The Underdiagnosis of Chronic Kidney Disease in Patients with a Documented Estimated Glomerular Filtration Rate and/or Urine Albumin–Creatinine Ratio in Germany
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Ethical Considerations
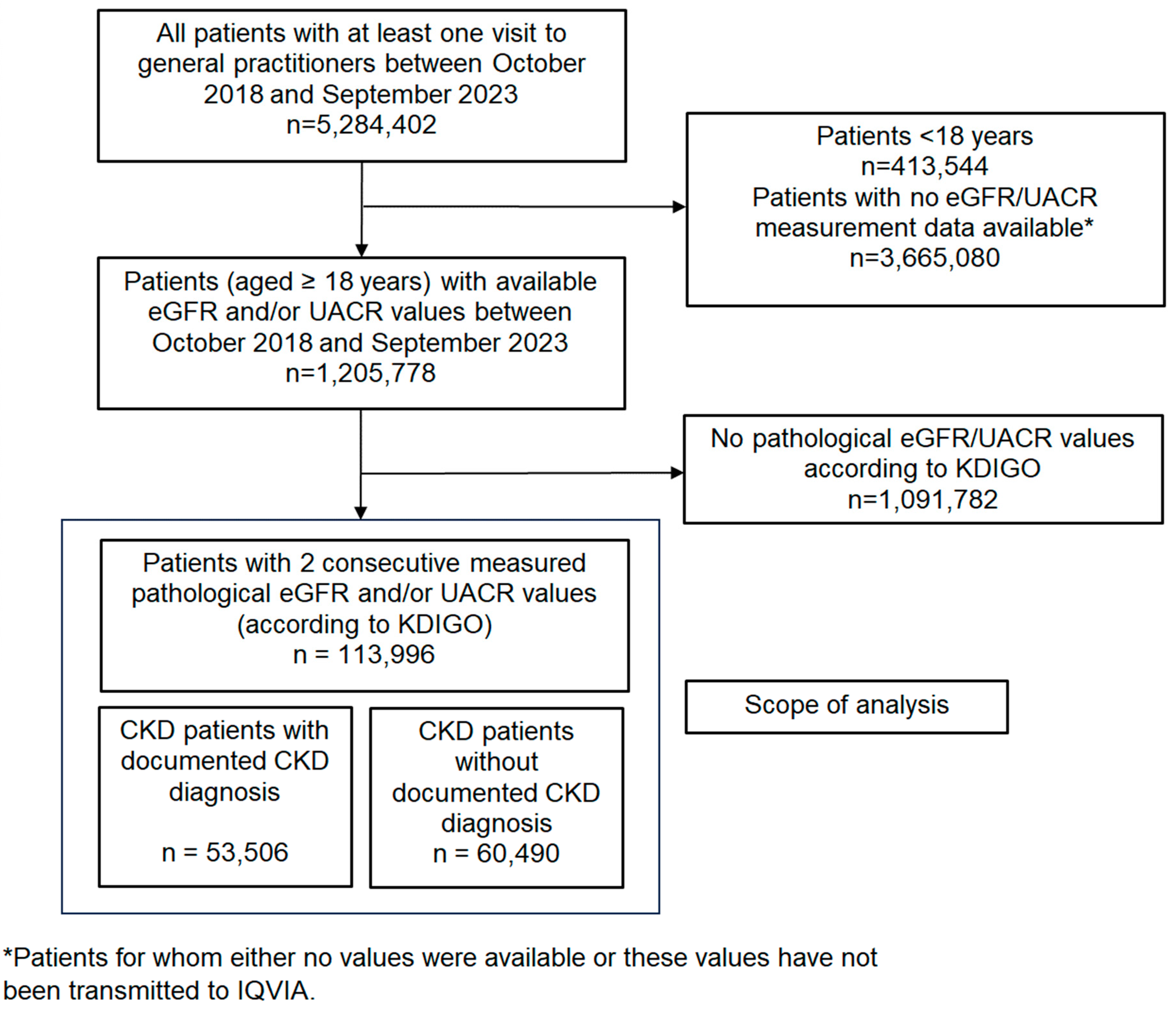
2.3. Study Population and Outcomes
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
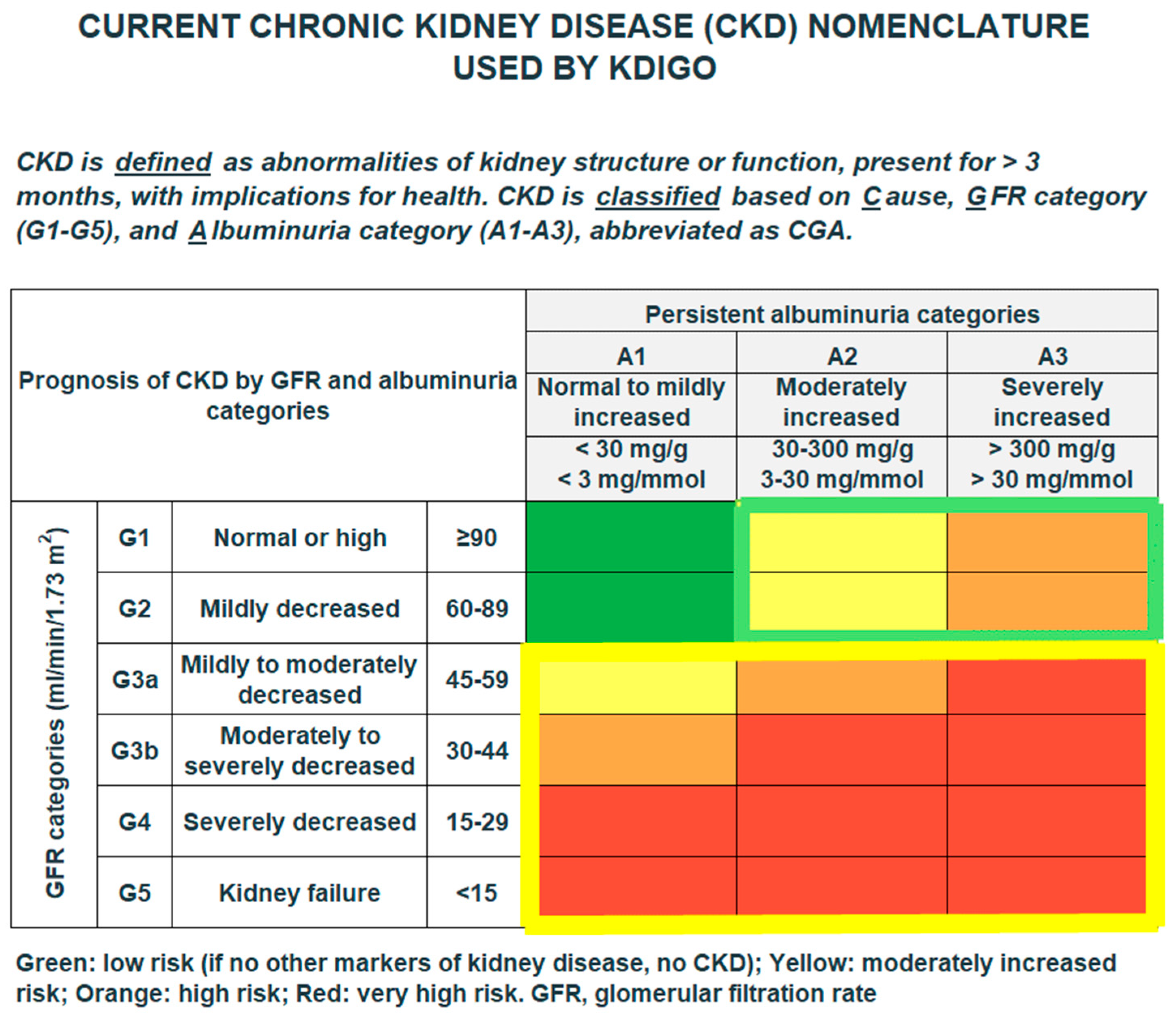
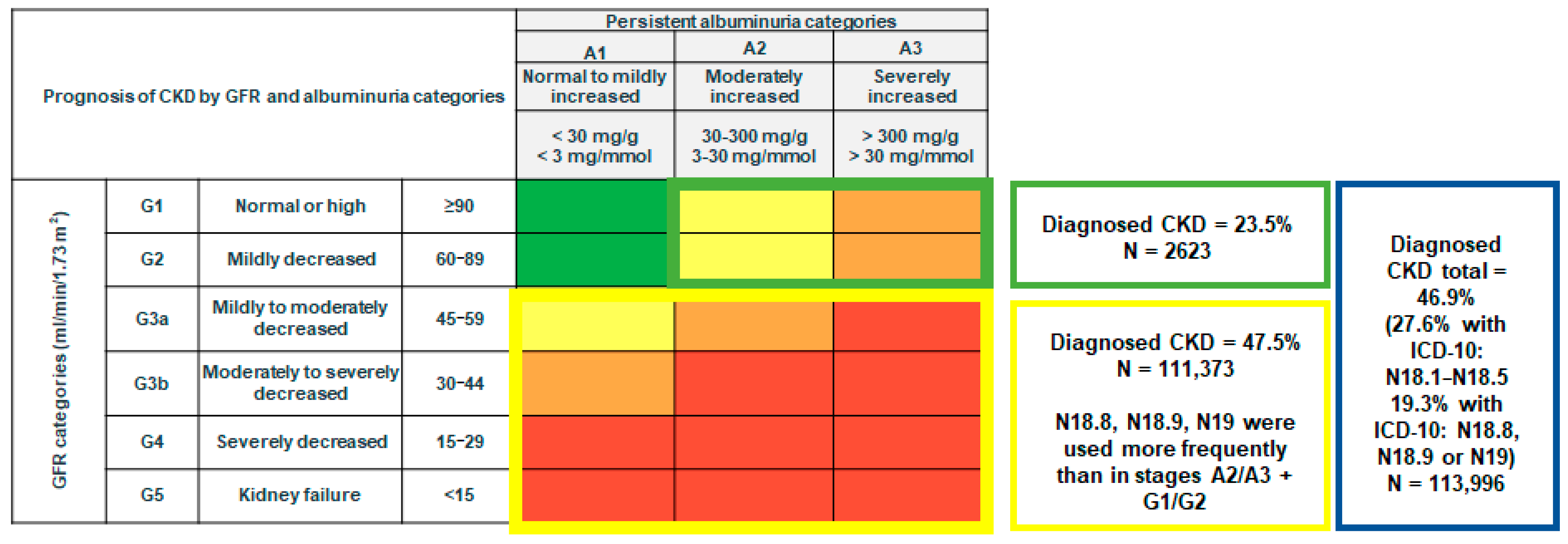
3.2. Diagnosed and Undiagnosed CKD
3.3. CKD by Age, Sex, Comorbidities, and eGFR
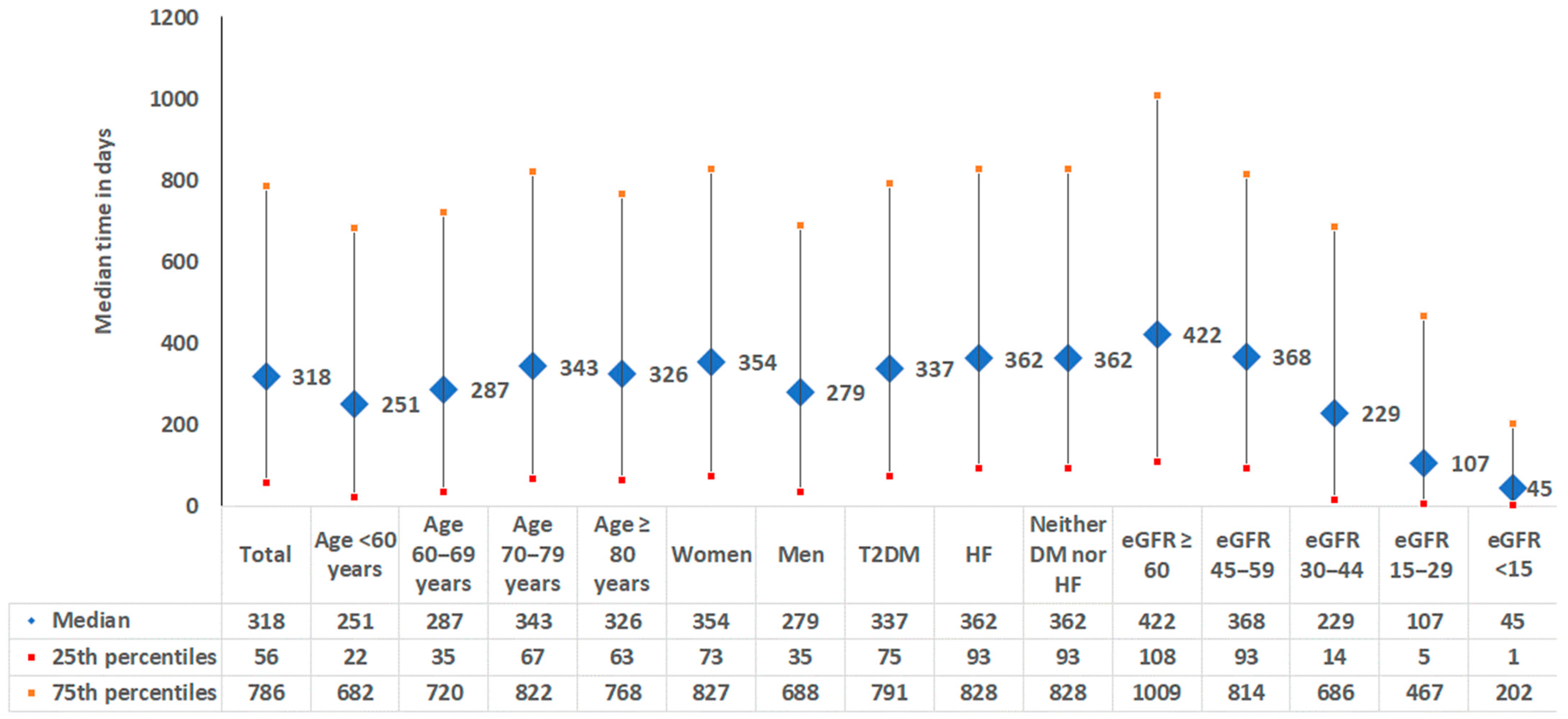
3.4. Time Between Pathological eGFR/UACR Measure and CKD Diagnosis Documentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jadoul, M.; Aoun, M.; Masimango Imani, M. The major global burden of chronic kidney disease. Lancet Glob. Health 2024, 12, e342–e343. [Google Scholar] [CrossRef] [PubMed]
- Aumann, N.; Baumeister, S.E.; Rettig, R.; Lieb, W.; Werner, A.; Döring, A.; Peters, A.; Koenig, W.; Hannemann, A.; Wallaschofski, H.; et al. Regional variation of chronic kidney disease in Germany: Results from two population-based surveys. Kidney Blood Press Res. 2015, 40, 231–243. [Google Scholar] [CrossRef]
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Stolpe, S.; Kowall, B.; Scholz, C.; Stang, A.; Blume, C. High Unawareness of Chronic Kidney Disease in Germany. Int. J. Environ. Res. Public Health 2021, 18, 11752. [Google Scholar] [CrossRef]
- Robson, B.; Deed, G.; Phoon, R.K. Improving the Detection and Management of Kidney Health in Primary Care. J. Patient Exp. 2024, 11, 23743735241256464. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Assogba, G.F.; Couchoud, C.; Roudier, C.; Pornet, C.; Fosse, S.; Romon, I.; Druet, C.; Stengel, B.; Fagot-Campagna, A. Prevalence, screening and treatment of chronic kidney disease in people with type 2 diabetes in France: The ENTRED surveys (2001 and 2007). Diabetes Metab. 2012, 38, 558–566. [Google Scholar] [CrossRef]
