Variations in Oppel–Kundt Illusion Strength Among Depressive and Schizophrenia Spectrum Disorder Groups: Impact of Benzodiazepine Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Design
2.2. Apparatus
2.3. Stimuli and Procedure
2.4. Drugs
2.5. Statistical Analysis
3. Results
3.1. Mental Disorders and O-K Figures
3.2. Medication and O-K Figures
4. Discussion
4.1. Depression
4.2. Schizophrenia Spectrum Disorder
4.3. Benzodiazepines
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| O-K | Oppel–Kundt |
| BZD | benzodiazepine |
| LUHS | Lithuanian University of Health Sciences |
| SSDG | schizophrenia spectrum disorder group |
| DDG | depressive disorder group |
| SSDs | schizophrenia spectrum disorders |
References
- Kundt, A. Untersuchungen Über Augenwass Und Optische Täuschungee. (Researches on Ocular Size and Optic Illusions). Ann. Der Phys. 1863, 120, 118–158. [Google Scholar] [CrossRef]
- Bertulis, A.; Surkys, T.; Bulatov, A.; Bielevičius, A. Temporal Dynamics of the Oppel-Kundt Illusion Compared to the Müller-Lyer Illusion. Acta Neurobiol. Exp. 2014, 74, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Bulatov, A.; Bulatova, N.; Surkys, T.; Mickienė, L. An Effect of Continuous Contextual Filling in the Filled-Space Illusion. Acta Neurobiol. Exp. 2017, 77, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Bulatov, A.; Marma, V.; Bulatova, N.; Mickienė, L. The Filled-Space Illusion Induced by a Single-Dot Distractor. Available online: https://pubmed.ncbi.nlm.nih.gov/31038484/ (accessed on 26 August 2020).
- Marma, V.; Bulatov, A.; Bulatova, N. Dependence of the Filled-Space Illusion on the Size and Location of Contextual Distractors. Acta Neurobiol. Exp. 2020, 80, 139–159. [Google Scholar] [CrossRef]
- Bertulis, A.; Surkys, T.; Bulatov, A.; Gutauskas, A. Three-Part Oppel-Kundt Illusory Figure. Medicina 2009, 45, 871. [Google Scholar] [CrossRef]
