Esomeprazole’s Role in Enhancing Colonic Anastomotic Healing Post-Ischemic Injury in the Rat Model
Abstract
1. Introduction
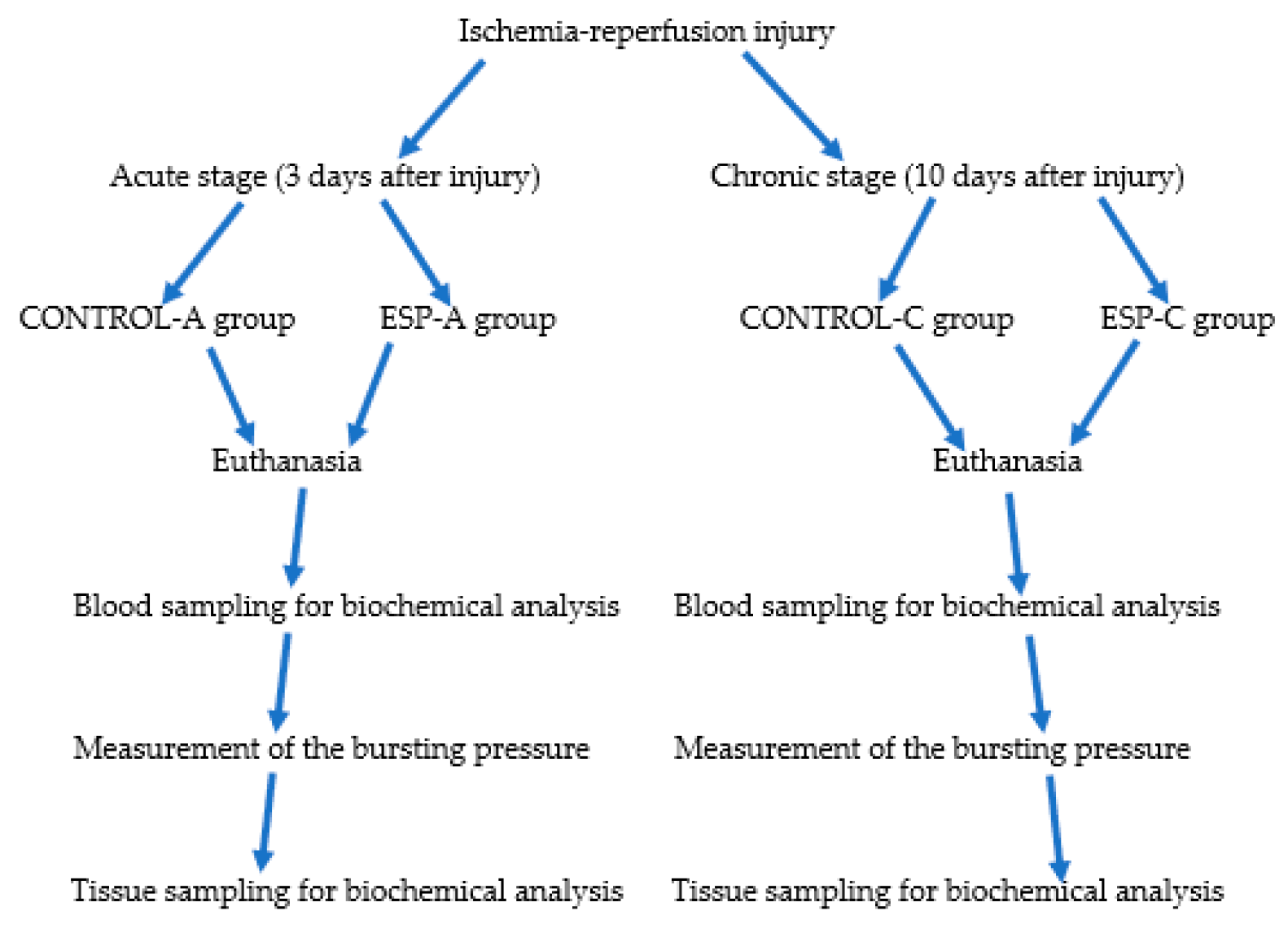
2. Materials and Methods
2.1. Animals
2.1.1. Acute Period Groups
- −
- CONTROL-A group (45 min of ischemia and then 45 min of reperfusion was performed over the colon mesentery, and then colon transection and end-to-end colon anastomosis was applied, and a single daily dose of 0.5 mL saline was administered intraperitoneally for three days, n = 10).
