Retinal Microvascular Profile of Patients with Coronary Artery Disease
Abstract
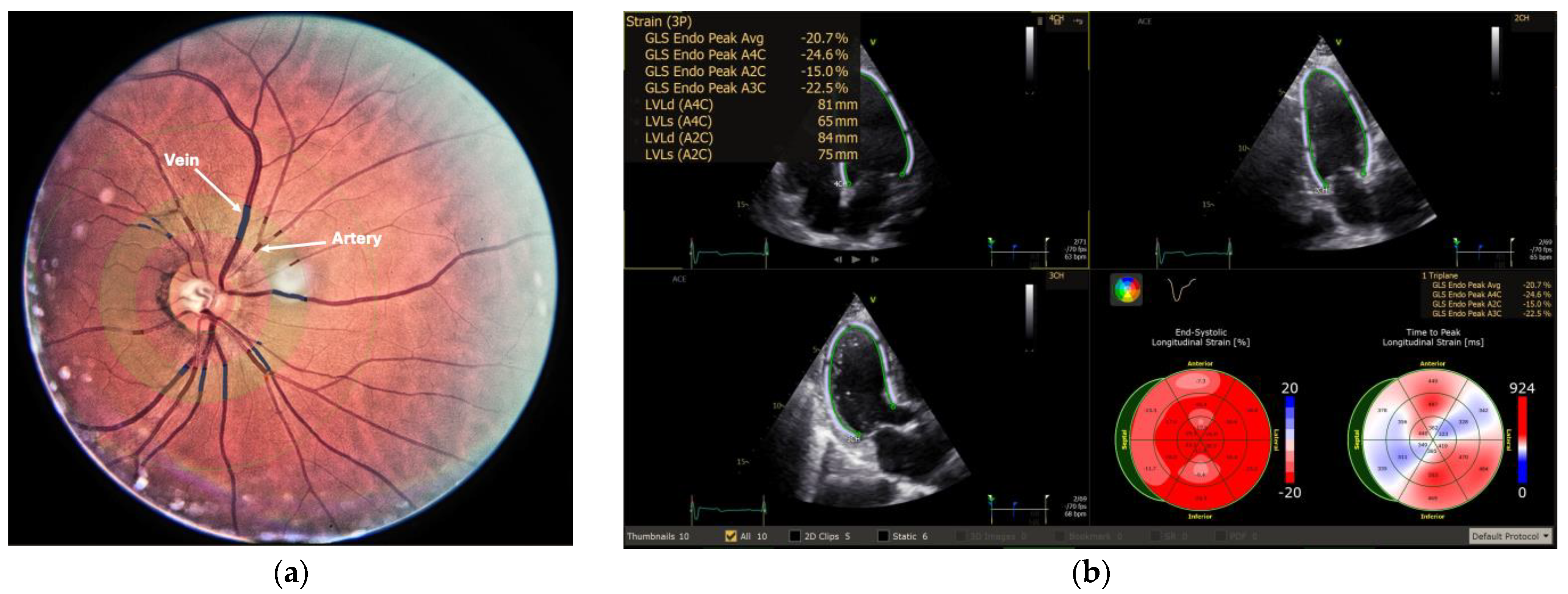
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Leading Causes of Death by Rate Worldwide. 2021. Available online: https://www.statista.com/statistics/1480277/rates-of-the-leading-causes-of-death-in-worldwide/ (accessed on 2 October 2024).
- Our World in Data. Life Expectancy Projections. Available online: https://ourworldindata.org/grapher/future-life-expectancy-projections (accessed on 2 October 2024).
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 2 October 2024).