- Alfego, D.; Ennis, J.; Gillespie, B.; Lewis, M.J.; Montgomery, E.; Ferrè, S.; Vassalotti, J.A.; Letovsky, S. Chronic Kidney Disease Testing Among At-Risk Adults in the U.S. Remains Low: Real-World Evidence From a National Laboratory Database. Diabetes Care 2021, 44, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Wanner C, Schaeffner E, Frese T, Weber C, Stahl P, Scherg F, Burckhardt F, Opfermann U, Radowsky F, Mader F. InspeCKD-Analysis of the use of diagnostics in patients at high risk for chronic kidney disease in German general practitioner (GP) practices. MMW Fortschr. Med. 2024, 166 (Suppl. 4), 9–17. [CrossRef]
- Szczech, L.A.; Stewart, R.C.; Su, H.L.; DeLoskey, R.J.; Astor, B.C.; Fox, C.H.; McCullough, P.A.; Vassalotti, J.A. Primary care detection of chronic kidney disease in adults with type-2 diabetes: The ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS ONE 2014, 9, e110535. [Google Scholar] [CrossRef]
- Diamantidis, C.J.; Hale, S.L.; Wang, V.; Smith, V.A.; Scholle, S.H.; Maciejewski, M.L. Lab-based and diagnosis-based chronic kidney disease recognition and staging concordance. BMC Nephrol. 2019, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ikenoue, T.; Saito, Y.; Fukuma, S. Undiagnosed and untreated chronic kidney disease and its impact on renal outcomes in the Japanese middle-aged general population. J. Epidemiol. Community Health 2019, 73, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Wanner, C.; Schich, M.; Kotseva, K.; Wood, D.; Hartmann, K.; Fette, G.; Rücker, V.; Oezkur, M.; Störk, S.; et al. Patient’s and physician’s awareness of kidney disease in coronary heart disease patients-a cross-sectional analysis of the German subset of the EUROASPIRE IV survey. BMC Nephrol. 2017, 18, 321. [Google Scholar] [CrossRef]
