Carotid Artery Stenting Intervention to Enhance Global Brain Blood Flow and Cognition in Carotid Artery Disease: Preliminary Findings from a Prospective Follow-Up MRI Study
Abstract
1. Introduction
2. Materials and Methods
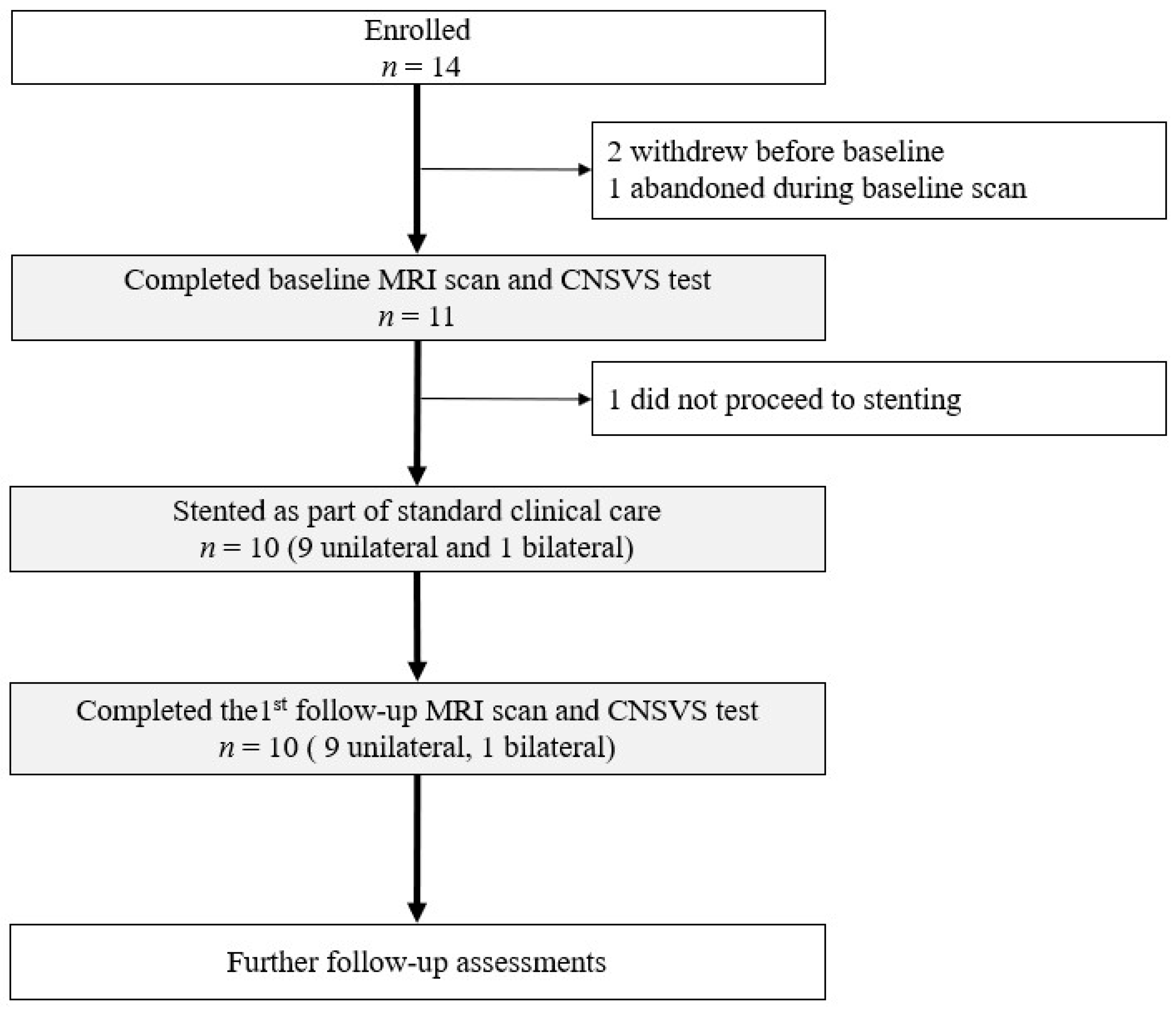
2.1. Study Design, Participant, and Clinical Care with CAS
2.2. Stenosis Quantification and Vascular Configuration
2.3. MRI Acquisition
2.4. Flow Quantification from Flow MRI
2.5. Neuropsychological Testing
2.6. Statistical Evaluation
3. Results
3.1. Participant Demographics and Characteristics
3.2. Inter-Rater Reliability of Flow Quantification
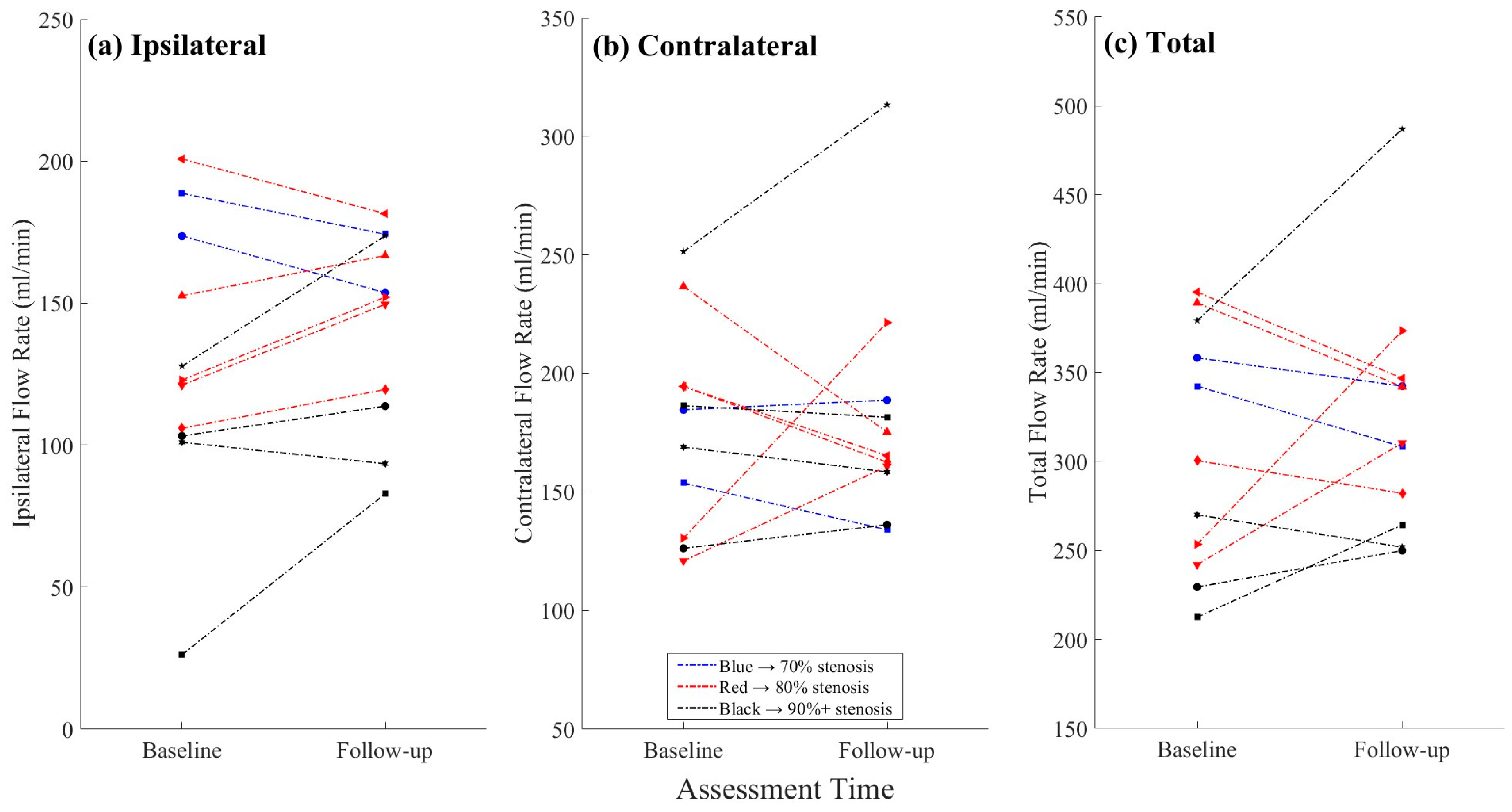
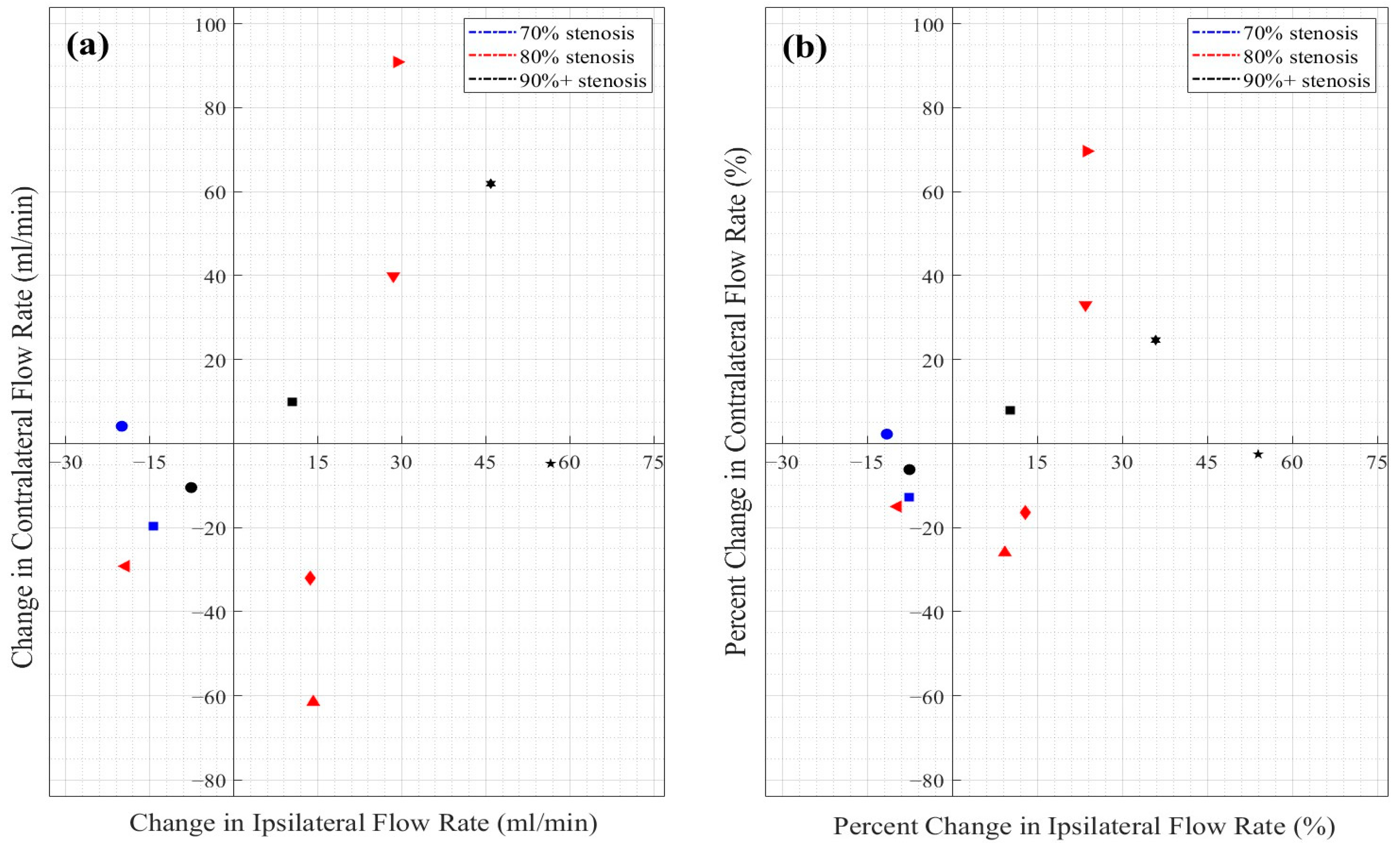
3.3. Objective 1: Flow Changes in the Treated vs. Non-Treated ICAs
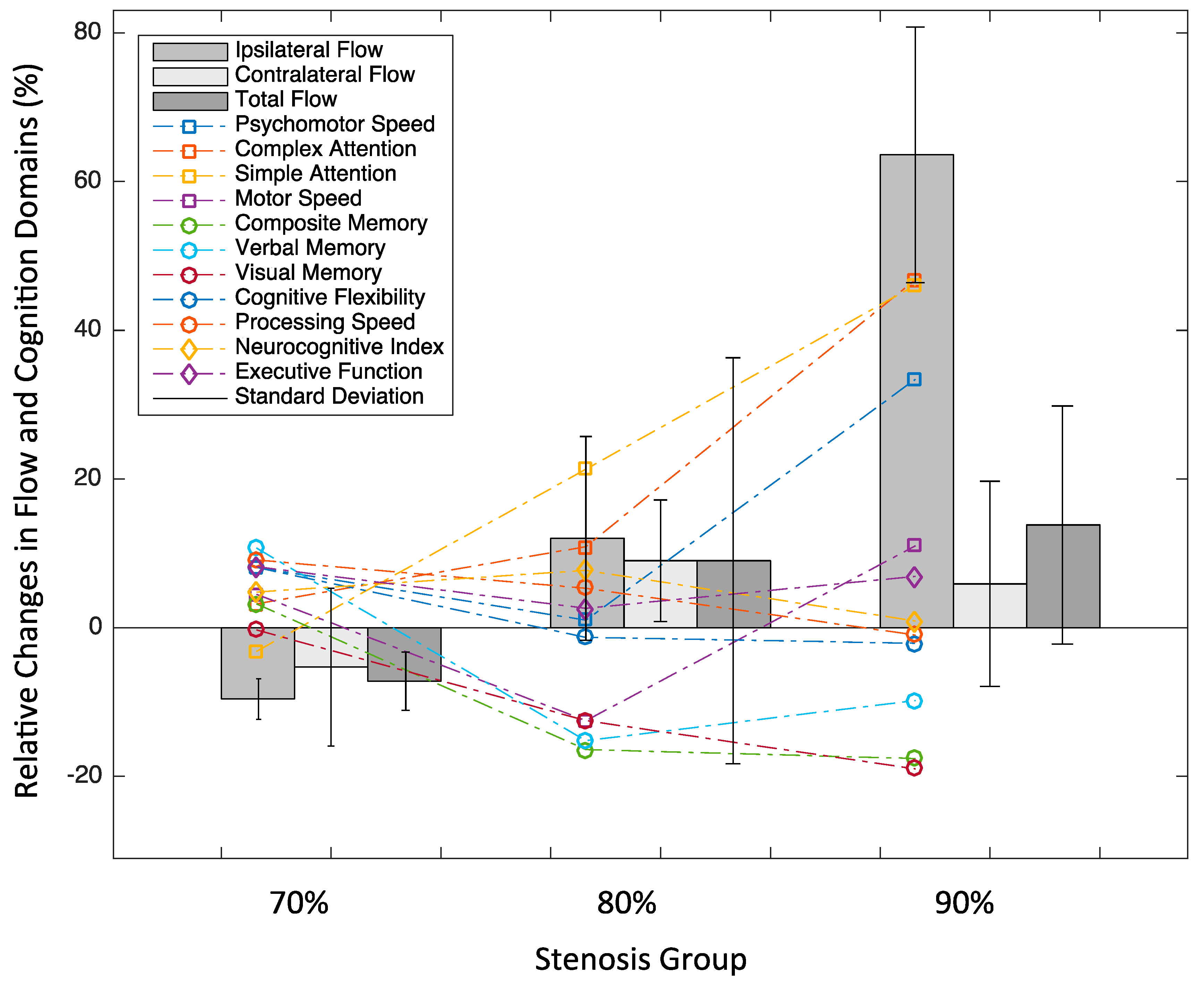
3.4. Objective 2: ICA Flow Changes by Stenosis Severity
3.5. Objective 3: Cognitive Changes in Relation to Flow Changes
4. Discussion
4.1. Summary of Main Findings
4.2. Interpretation
4.3. Limitations
4.4. Future Directions
4.5. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACA | anterior cerebral artery |
| ASL | arterial spin labeling |
| BMI | Body Mass Index |
| CAS | carotid artery stenting |
| CEA | carotid endarterectomy |
| CI | confidence interval |
| CNSVS | CNS Vital Signs |
| DICE | Dice similarity coefficient |
| FOV | Field of View |
| ICA | internal carotid artery |
| MCA | middle cerebral artery |
| MRI | magnetic resonance imaging |
| NCI | Neurocognitive Index |
| ROI | region of interest |
References
- Youn, S.W.; Kim, H.K.; Do, Y.R.; Do, J.K.; Kwon, O.C.; Lee, N.; Lee, H.J.; Lee, J. Haemodynamic alterations in cerebral blood vessels after carotid artery revascularisation: Quantitative analysis using 2D phase-contrast MRI. Eur. Radiol. 2013, 23, 2880–2890. [Google Scholar] [CrossRef] [PubMed]