- Manfredini, A.; Mancini, F.; Posteraro, L.; Savazzi, S. The Two Sides of Spatial Representation in Neglect Patients: The Same Spatial Distortion for Different Patterns of Performance. Neuropsychologia 2013, 51, 1867–1877. [Google Scholar] [CrossRef]
- Silverstein, S.M.; Keane, B.P. Perceptual Organization Impairment in Schizophrenia and Associated Brain Mechanisms: Review of Research from 2005 to 2010. Schizophr. Bull. 2011, 37, 690–699. [Google Scholar] [CrossRef]
- Clifford, C.W.G. The Tilt Illusion: Phenomenology and Functional Implications. Vis. Res. 2014, 104, 3–11. [Google Scholar] [CrossRef]
- Pessoa, V.F.; Monge-Fuentes, V.; Simon, C.Y.; Suganuma, E.; Tavares, M.C.H. The Müller-Lyer Illusion as a Tool for Schizophrenia Screening. Rev. Neurosci. 2008, 19, 91–100. [Google Scholar] [CrossRef]
- Diržius, E.; Liutkevičius, J.; Žukauskaitė, G.; Leskauskas, D.; Bulatov, A. Müller-Lyer Illusion Manifestation Peculiarities among People with Schizophrenia and Bipolar Disorders: A Pilot Study. Biol. Psychiatry Psychopharmacol. 2013, 15, 43–46. [Google Scholar]
- King, D.J.; Hodgekins, J.; Chouinard, P.A.; Chouinard, V.A.; Sperandio, I. A Review of Abnormalities in the Perception of Visual Illusions in Schizophrenia. Psychon. Bull. Rev. 2017, 24, 734–751. [Google Scholar] [CrossRef] [PubMed]
- Haß, K.; Sinke, C.; Reese, T.; Roy, M.; Wiswede, D.; Dillo, W.; Oranje, B.; Szycik, G.R. Enlarged Temporal Integration Window in Schizophrenia Indicated by the Double-Flash Illusion. Cogn. Neuropsychiatry 2017, 22, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; Rosen, R. Schizophrenia and the Eye. Schizophr. Res. Cogn. 2015, 2, 46–55. [Google Scholar] [CrossRef]
- Fam, J.; Rush, A.J.; Haaland, B.; Barbier, S.; Luu, C. Visual Contrast Sensitivity in Major Depressive Disorder. J. Psychosom. Res. 2013, 75, 83–86. [Google Scholar] [CrossRef]