- Luca, A.C.; David, S.G.; David, A.G.; Țarcă, V.; Pădureț, I.A.; Mîndru, D.E.; Roșu, S.T.; Roșu, E.V.; Adumitrăchioaiei, H.; Bernic, J.; et al. Atherosclerosis from Newborn to Adult—Epidemiology, Pathological Aspects and Risk Factors. Life 2023, 13, 2056. [Google Scholar] [CrossRef] [PubMed]
- Almohtasib, Y.; Fancher, A.J.; Sawalha, K. Emerging Trends in Atherosclerosis: Time to Address Atherosclerosis From a Younger Age. Cureus 2023, 16, e56635. [Google Scholar] [CrossRef] [PubMed]
- Scheie, H.G. Evaluation of Ophthalmoscopic Changes of Hypertension and Arteriolar Sclerosis. AMA Arch. Ophthalmol. 1953, 49, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Wong, T.Y. Microvascular Changes in the Retina as a Risk Marker for Cardiovascular Disease. Curr. Cardiovasc. Risk Rep. 2009, 3, 51–58. [Google Scholar] [CrossRef]
- Liebreich, R. Ophthalmoskopische Notizen. Arch. Für Ophthalmol. 1859, 5, 241–268. [Google Scholar]
- Gunn, M. Ophthalmoscopic Evidence of General Arterial Disease. Trans. Ophthalmol. Soc. UK 1898, 18, 356–381. [Google Scholar]
- Salus, R. A Contribution to the Diagnosis of Arteriosclerosis and Hypertension. Am. J. Ophthalmol. 1958, 45, 81–92. [Google Scholar] [CrossRef]
- Friedenwald, H. The Doyne Memorial Lecture: Pathological Changes in the Retinal Blood Vessels in Arteriosclerosis and Hypertension. Trans. Ophthalmol. Soc. UK 1930, 50, 452–531. [Google Scholar]
- Keith, N.M.; Wagener, H.P.; Barker, N.W. Some Different Types of Essential Hypertension: Their Course and Prognosis. Am. J. Med. Sci. 1939, 197, 332–343. [Google Scholar]
- Chandra, A.; Seidelmann, S.B.; Claggett, B.L.; Klein, B.E.; Klein, R.; Shah, A.M.; Solomon, S.D. The Association of Retinal Vessel Calibres with Heart Failure and Long-Term Alterations in Cardiac Structure and Function: The Atherosclerosis Risk in Communities (ARIC) Study. Eur. J. Heart Fail. 2019, 21, 1207–1215. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Couper, D.J.; Cooper, L.S.; Shahar, E.; Hubbard, L.D.; Wofford, M.R.; Sharrett, A.R. Retinal microvascular abnormalities and incident stroke: The Atherosclerosis Risk in Communities Study. Lancet 2001, 358, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Xie, J.; Cheung, N.; Lamoureux, E.; Klein, R.; Klein, B.E.; Cotch, M.F.; Sharrett, A.R.; Shea, S.; Wong, T.Y.; et al. Retinal microvascular signs and risk of stroke: The Multi-Ethnic Study of Atherosclerosis (MESA). Stroke 2012, 43, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Bravo, P.E.; Gupta, A.; Farhad, H.; Klein, B.E.; Klein, R.; Di Carli, M.; Solomon, S.D. Retinal Vessel Calibers in Predicting Long-Term Cardiovascular Outcomes: The Atherosclerosis Risk in Communities Study. Circulation 2016, 134, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Assmann, G.; Schulte, H.; Cullen, P.; Seedorf, U. Assessing Risk of Myocardial Infarction and Stroke: New Data from the Prospective Cardiovascular Münster (PROCAM) Study. Eur. J. Clin. Investig. 2007, 37, 925–932. [Google Scholar] [CrossRef]
- Su, C.H.; Lo, C.H.; Chen, H.H.; Tsai, C.F.; Yip, H.T.; Hsu, K.C.; Hsu, C.Y.; Kao, C.-H. CHA2DS2-VASc Score as an Independent Outcome Predictor in Patients Hospitalized with Acute Ischemic Stroke. PLoS ONE 2022, 17, e0270823. [Google Scholar] [CrossRef]