- Piegza, M.; Jaworska, I.; Piegza, J.; Bujak, K.; Dębski, P.; Leksowska, A.; Gorczyca, P.; Gąsior, M.; Pudlo, R. Cognitive functions after carotid artery stenting—1-year follow-up study. J. Clin. Med. 2022, 11, 3019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chadha, D.; Chaddha, A.; Chauhan, R.; Singh, N.; Kamal, P.; Mishra, A.; Kaur, N. The safety and long-term efficacy of carotid artery stenting: An all-comers registry. Cureus 2022, 14, e32060. [Google Scholar] [CrossRef]
- Chinda, B.; Liang, S.; Siu, W.; Medvedev, G.; Song, X. Functional MRI evaluation of the effect of carotid artery stenting: A case study demonstrating cognitive improvement. Acta Radiol. Open 2021, 10, 2058460120988822. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, A.; Gieowarsingh, S.; Spagnolo, B.; Manetti, R.; Liso, A.; Furgieri, A.; Barattoni, M.C.; Ghetti, L.; Tavazzi, L.; Castriota, F. Safety, efficacy and long-term durability of endovascular therapy for carotid artery disease: The tailored-carotid artery stenting experience of a single high-volume centre (tailored-CASE Registry). EuroIntervention 2009, 5, 589–598. [Google Scholar] [CrossRef]
- Elserwi, A.; Amer, T.; Soliman, N.; Gaballa, G.M.; Elmokadem, A.H. Efficacy and safety of carotid artery stenting for stroke prevention. Egypt. J. Radiol. Nucl. Med. 2016, 47, 185–192. [Google Scholar] [CrossRef]
- Lal, B.K.; Younes, M.; Cruz, G.; Kapadia, I.; Jamil, Z.; Pappas, P.J. Cognitive changes after surgery vs stenting for carotid artery stenosis. J. Vasc. Surg. 2011, 54, 691–698. [Google Scholar] [CrossRef]