- −
- ESP-A group (After 45 min of ischemia and then 45 min of reperfusion, the colon mesentery, colon transection, and end-to-end colon anastomosis were applied, and a single daily dose of 10 mg/day esomeprazole was administered intraperitoneally for three days, n = 10).
2.1.2. Chronic Stage Groups
- −
- CONTROL-C group (45 min of ischemia and then 45 min of reperfusion was performed over the colon mesentery, and then colon transection and end-to-end colon anastomosis were applied, and a single daily dose of 0.5 mL saline was administered intraperitoneally for ten days, n = 10).
- −
- ESP-C group (45 min of ischemia and then 45 min of reperfusion were performed over the colon mesentery, and then colon transection and end-to-end colon anastomosis were applied, and a single daily dose of 10 mg/day esomeprazole was administered intraperitoneally for ten days, n = 10).
2.2. Surgery
2.3. Anastomotic Burst Pressure Measurement
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Acute Stage Results
3.2. Chronic Stage Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pommergaard, H.C.; Gessler, B.; Burcharth, J.; Angenete, E.; Haglind, E.; Rosenberg, J. Preoperative risk factors for anastomotic leakage after resection for colorectal cancer: A systematic review and meta-analysis. Color. Dis. 2014, 16, 662–671. [Google Scholar] [CrossRef]
- Nesbakken, A.; Nygaard, K.; Lunde, O. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br. J. Surg. 2001, 88, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, A.; Merola, G.; De Palma, G.D.; Sodo, M.; Pirozzi, F.; Bracale, U.M.; Bracale, U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J. Gastroenterol. 2018, 24, 2247. [Google Scholar] [CrossRef]
- Chadi, S.A.; Fingerhut, A.; Berho, M.; DeMeester, S.R.; Fleshman, J.W.; Hyman, N.H.; Margolin, D.A.; Martz, J.E.; McLemore, E.C.; Molena, D. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J. Gastrointest. Surg. 2016, 20, 2035–2051. [Google Scholar] [CrossRef]