- Bubl, E.; Tebartz Van Elst, L.; Gondan, M.; Ebert, D.; Greenlee, M.W. Vision in Depressive Disorder. World J. Biol. Psychiatry 2009, 10, 377–384. [Google Scholar] [CrossRef]
- Beckers, T.; Wagemans, J.; Boucart, M.; Giersch, A. Different Effects of Lorazepam and Diazepam on Perceptual Integration. Vis. Res. 2001, 41, 2297–2303. [Google Scholar] [CrossRef]
- Gelbtuch, M.H.; Calvert, J.E.; Harris, J.P.; Phillipson, O.T. Modification of Visual Orientation Illusions by Drugs Which Influence Dopamine and GABA Neurones: Differential Effects on Simultaneous and Successive Illusions. Psychopharmacology 1986, 90, 379–383. [Google Scholar] [CrossRef]
- Giersch, A.; Boucart, M.; Danion, J.M.; Vidailhet, P.; Legrand, F. Effects of Lorazepam on Perceptual Integration of Visual Forms in Healthy Volunteers. Psychopharmacology 1995, 119, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Giersch, A.; Herzog, M.H. Lorazepam Strongly Prolongs Visuals Information Processing. Neuropsychopharmacology 2004, 29, 1386–1394. [Google Scholar] [CrossRef]
- Harris, J.P.; Phillipson, O.T. Effects of Lorazepam on Human Contrast Sensitivity. Psychopharmacology 1995, 117, 379–384. [Google Scholar] [CrossRef]
- Pompéia, S.; Pradella-Hallinan, M.; Manzano, G.M.; Bueno, O.F.A. Effects of Lorazepam on Visual Perceptual Abilities. Hum. Psychopharmacol. 2008, 23, 183–192. [Google Scholar] [CrossRef]
- Wagemans, J.; Notebaert, W.; Boucart, M. Lorazepam but Not Diazepam Impairs Identification of Pictures on the Basis of Specific Contour Fragments. Psychopharmacology 1998, 138, 326–333. [Google Scholar] [CrossRef]
- Letourneau, J.E. The Oppel-Kundt and the Müller-Lyer Illusions among Schizophrenics. Percept. Mot. Ski. 1974, 39, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, J.; Lavoie, G.; Delorme, A. La Perception Des Illusions de Mueller-Lyer et D’Oppel-Kundt Chez Les Deficients Mentaux. Can. J. Psychol. 1963, 17, 259–263. [Google Scholar] [PubMed]