- Lehrner, J.; Willfort, A.; Mlekusch, I.; Guttmann, G.; Minar, E.; Ahmadi, R.; Lalouschek, W.; Deecke, L.; Lang, W. Neuropsychological outcome 6 months after unilateral carotid stenting. J. Clin. Exp. Neuropsychol. 2005, 27, 859–866. [Google Scholar] [CrossRef]
- Tiemann, L.; Reidt, J.H.; Esposito, L.; Sander, D.; Theiss, W.; Poppert, H. Neuropsychological sequelae of carotid angioplasty with stent placement: Correlation with ischemic lesions in diffusion weighted imaging. PLoS ONE 2009, 4, e7001. [Google Scholar] [CrossRef]
- De Rango, P.; Caso, V.; Leys, D.; Paciaroni, M.; Lenti, M.; Cao, P. The role of carotid artery stenting and carotid endarterectomy in cognitive performance. Stroke 2008, 39, 3116–3127. [Google Scholar] [CrossRef]
- Grunwald, I.Q.; Papanagiotou, P.; Reith, W.; Backens, M.; Supprian, T.; Politi, M.; Vedder, V.; Zercher, K.; Muscalla, B.; Haass, A.; et al. Influence of carotid artery stenting on cognitive function. Neuroradiology 2010, 52, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.Y.; Sun, Q.J.; Yang, H.; Zhang, M.X.; Ban, R.; Xu, G.L.; Wu, Y.P.; Wang, L.X.; Du, Y.F. Effect of carotid artery stenting on cognitive function in patients with internal carotid artery stenosis and cerebral lacunar infarction: A 3-year follow-up study in China. PLoS ONE 2015, 10, e0129917. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Arbe, G.; García-Eroles, L.; Oliveres, M.; Parra, O.; Massons, J. Infarctions in the vascular territory of the posterior cerebral artery: Clinical features in 232 patients. BMC Res. Notes 2011, 4, 329. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Wei, Z.; Zhang, Y. Long-term prognosis of patients with stroke associated with middle cerebral artery occlusion: Single-centre registration study. Arch. Med. Sci. 2019, 18, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef]