- Baixauli, J.; Kiran, R.P.; Delaney, C.P. Investigation and management of ischemic colitis. Clevel. Clin. J. Med. 2003, 70, 920–936. [Google Scholar] [CrossRef] [PubMed]
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Ischemia—Reperfusion injury of the intestine and protective strategies against injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef]
- Washington, C.; Carmichael, J.C. Management of ischemic colitis. Clin. Colon Rectal Surg. 2012, 25, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Gumz, M.L.; Lynch, I.J.; Greenlee, M.M.; Cain, B.D.; Wingo, C.S. The renal H+-K+-ATPases: Physiology, regulation, and structure. Am. J. Physiol.-Ren. Physiol. 2010, 298, F12–F21. [Google Scholar] [CrossRef]
- Ozkan, T.A.; Karakoyunlu, N.; Polat, R.; Sarıbaş, G.S.; Şener, N.C.; Özdemir, S.; Peker, K.; Ünal, D.; Tuygun, C. An evaluation of the protective effect of esomeprazole in an experimental model of renal ischemia–reperfusion. Int. Urol. Nephrol. 2018, 50, 217–223. [Google Scholar] [CrossRef]
- Zhou, G.; Peng, Y.; Guo, M.; Qu, C.; Luo, S.; Jiang, Y.; Chen, D.; Wang, X.; Guo, Y. Esomeprazole inhibits endoplasmic reticulum stress and ameliorates myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2022, 627, 84–90. [Google Scholar] [CrossRef]
- Abed, M.N.; Alassaf, F.A.; Jasim, M.H.; Alfahad, M.; Qazzaz, M.E. Comparison of antioxidant effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole. Pharmacology 2020, 105, 645–651. [Google Scholar] [CrossRef]
- Eltahir, H.M.; Nazmy, M.H. Esomeprazole ameliorates CCl4 induced liver fibrosis in rats via modulating oxidative stress, inflammatory, fibrogenic and apoptotic markers. Biomed. Pharmacother. 2018, 97, 1356–1365. [Google Scholar] [CrossRef]
- Ghebremariam, Y.T.; Cooke, J.P.; Gerhart, W.; Griego, C.; Brower, J.B.; Doyle-Eisele, M.; Moeller, B.C.; Zhou, Q.; Ho, L.; de Andrade, J. Pleiotropic effect of the proton pump inhibitor esomeprazole leading to suppression of lung inflammation and fibrosis. J. Transl. Med. 2015, 13, 249. [Google Scholar] [CrossRef]