- Hsiao, L.L. Raising awareness, screening and prevention of chronic kidney disease: It takes more than a village. Nephrology 2018, 23 (Suppl. 4), 107–111. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Moriyama, T.; Schneider, M.P.; Virgitti, J.B.; De Nicola, L.; Arnold, M.; Barone, S.; Peach, E.; Wittbrodt, E.; Chen, H.; et al. Prevalence of undiagnosed stage 3 chronic kidney disease in France, Germany, Italy, Japan and the USA: Results from the multinational observational REVEAL-CKD study. BMJ Open 2023, 13, e067386. [Google Scholar] [CrossRef]
- Sundström, J.; Bodegard, J.; Bollmann, A.; Vervloet, M.G.; Mark, P.B.; Karasik, A.; Taveira-Gomes, T.; Botana, M.; Birkeland, K.I.; Thuresson, M.; et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 24 million patients from 11 countries: The CaReMe CKD study. Lancet Reg. Health Eur. 2022, 20, 100438. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Alicic, R.Z.; Duru, O.K.; Jones, C.R.; Daratha, K.B.; Nicholas, S.B.; McPherson, S.M.; Neumiller, J.J.; Bell, D.S.; Mangione, C.M.; et al. Clinical Characteristics of and Risk Factors for Chronic Kidney Disease Among Adults and Children: An Analysis of the CURE-CKD Registry. JAMA Netw. Open 2019, 2, e1918169. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Rathmann, W.; Bongaerts, B.; Carius, H.J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef]
- Kostev, K.; Lucas, A.; Jacob, L. Frequency of Blood Pressure and Estimated Glomerular Filtration Rate Testing in Type 2 Diabetes Mellitus: A Retrospective Study with 43,509 Patients. Exp. Clin. Endocrinol. Diabetes 2019, 127, 455–460. [Google Scholar] [CrossRef]