- Fang, H.; Song, B.; Cheng, B.; Wong, K.S.; Xu, Y.M.; Ho, S.S.Y.; Chen, X.Y. Compensatory patterns of collateral flow in stroke patients with unilateral and bilateral carotid stenosis. BMC Neurol. 2016, 16, 39. [Google Scholar] [CrossRef]
- Markl, M.; Frydrychowicz, A.; Kozerke, S.; Hope, M.; Wieben, O. 4D flow MRI. J. Magn. Reson. Imaging 2012, 36, 1015–1036. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Int. J. Nurs. Stud. 2010, 47, 361–371. [Google Scholar] [CrossRef]
- Gualtieri, C.T.; Johnson, L.G. A computerized test battery sensitive to mild and severe brain injury. Medscape J. Med. 2008, 10, 90. [Google Scholar]
- Vértes, M.; Nguyen, D.T.; Székely, G.; Bérczi, Á.; Dósa, E. Middle and distal common carotid artery stenting: Long-term patency rates and risk factors for in-stent restenosis. Cardiovasc. Intervent. Radiol. 2020, 43, 1134–1142. [Google Scholar] [CrossRef]
- Eckstein, H.H.; Eichbaum, M.; Klemm, K.; Doerfler, A.; Ringleb, P.; Bruckner, T.; Allenberg, J.-R. Improvement of carotid blood flow after carotid endarterectomy—Evaluation using intraoperative ultrasound flow measurement. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 168–174. [Google Scholar] [CrossRef]
- Chen, J.H.; Wu, M.H.; Luo, C.B.; Lirng, J.F.; Chen, S.T.; Wu, C.H.; Guo, W.-Y.; Chang, F.-C. Long-term imaging follow-up to evaluate restenosis in patients with carotid stenosis after angioplasty and stenting. J. Chin. Med. Assoc. 2021, 84, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Tang, X.; Chen, Z. Carotid artery stenting for patients with carotid stenosis and contralateral carotid artery occlusion: A 12-year experience. Ann. Vasc. Surg. 2023, 92, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.; Cullinane, M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001, 124 Pt 3, 457–467. [Google Scholar] [CrossRef]