- Pham, N.; Ludwig, M.S.; Wang, M.; Ebrahimpour, A.; Bonnen, M.D.; Diwan, A.H.; Kim, S.J.; Bryan, J.; Newton, J.M.; Sikora, A.G. Topical esomeprazole mitigates radiation-induced dermal inflammation and fibrosis. Radiat. Res. 2019, 192, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, X.; Chen, R.; Chen, R.; Li, T.; Wu, W.; Huang, Z. Esomeprazole alleviates the damage to stress ulcer in rats through not only its antisecretory effect but its antioxidant effect by inactivating the p38 MAPK and NF-κB signaling pathways. Drug Des. Dev. Ther. 2019, 13, 2969–2984. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Tandoğan, Y.İ.; Aydin, O.; Pehlivanli, F.; Aydinuraz, K.; Daphan, Ç.E.; Kaplan, İ. Therapeutic Effects of Esomeprazole on Pancreatic and Lung Injury in Acute Pancreatitis: An Experimental Study. Medicina 2025, 61, 200. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal route of drug administration: Should it be used in experimental animal studies? Pharm. Res. 2020, 37, 12. [Google Scholar] [CrossRef]
- Schoenberg, M.H.; Beger, H.G. Reperfusion injury after intestinal ischemia. Crit. Care Med. 1993, 21, 1376–1386. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Montalto, M.C.; Hart, M.L.; Jordan, J.E.; Wada, K.; Stahl, G.L. Role for complement in mediating intestinal nitric oxide synthase-2 and superoxide dismutase expression. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 285, G197–G206. [Google Scholar] [CrossRef]
- Paterson, I.S.; Klausner, J.M.; Goldman, G.; Kobzik, L.; Welbourn, R.; Valeri, C.R.; Shepro, D.; Hechtman, H.B. Thromboxane mediates the ischemia-induced neutrophil oxidative burst. Surgery 1989, 106, 224–229. [Google Scholar] [PubMed]
- Nathan, C.; Xie, Q. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994, 269, 13725–13728. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, W.T.; Diegelmann, R.F. Growth factors in wound healing. Clin. Dermatol. 1994, 12, 157–169. [Google Scholar] [CrossRef]
- Strodtbeck, F. Physiology of wound healing. Newborn Infant Nurs. Rev. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Steinbrech, D.S.; Longaker, M.T.; Mehrara, B.J.; Saadeh, P.B.; Chin, G.S.; Gerrets, R.P.; Chau, D.C.; Rowe, N.M.; Gittes, G.K. Fibroblast response to hypoxia: The relationship between angiogenesis and matrix regulation. J. Surg. Res. 1999, 84, 127–133. [Google Scholar] [CrossRef]