- Sperandio, I.; Chouinard, P.A.; Paice, E.; Griffiths-King, D.J.; Hodgekins, J. Visual Illusions in Young People Reporting Psychotic-like Experiences. J. Behav. Ther. Exp. Psychiatry 2023, 79, 101839. [Google Scholar] [CrossRef]
- Overall, J.E.; Gorham, D.R. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Coren, S.; Girgus, J.S. Differentiation and Decrement in the Mueller-Lyer Illusion. Percept. Psychophys. 1972, 12, 466–470. [Google Scholar] [CrossRef]
- Judd, C.H. Practice and Its Effects on the Perception of Illusions. Psychol. Rev. 1902, 9, 27–39. [Google Scholar] [CrossRef]
- Predebon, J. Decrement of the Müller-Lyer and Poggendorff Illusions: The Effects of Inspection and Practice. Psychol. Res. 2005, 70, 384–394. [Google Scholar] [CrossRef]
- The Ashton Manual; Ashton, H. , Ed.; Routledge: London, UK; New York, NY, USA, 2002; pp. 15–16. [Google Scholar]
- Woods, S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry 2003, 64, 663–667. [Google Scholar] [CrossRef]
- Bubl, E.; Ebert, D.; Kern, E.; Van Elst, L.T.; Bach, M. Effect of Antidepressive Therapy on Retinal Contrast Processing in Depressive Disorder. Br. J. Psychiatry 2012, 201, 151–158. [Google Scholar] [CrossRef]
- Friedel, E.B.N.; Tebartz van Elst, L.; Schmelz, C.; Ebert, D.; Maier, S.; Endres, D.; Runge, K.; Domschke, K.; Bubl, E.; Kornmeier, J.; et al. Replication of Reduced Pattern Electroretinogram Amplitudes in Depression with Improved Recording Parameters. Front. Med. 2021, 8, 732222. [Google Scholar] [CrossRef]
- Luo, X.; Frishman, L.J. Retinal Pathway Origins of the Pattern Electroretinogram (PERG). Investig. Ophthalmol. Vis. Sci. 2011, 52, 8571. [Google Scholar] [CrossRef]