- Fercho, K.A.; Scholl, J.L.; Kc, B.; Bosch, T.J.; Baugh, L.A. Sensorimotor control of object manipulation following middle cerebral artery (MCA) stroke. Neuropsychologia 2023, 182, 108525. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, C.T.; Johnson, L.G. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch. Clin. Neuropsychol. 2006, 21, 623–633. [Google Scholar] [CrossRef]
- Huang, D.; Guo, Y.; Guan, X.; Pan, L.; Zhu, Z.; Chen, Z.; Dijkhuizen, R.M.; Duering, M.; Yu, F.; Boltze, J.; et al. Recent advances in arterial spin labeling perfusion MRI in patients with vascular cognitive impairment. J. Cereb. Blood Flow. Metab. 2023, 43, 173–184. [Google Scholar] [CrossRef]
- van der Thiel, M.; Rodriguez, C.; Van De Ville, D.; Giannakopoulos, P.; Haller, S. Regional cerebral perfusion and cerebrovascular reactivity in elderly controls with subtle cognitive deficits. Front. Aging Neurosci. 2019, 11, 19. [Google Scholar] [CrossRef]
- Grajauskas, L.A.; Guo, H.; D’Arcy, R.C.N.; Song, X. Toward MRI-based whole-brain health assessment: The brain atrophy and lesion index (BALI). Aging Med. 2018, 1, 55–63. [Google Scholar] [CrossRef]
- Grajauskas, L.A.; Siu, W.; Medvedev, G.; Guo, H.; D’Arcy, R.C.N.; Song, X. MRI-based evaluation of structural degeneration in the ageing brain: Pathophysiology and assessment. Ageing Res. Rev. 2019, 49, 67–82. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, Y.; Zhang, M.; Ling, Y.; Yao, X.; Hu, M. Prevalence and adverse outcomes of pre-operative frailty in patients undergoing carotid artery revascularization: A meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1297848. [Google Scholar] [CrossRef]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.M.C.S.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular impairment, muscle atrophy, and cognitive decline: Critical age-related conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, M.A.M.; Gerstein, H.; Yusuf, S.; Leong, D.P. Accumulation of deficits as a key risk factor for cardiovascular morbidity and mortality: A pooled analysis of 154 000 individuals. J. Am. Heart Assoc. 2020, 9, e014686. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mitnitski, A.; Rockwood, K. Age-related deficit accumulation and the risk of late-life dementia. Alzheimers Res. Ther. 2014, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, R.I.; Dannels, W. Use of standard gradients with compound oblique angulation for optimal quantitative MR flow imaging in oblique vessels. AJR Am. J. Roentgenol. 1987, 148, 405–409. [Google Scholar] [CrossRef]
- Bernstein, M.A.; Zhou, X.J.; Polzin, J.A.; King, K.F.; Ganin, A.; Pelc, N.J.; Glover, G.H. Concomitant gradient terms in phase contrast MR: Analysis and correction. Magn. Reson. Med. 1998, 39, 300–308. [Google Scholar] [CrossRef]


| Demographics | n | 10 |
| Age (years) | 77.0 ± 5.6 (range: 69–86) | |
| Gender, male | 70% | |
| Weight (kg) | 73.9 ± 9.2 (range: 59–93) | |
| Height (m) | 1.63 ± 0.15 (range: 1.30–1.83) | |
| BMI (kg/m2) | 28.65 ± 7.83 (range: 21.46–48.52) | |
| Retirement Status | Retired (80%); Self-Employed (20%) | |
| Education | High school (70%); University (10%); Diploma (20%) | |
| Ethnicity | South Asian (40%); Caucasian (50%); Asian (10%) | |