- Thornton, F.J.; Barbul, A. Healing in the gastrointestinal tract. Surg. Clin. N. Am. 1997, 77, 549–573. [Google Scholar] [CrossRef]
- Uysal, E.; Dokur, M. Comparison of effects of the tacrolimus and cyclosporine A on the colon anastomosis recovery of rats. Ann. Surg. Treat. Res. 2017, 92, 402–410. [Google Scholar] [CrossRef]

| CONTROL-A | ESP-A | 95% CI | |||||
|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD/ Median (min–max) | Mean ± SD/ Median (min–max) | t/Z | p | Lower | Upper | |
| Serum | CAT | 106.32 (88.61–148.63) | 40 (29.65–52.31) | −3.361 † | 0.001 | - | - |
| NO | 71.01 ± 6.88 | 37.71 ± 14.11 | 6.003 * | <0.001 | 21.408 | 45.209 | |
| RGT | 1072.45 (1010.70–1211) | 581.55 (426.30–766.20) | −3.361 † | 0.001 | - | - | |
| SOD | 7.39 ± 0.56 | 4.70 ± 1.14 | 6.002 * | <0.001 | 1.727 | 3.651 | |
| TNF | 1810.97 ± 348.41 | 3058.29 ± 324.21 | −7.413 * | <0.001 | −1608.214 | −886.432 | |
| IL6 | 153.83 ± 37.23 | 274.85 ± 31.68 | −7.002 * | <0.001 | −158.100 | −83.955 | |
| ALT | 76.87 ± 21.18 | 152.69 ± 19.71 | −7.413 * | <0.001 | −97.761 | −53.884 | |
| AST | 7.67 ± 1.57 | 13.29 ± 1.47 | −7.404 * | <0.001 | −7.254 | −3.996 | |
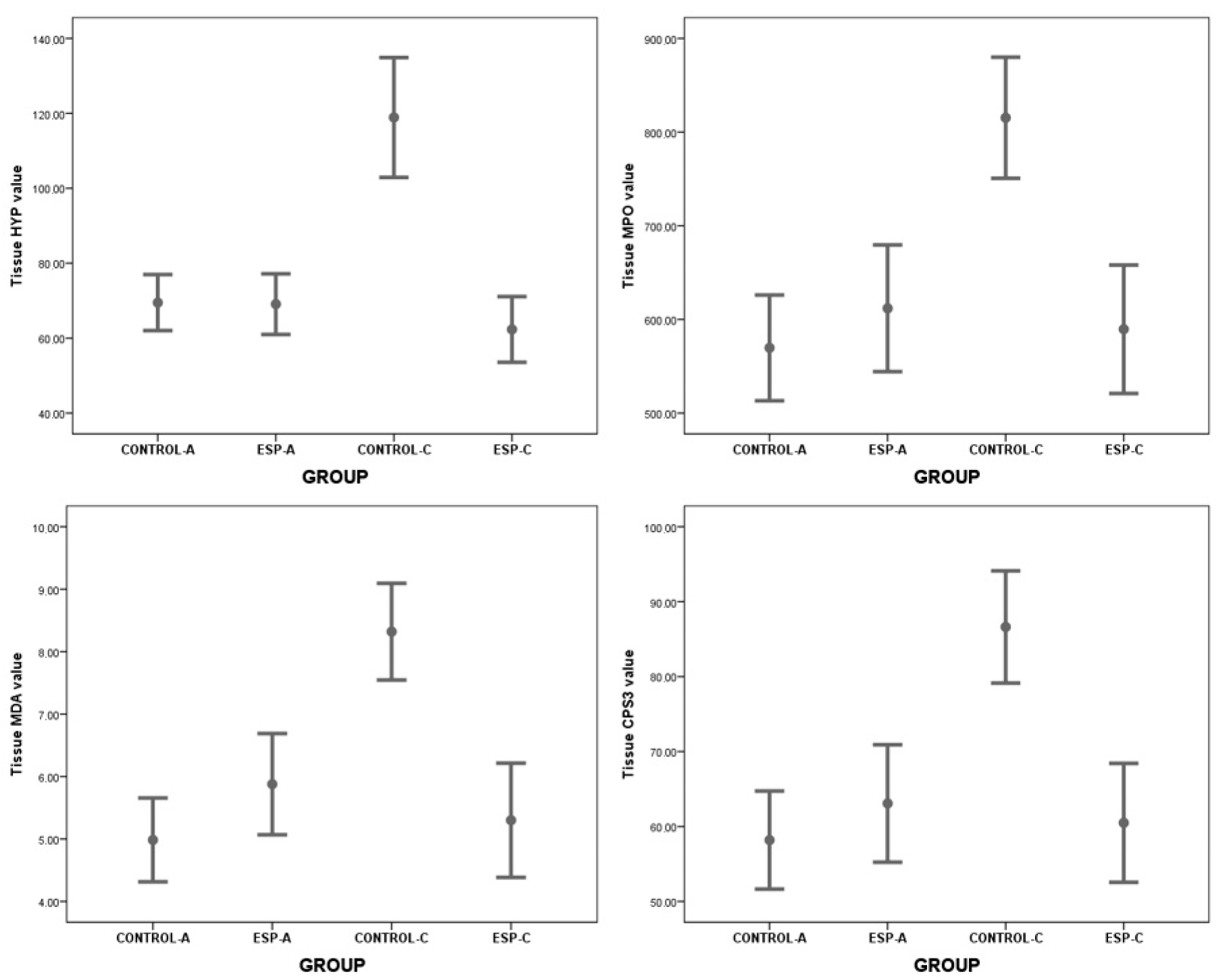
| Tissue | HYP | 69.48 ± 8.95 | 69.08 ± 9.69 | 0.086 * | 0.932 | −9.600 | 10.405 |
| MPO | 569.62 ± 67.56 | 611.85 ± 80.94 | −1.133 * | 0.276 | −122.178 | 37.717 | |
| MDA | 4.99 ± 0.80 | 5.88 ± 0.97 | −2.006 * | 0.065 | −1.847 | 0.062 | |
| CPS3 | 58.19 ± 7.82 | 63.08 ± 9.37 | −1.133 * | 0.276 | −14.142 | 4.365 | |
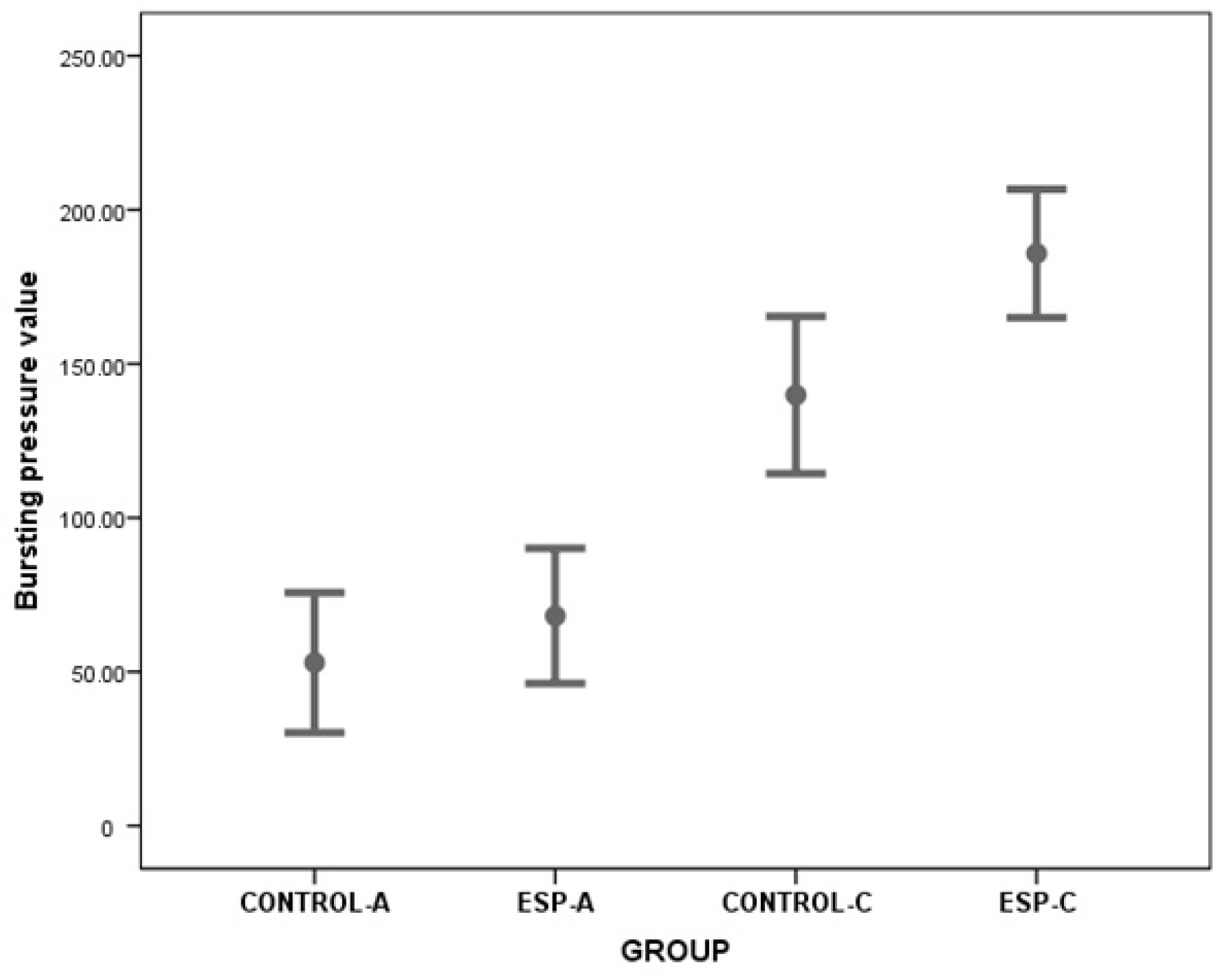
| BP | 53.00 ± 27.21 | 68.15 ± 26.23 | −1.134 * | 0.276 | −43.810 | 13.508 | |
| CONTROL-C | ESP-C | 95% CI | |||||
|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD/ Median (min–max) | Mean ± SD/ Median (min–max) | t/Z | p | Lower | Upper | |
| Serum | CAT | 59.00 ± 8.40 | 88.86 ± 11.81 | −6.366 * | <0.001 | −40.189 | −20.232 |