- Bubl, E.; Kern, E.; Ebert, D.; Riedel, A.; Tebartz van Elst, L.; Bach, M. Retinal Dysfunction of Contrast Processing in Major Depression Also Apparent in Cortical Activity. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Pickard, G.E.; So, K.F.; Pu, M. Dorsal Raphe Nucleus Projecting Retinal Ganglion Cells: Why Y Cells? Neurosci. Biobehav. Rev. 2015, 57, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Kogata, T.; Iidaka, T. A Review of Impaired Visual Processing and the Daily Visual World in Patients with Schizophrenia. Nagoya J. Med. Sci. 2018, 80, 317–328. [Google Scholar] [CrossRef]
- Parnas, J.; Vianin, P.; Saebye, D.; Jansson, L.; Volmer-Larsen, a; Bovet, P. Visual Binding Abilities in the Initial and Advanced Stages of Schizophrenia. Acta Psychiatr. Scand. 2001, 103, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.L.; Costa, D.L.; Pessoa, V.F.; Caixeta, F.V.; Maior, R.S. Systematic Review of Visual Illusions in Schizophrenia. Schizophr. Res. 2023, 252, 13–22. [Google Scholar] [CrossRef]
- Coren, S.; Ward, L.M. Levels of Processing in Visual Illusions: The Combination and Interaction of Distortion-Producing Mechanisms. J. Exp. Psychol. Hum. Percept. Perform. 1979, 5, 324–335. [Google Scholar] [CrossRef]
- Bulatov, A.; Bulatova, N.; Marma, V.; Kučinskas, L. Quantitative Study of Asymmetry in the Manifestation of the Wings-in and Wings-out Versions of the Müller-Lyer Illusion. Atten. Percept. Psychophys. 2022, 84, 560–575. [Google Scholar] [CrossRef]
- Elliott, M.A.; Giersch, A.; Seifert, D. Some Facilitatory Effects of Lorazepam on Dynamic Visual Binding. Psychopharmacology 2006, 184, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.N.; Weingartner, H.J.; Andreason, P.; George, D.T. An Effect of Triazolam on Visual Attention and Information Processing. Psychopharmacology 1995, 121, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xie, J.; Yuan, Y.; Cheng, Z.; Han, X.; Yang, L.; Yu, X.; Shi, C. Neurocognitive Effects of Atypical Antipsychotics in Patients with First-Episode Schizophrenia. Nord. J. Psychiatry 2020, 74, 594–601. [Google Scholar] [CrossRef] [PubMed]
