.; Alvarez, C.A.; Musialek, P.; Grunwald, I.Q. Thermography in Stroke—A Systematic Review. Medicina 2025, 61, 854. https://doi.org/10.3390/medicina61050854
Podlasek A, Petrov I, Stankov Z, Snyder K, Alvarez CA, Musialek P, Grunwald IQ. Thermography in Stroke—A Systematic Review. Medicina. 2025; 61(5):854. https://doi.org/10.3390/medicina61050854
Chicago/Turabian StylePodlasek, Anna, Ivo Petrov, Zoran Stankov, Kenneth Snyder, Carlos Alejandro Alvarez, Piotr Musialek, and Iris Q. Grunwald. 2025. "Thermography in Stroke—A Systematic Review" Medicina 61, no. 5: 854. https://doi.org/10.3390/medicina61050854
APA StylePodlasek, A., Petrov, I., Stankov, Z., Snyder, K., Alvarez, C. A., Musialek, P., & Grunwald, I. Q. (2025). Thermography in Stroke—A Systematic Review. Medicina, 61(5), 854. https://doi.org/10.3390/medicina61050854