| NO | 28.65 (20.19–40.16) | 63.69 (50.46–72.38) | −3.674 † | <0.001 | - | - | |
| RGT | 868.65 (677.40–1037.25) | 1030.38 (986.25–1160.70) | −3.103 † | 0.002 | - | - | |
| SOD | 3.97 (3.29–4.90) | 6.81 (5.73–7351) | −3.674 † | <0.001 | - | - | |
| TNF | 3984.51 (3412.29–4960.28) | 1983.39 (1769.88–2156.85) | −3.674 † | <0.001 | - | - | |
| IL6 | 353.53 (309.45–460.73) | 169.81 (148.94–186.75) | −3.674 † | <0.001 | - | - | |
| ALT | 206.73 (174.21–211.31) | 87.35 (74.37–97.89) | −3.674 † | <0.001 | - | - | |
| AST | 17.47 (14.89–21.87) | 8.45 (7.48–9.23) | −3.674 † | <0.001 | - | - | |
| Tissue | HYP | 118.90 ± 20.81 | 62.32 ± 12.25 | 7.315 * | <0.001 | 40.257 | 72.893 |
| MPO | 815.29 ± 84.12 | 589.53 ± 95.86 | 5.428 * | <0.001 | 138.007 | 313.521 | |
| MDA | 8.32 ± 1.01 | 5.30 ± 1.28 | 5.670 * | <0.001 | 1.896 | 4.144 | |
| CPS3 | 86.62 ± 9.74 | 60.49 ± 11.10 | 5.428 * | <0.001 | 15.976 | 36.293 | |
| BP | 139.89 ± 33.19 | 185.84 ± 27.14 | −3.216 * | 0.005 | −76.340 | −15.659 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pehlivanli, F.; Aydin, O.; Mısırlıgil, M.S.; Peker, K.; Kaplan, İ. Esomeprazole’s Role in Enhancing Colonic Anastomotic Healing Post-Ischemic Injury in the Rat Model. Medicina 2025, 61, 851. https://doi.org/10.3390/medicina61050851
Pehlivanli F, Aydin O, Mısırlıgil MS, Peker K, Kaplan İ. Esomeprazole’s Role in Enhancing Colonic Anastomotic Healing Post-Ischemic Injury in the Rat Model. Medicina. 2025; 61(5):851. https://doi.org/10.3390/medicina61050851
Chicago/Turabian StylePehlivanli, Faruk, Oktay Aydin, Mehmet Selçuk Mısırlıgil, Kevser Peker, and İlker Kaplan. 2025. "Esomeprazole’s Role in Enhancing Colonic Anastomotic Healing Post-Ischemic Injury in the Rat Model" Medicina 61, no. 5: 851. https://doi.org/10.3390/medicina61050851
APA StylePehlivanli, F., Aydin, O., Mısırlıgil, M. S., Peker, K., & Kaplan, İ. (2025). Esomeprazole’s Role in Enhancing Colonic Anastomotic Healing Post-Ischemic Injury in the Rat Model. Medicina, 61(5), 851. https://doi.org/10.3390/medicina61050851