- Roderburg, C.; Krieg, S.; Krieg, A.; Demir, M.; Luedde, T.; Kostev, K.; Loosen, S.H. Non-alcoholic fatty liver disease (NAFLD) is associated with an increased incidence of chronic kidney disease (CKD). Eur. J. Med. Res. 2023, 28, 153. [Google Scholar] [CrossRef] [PubMed]
- Chudleigh, R.A.; Ollerton, R.L.; Dunseath, G.; Peter, R.; Harvey, J.N.; Luzio, S.; Owens, D.R. Performance of the revised ‘175′ Modification of Diet in Renal Disease equation in patients with type 2 diabetes. Diabetologia 2008, 51, 1714–1718. [Google Scholar] [CrossRef]
- Al-Maqbali, S.R.; Mula-Abed, W.A. Comparison between Three Different Equations for the Estimation of Glomerular Filtration Rate in Omani Patients with Type 2 Diabetes Mellitus. Sultan Qaboos Univ. Med J. 2014, 14, e197–e203. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Maruyama, S.; Chishima, N.; Akiyama, H.; Shimamoto, K.; Inokuchi, S.; Yokota, K.; Ozaki, A. Population characteristics and diagnosis rate of chronic kidney disease by eGFR and proteinuria in Japanese clinical practice: An observational database study. Sci. Rep. 2024, 14, 5172. [Google Scholar] [CrossRef]
- Bikbov, B.; Perico, N.; Remuzzi, G.; on behalf of the GBD Genitourinary Diseases Expert Group. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron 2018, 139, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, M.J.; Krenn, S.; Kurnikowski, A.; Bretschneider, P.; Sattler, M.; Schwaiger, E.; Antlanger, M.; Gauckler, P.; Pirklbauer, M.; Brunner, M.; et al. Chronic kidney disease is more prevalent among women but more men than women are under nephrological care: Analysis from six outpatient clinics in Austria 2019. Wien. Klin. Wochenschr. 2023, 135, 89–96. [Google Scholar] [CrossRef]