| Marital Status | Married (70%); Single (10%); Widowed (20%) | |
| Living Situation | Living with family (80%); Living alone (20%) | |
| Handedness | Right (100%) | |
| Comorbidities | Hypertension | 30% |
| Diabetes | 40% | |
| Hyper-cholesterolaemia | 10% | |
| Atrial Fibrillation | 10% | |
| Ischemic Heart Disease | 20% | |
| Stroke | 10% | |
| Transient Ischemic Attack | 20% | |
| Renal Problems | 10% | |
| Lifestyle | Alcohol Use | 10% |
| Physical Activity | No (50%); Light (20%); Moderate (30%) | |
| Smoking | 0% | |
| Medication | Blood Pressure Medications | 40% |
| Antiplatelet | 40% | |
| Anti-coagulant | 20% | |
| Other Med | 40% | |
| Flow Measures | With Isolated MCA | 60% |
| Baseline Ipsilateral Flow Rate (mL/min) | 129.4 ± 48.9 | |
| Follow-up Ipsilateral Flow Rate (mL/min) | 141.9 ± 34.2 | |
| Baseline Contralateral Flow Rate (mL/min) | 177.1 ± 42.8 | |
| Follow-up Contralateral Flow Rate (mL/min) | 181.6 ± 49.9 |
| Participant ID | Isolated MCA | Ipsilateral Stenosis (%) | Baseline Ipsilateral Flow (mL/min) | First Follow-Up Ipsilateral Flow (mL/min) | Post-Stenting Ipsilateral Flow (% Change) | Contralateral Stenosis (%) | Baseline Contralateral Flow (mL/min) | First Follow-Up Contralateral Flow (mL/min) | Post-Stenting Contralateral Flow (% Change) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | y | 90 | 127.8 | 173.7 | 35.9 | 0 | 251.4 | 313.3 | 24.6 |
| 2 | y | 80 | 105.9 | 119.6 | 12.9 | 0 | 194.5 | 162.6 | −16.4 |
| 3 | y | 95 | 26.2 | 82.8 | 216.4 | 0 | 186.3 | 181.5 | −2.6 |
| 4 | y | 80 | 152.6 | 166.8 | 9.3 | 70 | 236.7 | 175.2 | −26.0 |
| 5 | y | 90 | 101.0 | 93.4 | −7.5 | 0 | 168.9 | 158.4 | −6.3 |