- Luyten, L.J.; Dockx, Y.; Madhloum, N.; Sleurs, H.; Gerrits, N.; Janssen, B.G.; Neven, K.Y.; Plusquin, M.; Provost, E.B.; De Boever, P.; et al. Association of Retinal Microvascular Characteristics with Short-Term Memory Performance in Children Aged 4 to 5 Years. JAMA Netw. Open 2020, 3, e2011537. [Google Scholar] [CrossRef]
- Knudtson, M.D.; Lee, K.E.; Hubbard, L.D.; Wong, T.Y.; Klein, R.; Klein, B.E. Revised Formulas for Summarizing Retinal Vessel Diameters. Curr. Eye Res. 2003, 27, 143–149. [Google Scholar] [CrossRef]
- McGowan, A.; Silvestri, G.; Moore, E.; Silvestri, V.; Patterson, C.C.; Maxwell, A.P.; McKay, G.J. Evaluation of the Retinal Vasculature in Hypertension and Chronic Kidney Disease in an Elderly Population of Irish Nuns. PLoS ONE 2015, 10, e0136434. [Google Scholar] [CrossRef]
- Dervenis, N.; Coleman, A.L.; Harris, A.; Wilson, M.R.; Yu, F.; Anastasopoulos, E.; Founti, P.; Pappas, T.; Kilintzis, V.; Topouzis, F. Factors Associated with Retinal Vessel Diameters in an Elderly Population: The Thessaloniki Eye Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2208. [Google Scholar] [CrossRef]
- Liang, C.; Gu, C.; Wang, N. Retinal Vascular Caliber in Coronary Heart Disease and Its Risk Factors. Ophthalmic Res. 2023, 66, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Rudnicka, A.R.; Welikala, R.A.; Fraz, M.M.; Barman, S.A.; Luben, R.; Hayat, S.A.; Khaw, K.-T.; Strachan, D.P.; Whincup, P.H.; et al. Retinal Vasculometry Associations with Cardiometabolic Risk Factors in the European Prospective Investigation of Cancer—Norfolk Study. Ophthalmology 2019, 126, 96–106. [Google Scholar] [CrossRef] [PubMed]
- French, C.; Cubbidge, R.P.; Heitmar, R. The Application of Arterio-Venous Ratio (AVR) Cut-Off Values in Clinic to Stratify Cardiovascular Risk in Patients. Ophthalmic Physiol. Opt. 2022, 42, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Jiang, L.; Wang, Z.; Liu, W.; Zuo, H. Diagnostic Accuracy of Global Longitudinal Strain for Detecting Exercise Intolerance in Patients with Ischemic Heart Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 10. [Google Scholar] [CrossRef]
- Tsougos, E.; Angelidis, G.; Gialafos, E.; Tzavara, C.; Tzifos, V.; Tsougos, I.; Georgoulias, P. Myocardial Strain May Predict Exercise Tolerance in Patients with Reduced and Mid-Range Ejection Fraction. Hell. J. Cardiol. 2018, 59, 331–335. [Google Scholar] [CrossRef]
- Seecheran, N.A.; Rafeeq, S.; Maharaj, N.; Swarath, S.; Seecheran, V.; Seecheran, R.; Seebalack, V.; Jagdeo, C.-L.; Quert, A.Y.L.; Giddings, S.; et al. Correlation of Retinal Artery Diameter with Coronary Artery Disease: The Retina CAD Pilot Study—Are the Eyes the Windows to the Heart? Cardiol. Ther. 2023, 12, 499–509. [Google Scholar] [CrossRef]
- Wang, N.; Liang, C. Relationship of Gensini Score with Retinal Vessel Diameter and Arteriovenous Ratio in Senile CHD. Open Life Sci. 2021, 16, 737–745. [Google Scholar] [CrossRef]
- Wei, F.F.; Thijs, L.; Melgarejo, J.D.; Cauwenberghs, N.; Zhang, Z.Y.; Liu, C.; Kuznetsova, T.; Struijker-Boudier, H.A.J.; Verhamme, P.; Dong, Y.-G.; et al. Diastolic Left Ventricular Function in Relation to the Retinal Microvascular Fractal Dimension in a Flemish Population. Hypertens. Res. 2021, 44, 446–453. [Google Scholar] [CrossRef]