- Chesnaye, N.C.; Carrero, J.J.; Hecking, M.; Jager, K.J. Differences in the epidemiology, management and outcomes of kidney disease in men and women. Nat. Rev. Nephrol. 2024, 20, 7–20. [Google Scholar] [CrossRef]
- Ärnlöv, J.; Nowak, C. Association between albuminuria, incident cardiovascular events, and mortality in persons without hypertension, diabetes, and cardiovascular disease. Eur. J. Prev. Cardiol. 2022, 29, e4–e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Mavrakanas, T.A.; Tsoukas, M.A.; Brophy, J.M.; Sharma, A.; Gariani, K. SGLT-2 inhibitors improve cardiovascular and renal outcomes in patients with CKD: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 15922. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, A.; Abdul-Ghani, M.; Gazit, S.; Gross, A.; Livnat, I.; Greenbloom, M.; Yarden, A.; Khazim, K.; Shehadeh, N.; Melzer Cohen, C. Real-world effectiveness of sodium-glucose cotransporter-2 inhibitors on the progression of chronic kidney disease in patients without diabetes, with and without albuminuria. Diabetes Obes. Metab. 2024, 26, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- EMPA-KIDNEY Collaborative Group. Impact of primary kidney disease on the effects of empagliflozin in patients with chronic kidney disease: Secondary analyses of the Empa-Kidney trial. Lancet Diabetes Endocrinol. 2024, 12, 51–60. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.J.; Ang, K.; Lee, J.; Chan, C.; Gurung, R.L.; Zheng, H.; Tang, J.; Lim, S.C. Incident heart failure and the subsequent risk of progression to end stage kidney disease in individuals with type 2 diabetes. Cardiovasc. Diabetol. 2024, 23, 204. [Google Scholar] [CrossRef]