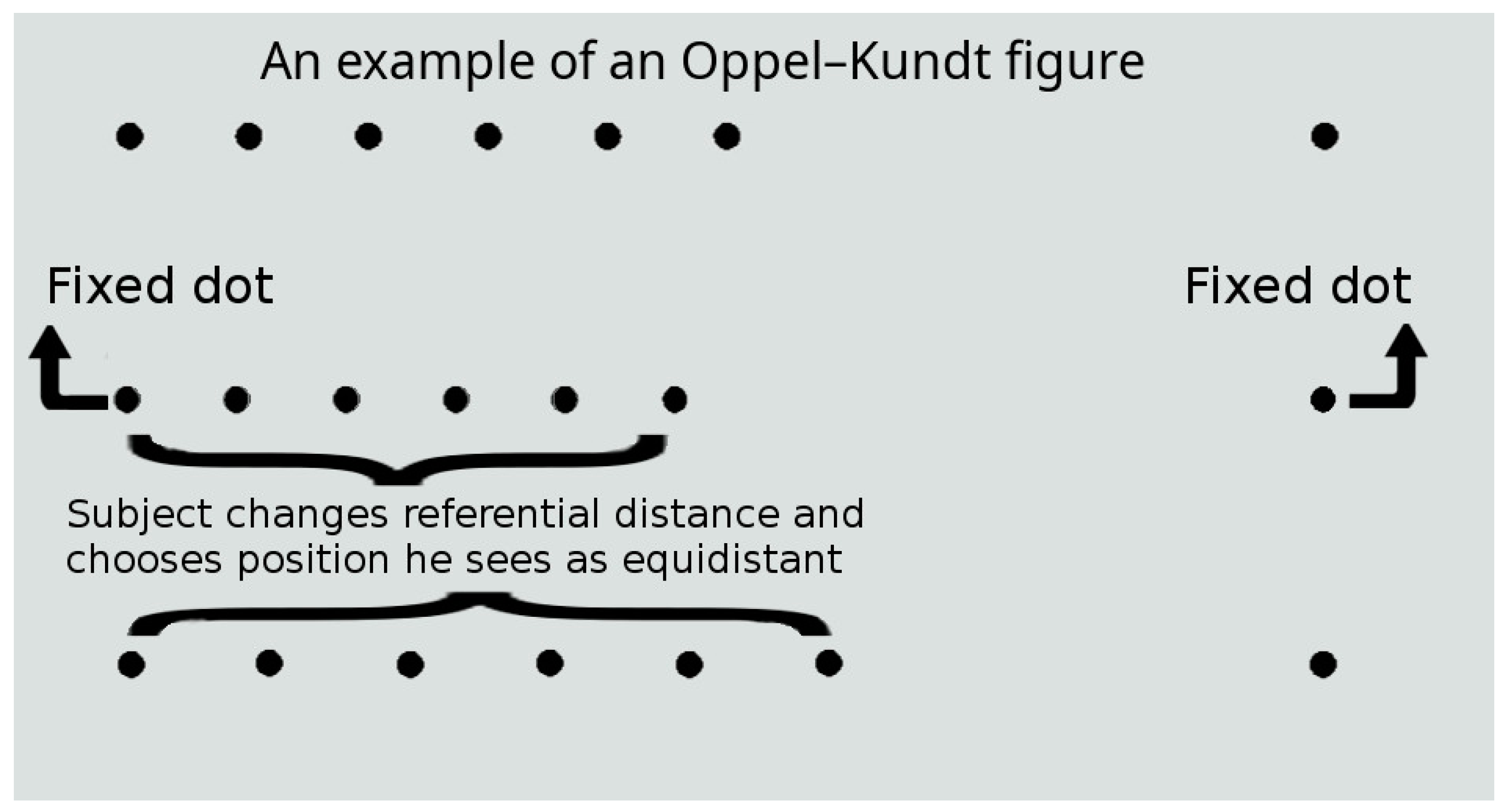
| Characteristic | Comparison | SSDG | DDG | p |
|---|---|---|---|---|
| Age, y median (min.–max.) | 28 (19–57) | 35 (18–63) | 31 (18–53) | 0.426 |
| Sex, male, n (%) | 18 (51.4) | 7 (36.8) | 10 (62.5) | 0.310 |
| Benzodiazepine usage, n (%) | - | 6 (31.6) | 11 (68.8) | 0.028 |
| Disease duration, months median (min.–max.) | - | 48 (1–360) | 5 (1–72) | 0.006 |
| Diazepam equivalents, median (min.–max.) | - | 5 (0–20) | 0 (0–30) | 0.892 |
| Chlorpromazine equivalents, median (min.–max.) | - | 200 (50–1000) | 125 (0–900) | 0.415 |
| No. of Dots | Comparison | SSDG | DDG | p | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
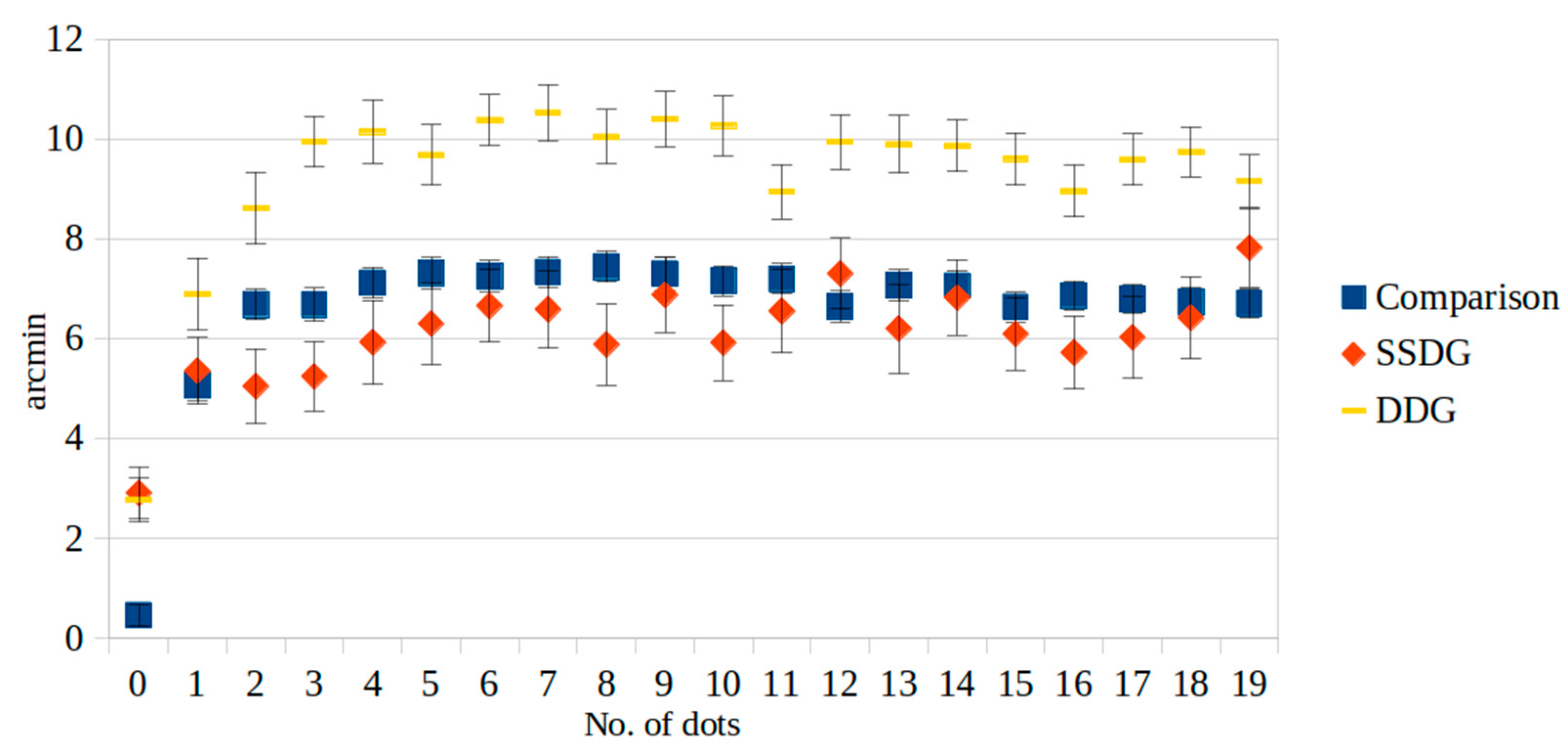
| 0 | 0.47 | 4.04 | 2.92 * | 7.00 | 2.78 * | 5.68 | <0.000 |
| 1 | 5.07 | 6.04 | 5.36 | 8.97 | 6.90 * | 9.15 | 0.042 |
| 2 | 6.69 | 5.77 | 5.05 ** | 10.07 | 8.62 * | 9.15 | <0.000 |
| 3 | 6.69 | 6.21 | 5.25 ** | 9.37 | 9.95 * | 6.44 | <0.000 |
| 4 | 7.12 | 5.91 | 5.93 ** | 11.38 | 10.15 * | 8.07 | <0.000 |
| 5 | 7.32 | 5.89 | 6.31 ** | 11.06 | 9.69 * | 7.65 | <0.000 |
| 6 | 7.26 | 5.85 | 6.66 ** | 9.81 | 10.39 * | 6.45 | <0.000 |
| 7 | 7.33 | 5.87 | 6.59 ** | 10.45 | 10.53 * | 7.15 | <0.000 |
| 8 | 7.45 | 5.78 | 5.89 ** | 10.99 | 10.05 * | 6.83 | <0.000 |
| 9 | 7.31 | 6.15 | 6.88 ** | 10.17 | 10.40 * | 7.04 | <0.000 |
| 10 | 7.16 | 5.68 | 5.92 ** | 10.26 | 10.27 * | 7.60 | <0.000 |
| 11 | 7.20 | 5.66 | 6.56 ** | 11.21 | 8.95 * | 6.89 | 0.013 |
| 12 | 6.65 | 5.77 | 7.31 ** | 9.56 | 9.95 * | 6.91 | <0.000 |
| 13 | 7.07 | 7.31 | 6.21 ** | 12.10 | 9.90 * | 7.28 | 0.002 |
| 14 | 7.06 | 5.65 | 6.83 ** | 10.13 | 9.87 * | 6.57 | <0.000 |