- Fu, Y.; Yusufu, M.; Wang, Y.; He, M.; Shi, D.; Wang, R. Association of Retinal Microvascular Density and Complexity with Incident Coronary Heart Disease. Atherosclerosis 2023, 380, 117196. [Google Scholar] [CrossRef]
- Sandoval-Garcia, E.; McLachlan, S.; Price, A.H.; MacGillivray, T.J.; Strachan, M.W.J.; Wilson, J.F.; Price, J.F. Retinal Arteriolar Tortuosity and Fractal Dimension Are Associated with Long-Term Cardiovascular Outcomes in People with Type 2 Diabetes. Diabetologia 2021, 64, 2215–2227. [Google Scholar] [CrossRef]
- Dinesen, S.; Jensen, P.S.; Bloksgaard, M.; Blindbæk, S.L.; De Mey, J.; Rasmussen, L.M.; Lindholt, J.S.; Grauslund, J. Retinal Vascular Fractal Dimensions and Their Association with Macrovascular Cardiac Disease. Ophthalmic Res. 2021, 64, 561–566. [Google Scholar] [CrossRef]
| Variable | Control | AHT | CAD | p |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 58.9 ± 9.15 | 60.43 ± 12.40 | 64.08 ± 10.90 | 0.0121 |
| Male gender (no., %) | 29 (53.70%) | 37 (60.66%) | 76 (76.77%) | 0.0083 |
| Current smoker (no., %) | 5 (9.26%) | 5 (8.20%) | 4 (4.04%) | n.s. |
| Diabetes mellitus (no., %) | 2 (3.70%) | 10 (16.39%) | 41 (41.41%) | <0.001 |
| Mild diabetic retinopathy (no., %) | 0% | 0% | 3 (3.03%) | - |
| AMI (no., %) | 0% | 0% | 2 (2.02%) | - |
| Stroke (no., %) | 0% | 3 (4.92%) | 4 (4.04%) | - |
| CKD (no., %) | 2 (3.70%) | 6 (6.59%) | 8 (8.08%) | n.s. |
| SBP (mmHg) (mean ± SD) | 127.52 ± 10.52 | 155.71 ± 18.06 | 147.02 ± 19.04 | <0.001 |
| Total cholesterol (mg/dL) (mean ± SD) | 189.5 ± 27.18 | 195 ± 32.57 | 162.76 ± 52.22 | <0.001 |
| HDL cholesterol (mg/dL) (mean ± SD) | 54.1 ± 10.51 | 45.38 ± 14.74 | 43.07 ± 10.89 | <0.001 |
| LDL cholesterol (mg/dL) (mean ± SD) | 139.2 ± 28.63 | 116.85 ± 28.82 | 97.88 ± 43.23 | <0.001 |
| TG (mg/dL) (mean ± SD) | 177.1 ± 106.3 | 172.15 ± 96.05 | 141.64 ± 78.59 | 0.0324 |
| Variable | Control | AHT | CAD | p |
|---|---|---|---|---|
| CRAE (µm, mean ± SD) | 172.79 ± 14.32 | 165.75 ± 17.67 | 157.59 ± 16.75 | <0.001 |
| CRVE (µm, mean ± SD) | 251.66 ± 20.72 | 253.53 ± 33.87 | 236.33 ± 28.89 | <0.001 |
| AVR (mean ± SD) | 0.69 ± 0.058 | 0.66 ± 0.082 | 0.67 ± 0.078 | n.s. |
| Fractal dimension (mean ± SD) | 1.43 ± 0.030 | 1.40 ± 0.043 | 1.42 ± 0.044 | <0.001 |
| Lacunarity (median ± IQR; 25–75%) | 1.063 ± 0.035 (1.044–1.079) | 1.065 ± 0.044 (1.043–1.087) | 1.073 ± 0.034 (1.054–1.088) | n.s. |
| Tortuosity index (median ± IQR; 25–75%) | 0.884 ± 0.011 (0.879–0.890) | 0.880 ± 0.019 (0.871–0.890) | 0.876 ± 0.025 (0.865–0.890) | 0.037 |
| Variable | Beta | Standard Error | Adjusted R2 | p | |
|---|---|---|---|---|---|
| CRAE | Single | −0.349 | 0.734 | 0.118 | <0.001 |
| +medical treatment | −0.100 | 0.003 | 0.641 | 0.023 | |
| CRVE | Single | −0.205 | 0.003 | 0.038 | 0.003 |
| +medical treatment | 0.015 | 0.002 | 0.633 | 0.004 | |
| Df | Single | −0.041 | 1.955 | −0.003 | 0.547 |
| +medical treatment | −0.150 | 1.157 | 0.655 | <0.001 | |
| Tortuosity index | Single | −0.191 | 4.800 | 0.032 | 0.005 |
| +medical treatment | −0.060 | 2.985 | 0.636 | 0.153 |