- Pasea, L.; Dashtban, A.; Mizani, M.; Bhuva, A.; Morris, T.; Mamza, J.B.; Banerjee, A. Risk factors, outcomes and healthcare utilisation in individuals with multimorbidity including heart failure, chronic kidney disease and type 2 diabetes mellitus: A national electronic health record study. Open Heart 2023, 10, e002332. [Google Scholar] [CrossRef]
- Shen, Y.; Cai, R.; Sun, J.; Dong, X.; Huang, R.; Tian, S.; Wang, S. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 2017, 55, 66–76. [Google Scholar] [CrossRef]
- Wu, M.; Teng, T.K.; Tay, W.; Ren, Q.; Tromp, J.; Ouwerkerk, W.; Chandramouli, C.; Huang, J.; Chan, Y.; Teramoto, K.; et al. Chronic kidney disease begets heart failure and vice versa: Temporal associations between heart failure events in relation to incident chronic kidney disease in type 2 diabetes. Diabetes Obes. Metab. 2023, 25, 707–715. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Ahmed, S.; El Nahas, M. Microalbuminuria: Marker or maker of cardiovascular disease. Nephron. Exp. Nephrol. 2011, 119 (Suppl. S1), e6–e10. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Parving, H.-H.; Henning, R.H. Microalbuminuria as an early marker for cardiovascular disease. J. Am. Soc. Nephrol. 2006, 17, 2100–2105. [Google Scholar] [CrossRef]

| Variable | N (%) or Mean (SD) |
|---|---|
| N | 113,996 |
| Age (mean, SD) | 76.5 (10.1) |
| Age group (N, %) | |
| Age <60 years | 7414 (6.5) |
| Age 60–69 years | 18,072 (15.9) |
| Age 70–79 years | 37,146 (32.6) |
| Age ≥ 80 years | 51,364 (45.1) |
| Sex (N, %) | |
| Women | 68,683 (60.2) |
| Men | 45,413 (39.8) |
| Comorbidities (N, %) | |
| Type 2 diabetes mellitus | 54,278 (47.6) |
| Heart failure | 33,846 (29.7) |
| Neither diabetes mellitus nor heart failure | 44,017 (38.6) |