| 6 | n | 90 | 103.2 | 113.7 | 10.2 | 50 | 126.2 | 136.1 | 7.9 |
| 7 | n | 80 | 121.2 | 149.6 | 23.5 | 50 | 120.9 | 160.8 | 33.0 |
| 8 | y | 80 | 122.9 | 152.1 | 23.8 | 0 | 130.5 | 221.4 | 69.7 |
| 9 | n | 80 | 200.8 | 181.5 | −9.6 | 0 | 194.5 | 165.3 | −15.0 |
| 10a | n | 70 | 173.7 | 153.7 | −11.5 | 70 | 184.6 | 188.7 | 2.2 |
| 10b | n | 70 | 188.7 | 174.3 | −7.6 | 0 | 153.7 | 134.0 | −12.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, R.; Summers, P.; Siu, W.; Medvedev, G.; Doesburg, S.M.; Song, X. Carotid Artery Stenting Intervention to Enhance Global Brain Blood Flow and Cognition in Carotid Artery Disease: Preliminary Findings from a Prospective Follow-Up MRI Study. Medicina 2025, 61, 848. https://doi.org/10.3390/medicina61050848
Kaur R, Summers P, Siu W, Medvedev G, Doesburg SM, Song X. Carotid Artery Stenting Intervention to Enhance Global Brain Blood Flow and Cognition in Carotid Artery Disease: Preliminary Findings from a Prospective Follow-Up MRI Study. Medicina. 2025; 61(5):848. https://doi.org/10.3390/medicina61050848
Chicago/Turabian StyleKaur, Raminder, Paul Summers, William Siu, George Medvedev, Sam M. Doesburg, and Xiaowei Song. 2025. "Carotid Artery Stenting Intervention to Enhance Global Brain Blood Flow and Cognition in Carotid Artery Disease: Preliminary Findings from a Prospective Follow-Up MRI Study" Medicina 61, no. 5: 848. https://doi.org/10.3390/medicina61050848
APA StyleKaur, R., Summers, P., Siu, W., Medvedev, G., Doesburg, S. M., & Song, X. (2025). Carotid Artery Stenting Intervention to Enhance Global Brain Blood Flow and Cognition in Carotid Artery Disease: Preliminary Findings from a Prospective Follow-Up MRI Study. Medicina, 61(5), 848. https://doi.org/10.3390/medicina61050848