| Variable | CRAE | CRVE | AVR | Df | Lacunarity | Tortuosity Index | |
|---|---|---|---|---|---|---|---|
| Age | Correlation coefficient | 0.034 | −0.07 | 0.117 | −0.271 | 0.198 | 0.010 |
| p value | 0.736 | 0.491 | 0.249 | 0.007 | 0.049 | 0.922 | |
| LDL | Correlation coefficient | −0.256 | −0.15 | −0.072 | −0.068 | −0.078 | 0.054 |
| p value | 0.022 | 0.185 | 0.524 | 0.548 | 0.492 | 0.636 | |
| HDL | Correlation coefficient | −0.167 | −0.220 | 0.076 | 0.021 | 0.067 | −0.070 |
| p value | 0.139 | 0.049 | 0.502 | 0.853 | 0.556 | 0.538 | |
| Triglycerides | Correlation coefficient | 0.007 | 0.123 | −0.125 | 0.058 | 0.044 | −0.069 |
| p value | 0.946 | 0.225 | 0.216 | 0.568 | 0.668 | 0.498 | |
| LVEF | Correlation coefficient | 0.022 | −0.033 | 0.066 | −0.102 | −0.087 | 0.097 |
| p value | 0.826 | 0.750 | 0.518 | 0.319 | 0.395 | 0.343 | |
| SBP | Correlation coefficient | −0.028 | −0.191 | −0.220 | 0.053 | 0.005 | −0.141 |
| p value | 0.040 | 0.065 | 0.034 | 0.615 | 0.964 | 0.176 | |
| DBP | Correlation coefficient | −0.014 | −0.040 | 0.009 | 0.050 | 0.035 | −0.106 |
| p value | 0.891 | 0.704 | 0.928 | 0.632 | 0.739 | 0.308 | |
| LV strain | Correlation coefficient | 0.030 | 0.058 | −0.038 | −0.137 | 0.049 | 0.256 |
| p value | 0.857 | 0.727 | 0.817 | 0.407 | 0.765 | 0.116 | |
| LA strain | Correlation coefficient | 0.077 | −0.133 | 0.231 | −0.052 | −0.182 | −0.095 |
| p value | 0.669 | 0.462 | 0.195 | 0.773 | 0.311 | 0.598 | |
| CHA2DS2-VASc Score | Correlation coefficient | 0.090 | 0.038 | 0.028 | −0.140 | −0.169 | −0.0933 |
| p value | 0.376 | 0.711 | 0.783 | 0.168 | 0.094 | 0.361 | |
| PROCAM score | Correlation coefficient | 0.245 | 0.404 | −0.258 | 0.115 | 0.100 | −0.056 |
| p value | 0.156 | 0.016 | 0.135 | 0.511 | 0.569 | 0.750 | |
| Agatston score | Correlation coefficient | 0.186 | 0.676 | −0.359 | −0.222 | −0.046 | 0.446 |
| p value | 0.523 | 0.008 | 0.208 | 0.445 | 0.876 | 0.110 |
| Variable | CRAE | CRVE | AVR | Df | Lacunarity | Tortuosity Index | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p Value | B | p Value | B | p Value | B | p Value | B | p Value | B | p Value | B | |
| Gender | 0.030 | −1.520 | 0.592 | −3.711 | 0.069 | −0.001 | 0.941 | −0.001 | 0.981 | 0.005 | 0.307 | 0.005 |
| Dyslipidaemia | 0.005 | −6.770 | 0.101 | −11.674 | 0.812 | −0.005 | 0.842 | −0.002 | 0.489 | −0.006 | 0.350 | 0.004 |
| Current smoker | 0.317 | 0.102 | 0.425 | −0.081 | 0.097 | 0.168 | 0.404 | 0.085 | 0.335 | 0.098 | 0.691 | 0.040 |
| Diabetes mellitus | 0.659 | 0.777 | 0.947 | −0.204 | 0.999 | 1.527 | 0.962 | 0.005 | 0.734 | 0.001 | 0.199 | −0.003 |
| Prior stroke | 0.680 | 3.553 | 0.398 | 12.553 | 0.544 | −0.025 | 0.811 | 0.005 | 0.285 | −0.019 | 0.786 | 0.003 |
| Prior AMI | 0.818 | 0.799 | 0.124 | 9.046 | 0.233 | −0.020 | 0.586 | 0.005 | 0.427 | 0.006 | 0.308 | −0.004 |
| NYHA class | 0.733 | −0.035 | 0.985 | −0.002 | 0.714 | −0.037 | 0.544 | 0.062 | 0.613 | 0.052 | 0.827 | −0.022 |
| Therapeutic intervention | 0.259 | −0.005 | 0.307 | −0.003 | 0.217 | −0.002 | 0.312 | 1.617 | 0.354 | 1.896 | 0.199 | −4.850 |
| Model | Unstandardised Coefficients | Standardised Coefficients | t | p | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| CRAE | |||||