| eGFR/UACR category (N, %) | |
| eGFR not available; UACR ≥ 30 mg/g | 652 (0.6) |
| eGFR ≥ 90 mL/min/1.73 m–UACR ≥ 30 mg/g | 813 (0.7) |
| eGFR 60–89 mL/min/1.73 m–UACR ≥ 30 mg/g | 1158 (1.0) |
| eGFR 45–59 mL/min/1.73 m | 81,543 (71.5) |
| eGFR 30–44 mL/min/1.73 m | 24,044 (21.1) |
| eGFR 15–29 mL/min/1.73 m | 5258 (4.6) |
| End-stage kidney disease (eGFR < 15 mL/min/1.73 m) | 528 (0.5) |
| UACR < 30 mg/g | 1119 (1.0) |
| UACR 30–300 mg/g | 2470 (2.2) |
| UACR > 300 mg/g | 1178 (1.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostev, K.; Lang, M.; Tröbs, S.-O.; Urbisch, S.; Gabler, M. The Underdiagnosis of Chronic Kidney Disease in Patients with a Documented Estimated Glomerular Filtration Rate and/or Urine Albumin–Creatinine Ratio in Germany. Medicina 2025, 61, 843. https://doi.org/10.3390/medicina61050843
Kostev K, Lang M, Tröbs S-O, Urbisch S, Gabler M. The Underdiagnosis of Chronic Kidney Disease in Patients with a Documented Estimated Glomerular Filtration Rate and/or Urine Albumin–Creatinine Ratio in Germany. Medicina. 2025; 61(5):843. https://doi.org/10.3390/medicina61050843
Chicago/Turabian StyleKostev, Karel, Martina Lang, Sven-Oliver Tröbs, Sophia Urbisch, and Maximilian Gabler. 2025. "The Underdiagnosis of Chronic Kidney Disease in Patients with a Documented Estimated Glomerular Filtration Rate and/or Urine Albumin–Creatinine Ratio in Germany" Medicina 61, no. 5: 843. https://doi.org/10.3390/medicina61050843
APA StyleKostev, K., Lang, M., Tröbs, S.-O., Urbisch, S., & Gabler, M. (2025). The Underdiagnosis of Chronic Kidney Disease in Patients with a Documented Estimated Glomerular Filtration Rate and/or Urine Albumin–Creatinine Ratio in Germany. Medicina, 61(5), 843. https://doi.org/10.3390/medicina61050843