| 15 | 6.64 | 5.38 | 6.10 ** | 9.74 | 9.60 * | 6.65 | <0.000 |
| 16 | 6.87 | 5.38 | 5.73 ** | 9.87 | 8.97 * | 6.63 | <0.000 |
| 17 | 6.80 | 5.50 | 6.03 ** | 11.16 | 9.59 * | 6.49 | <0.000 |
| 18 | 6.74 | 5.34 | 6.43 ** | 11.06 | 9.75 * | 6.36 | <0.000 |
| 19 | 6.71 | 5.32 | 7.83 | 10.63 | 9.16 * | 6.64 | <0.000 |
| No. of Dots | Comparison | SSDG | DDG | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BZD+ | BZD− | BZD+ | BZD− | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
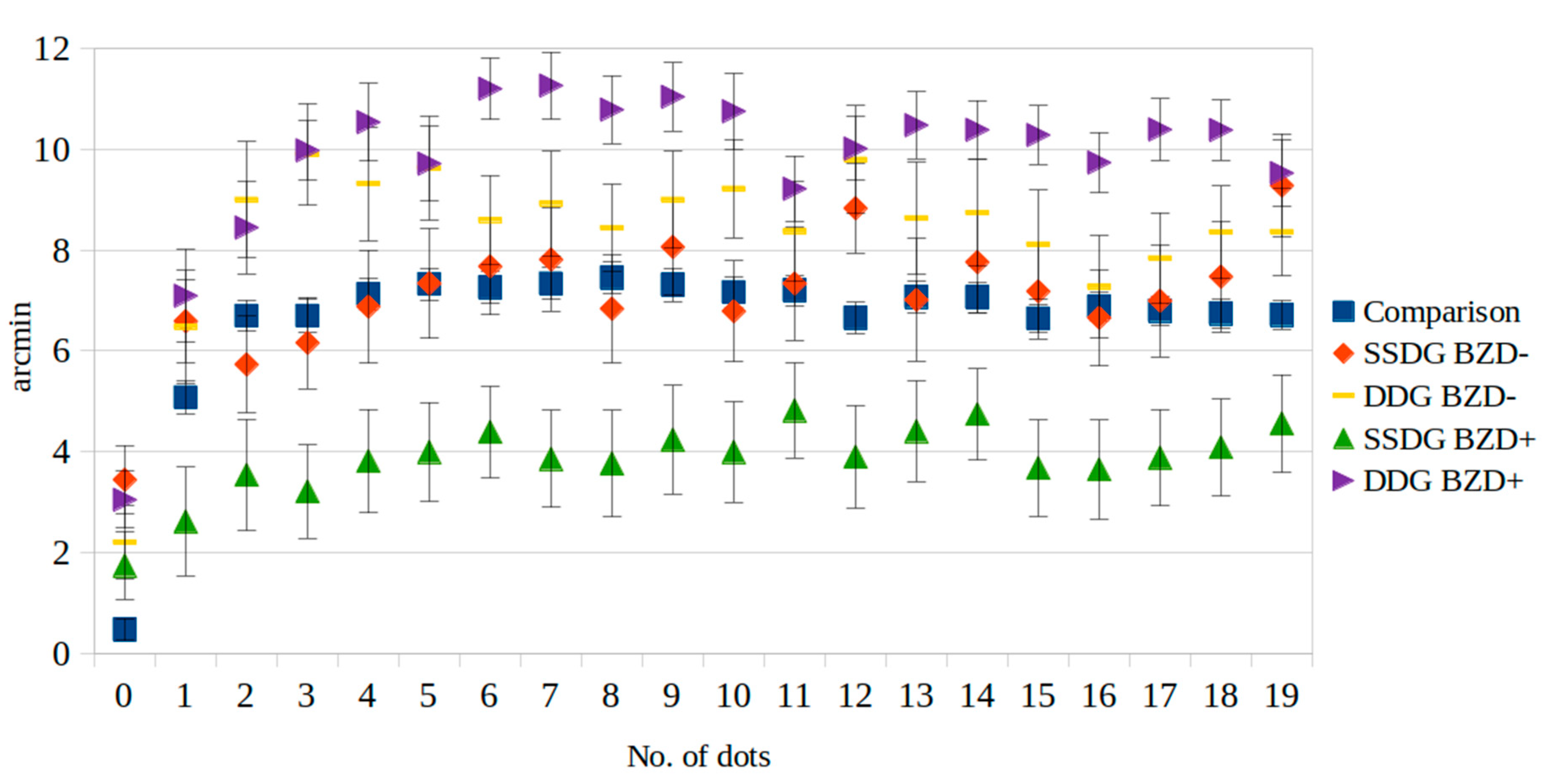
| 0 | 0.47 | 4.04 | 1.74 | 5.01 | 3.44 * | 7.69 | 3.04 * | 5.91 | 2.20 | 5.15 | <0.001 |
| 1 | 5.07 | 6.04 | 2.61 | 8.06 | 6.59 ** | 9.11 | 7.09 ** | 9.70 | 6.47 | 7.90 | 0.002 |
| 2 | 6.69 | 5.77 | 3.53 | 8.25 | 5.73 | 10.74 | 8.45 ** | 9.61 | 9.00 ** | 8.14 | <0.001 |
| 3 | 6.69 | 6.21 | 3.21 * | 6.93 | 6.16 *** | 10.17 | 9.98 *,** | 6.15 | 9.90 *,** | 7.10 | <0.001 |
| 4 | 7.12 | 5.91 | 3.81 * | 7.70 | 6.88 | 12.59 | 10.54 *,** | 8.12 | 9.31 ** | 7.97 | <0.001 |
| 5 | 7.32 | 5.89 | 3.99 * | 7.36 | 7.33 | 12.24 | 9.71 *,** | 7.85 | 9.63 ** | 7.27 | <0.001 |
| 6 | 7.26 | 5.85 | 4.39 * | 6.79 | 7.67 *** | 10.76 | 11.20 *,** | 6.41 | 8.60 ** | 6.25 | <0.001 |
| 7 | 7.33 | 5.87 | 3.86 * | 7.22 | 7.81 *** | 11.42 | 11.26 *,** | 6.97 | 8.92 ** | 7.33 | <0.001 |
| 8 | 7.45 | 5.78 | 3.76 * | 7.90 | 6.83 *** | 12.02 | 10.79 *,** | 7.05 | 8.44 ** | 6.11 | <0.001 |
| 9 | 7.31 | 6.15 | 4.24 | 8.06 | 8.06 | 10.80 | 11.04 *,** | 7.09 | 9.00 ** | 6.78 | <0.001 |
| 10 | 7.16 | 5.68 | 3.99 * | 7.45 | 6.78 *** | 11.21 | 10.75 *,** | 7.88 | 9.22 ** | 6.88 | <0.001 |
| 11 | 7.20 | 5.66 | 4.82 | 7.04 | 7.33 | 12.58 | 9.21 ** | 6.84 | 8.37 | 7.05 | 0.010 |
| 12 | 6.65 | 5.77 | 3.90 | 7.60 | 8.83 ** | 9.96 | 10.01 *,** | 6.65 | 9.80 ** | 7.52 | <0.001 |
| 13 | 7.07 | 7.31 | 4.40 | 7.46 | 7.01 | 13.61 | 10.48 *,** | 6.97 | 8.63 | 7.85 | <0.001 |
| 14 | 7.06 | 5.65 | 4.74 | 6.76 | 7.76 | 11.21 | 10.38 *,** | 6.11 | 8.74 ** | 7.44 | <0.001 |
| 15 | 6.64 | 5.38 | 3.67 * | 7.16 | 7.18 | 10.54 | 10.28 *,** | 6.09 | 8.11 ** | 7.61 | <0.001 |
| 16 | 6.87 | 5.38 | 3.64 * | 7.35 | 6.65 | 10.70 | 9.74 *,** | 6.24 | 7.27 | 7.19 | <0.001 |
| 17 | 6.80 | 5.50 | 3.88 * | 7.16 | 6.99 | 12.44 | 10.39 *,** | 6.41 | 7.84 ** | 6.36 | <0.001 |
| 18 | 6.74 | 5.34 | 4.08 | 7.18 | 7.47 | 12.28 | 10.38 *,** | 6.24 | 8.35 ** | 6.46 | <0.001 |
| 19 | 6.71 | 5.32 | 4.56 | 7.26 | 9.28 ** | 11.55 | 9.53 *,** | 6.86 | 8.36 ** | 6.13 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diržius, E.; Pakanavičiūtė, R.; Andriuškevičiūtė, D.; Leskauskas, D.; Bulatov, A. Variations in Oppel–Kundt Illusion Strength Among Depressive and Schizophrenia Spectrum Disorder Groups: Impact of Benzodiazepine Use. Medicina 2025, 61, 835. https://doi.org/10.3390/medicina61050835
Diržius E, Pakanavičiūtė R, Andriuškevičiūtė D, Leskauskas D, Bulatov A. Variations in Oppel–Kundt Illusion Strength Among Depressive and Schizophrenia Spectrum Disorder Groups: Impact of Benzodiazepine Use. Medicina. 2025; 61(5):835. https://doi.org/10.3390/medicina61050835
Chicago/Turabian StyleDiržius, Edgaras, Rasa Pakanavičiūtė, Deimantė Andriuškevičiūtė, Darius Leskauskas, and Aleksandr Bulatov. 2025. "Variations in Oppel–Kundt Illusion Strength Among Depressive and Schizophrenia Spectrum Disorder Groups: Impact of Benzodiazepine Use" Medicina 61, no. 5: 835. https://doi.org/10.3390/medicina61050835
APA StyleDiržius, E., Pakanavičiūtė, R., Andriuškevičiūtė, D., Leskauskas, D., & Bulatov, A. (2025). Variations in Oppel–Kundt Illusion Strength Among Depressive and Schizophrenia Spectrum Disorder Groups: Impact of Benzodiazepine Use. Medicina, 61(5), 835. https://doi.org/10.3390/medicina61050835