| (Constant) | 221.977 | 20.832 | 10.655 | 0.000 | |
| Dyslipidaemia | −15.926 | 5.603 | −0.430 | −2.842 | 0.009 |
| SBP | −17.091 | 5.983 | −0.493 | −2.856 | 0.008 |
| LV strain | 1.784 | 0.954 | 0.323 | 1.870 | 0.043 |
| LDL | −0.195 | 0.060 | −0.510 | −3.242 | 0.003 |
| CRVE | |||||
| (Constant) | 278.330 | 25.342 | 10.983 | 0.000 | |
| Agatston Score | 0.031 | 0.011 | 0.541 | 2.940 | 0.016 |
| HDL | −1.486 | 0.515 | −0.531 | −2.888 | 0.018 |
| AVR | |||||
| (Constant) | 0.542 | 0.063 | 8.639 | 0.000 | |
| SBP | −0.001 | 0.000 | −0.258 | −2.524 | 0.013 |
| TG | 0.000 | 0.000 | −0.195 | −1.907 | 0.060 |
| Df | |||||
| (Constant) | 1.704 | 0.083 | 20.409 | 0.000 | |
| LVEF | 0.004 | 0.001 | 0.629 | 3.014 | 0.006 |
| DM | −0.021 | 0.008 | −0.431 | −2.650 | 0.014 |
| AMI | −0.052 | 0.021 | −0.537 | −2.515 | 0.018 |
| Medical treatment | 0.035 | 0.011 | 0.508 | 3.088 | 0.005 |
| Dyslipidaemia | −0.050 | 0.018 | −0.465 | −2.752 | 0.011 |
| Age | −0.033 | 0.022 | −0.223 | −1.452 | 0.158 |
| Lacunarity | |||||
| (Constant) | 1.376 | 0.041 | 33.874 | 0.000 | |
| HDL | −0.001 | 0.000 | −0.569 | −3.693 | 0.014 |
| Age | 0.002 | 0.000 | 1.056 | 8.600 | 0.000 |
| LVEF | −0.002 | 0.000 | −0.484 | −4.734 | 0.005 |
| Agatston Score | 3.664 | 0.000 | 0.841 | 6.841 | 0.001 |
| Gender | −0.046 | 0.010 | −0.740 | −4.812 | 0.005 |
| Tortuosity index | |||||
| (Constant) | 0.905 | 0.020 | 45.227 | 0.000 | |
| Gender | 0.022 | 0.008 | 0.431 | 2.914 | 0.006 |
| LV strain | 0.002 | 0.001 | 0.292 | 2.026 | 0.050 |
| Medical treatment | 0.010 | 0.005 | 0.312 | 2.101 | 0.043 |
| q = 0.25 | q = 0.5 | q = 0.75 | |
|---|---|---|---|
| CRAE | |||
| Pseudo R Squared | 0.179 | 0.359 | 0.413 |
| MAE | 11.0223 | 8.7129 | 9.8379 |
| CRVE | |||
| Pseudo R Squared | 0.536 | 0.515 | 0.554 |
| MAE | 14.1492 | 12.4089 | 16.9146 |
| AVR | |||
| Pseudo R Squared | 0.363 | 0.261 | 0.418 |
| MAE | 0.0691 | 0.0631 | 0.0984 |
| Df | |||
| Pseudo R Squared | 0.359 | 0.303 | 0.324 |
| MAE | 0.0266 | 0.0246 | 0.0357 |
| Lacunarity | |||
| Pseudo R Squared | 0.376 | 0.269 | 0.575 |
| MAE | 0.0287 | 0.0254 | 0.0286 |
| Tortuosity index | |||
| Pseudo R Squared | 0.212 | 0.195 | 0.147 |
| MAE | 0.0208 | 0.0140 | 0.0192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, A.C.; Chistol, R.O.; Tinica, G.; Furnica, C.; Damian, S.I.; David, S.M.; Brînzaniuc, K.; Horvath, K.U. Retinal Microvascular Profile of Patients with Coronary Artery Disease. Medicina 2025, 61, 834. https://doi.org/10.3390/medicina61050834
Rusu AC, Chistol RO, Tinica G, Furnica C, Damian SI, David SM, Brînzaniuc K, Horvath KU. Retinal Microvascular Profile of Patients with Coronary Artery Disease. Medicina. 2025; 61(5):834. https://doi.org/10.3390/medicina61050834
Chicago/Turabian StyleRusu, Alexandra Cristina, Raluca Ozana Chistol, Grigore Tinica, Cristina Furnica, Simona Irina Damian, Sofia Mihaela David, Klara Brînzaniuc, and Karin Ursula Horvath. 2025. "Retinal Microvascular Profile of Patients with Coronary Artery Disease" Medicina 61, no. 5: 834. https://doi.org/10.3390/medicina61050834
APA StyleRusu, A. C., Chistol, R. O., Tinica, G., Furnica, C., Damian, S. I., David, S. M., Brînzaniuc, K., & Horvath, K. U. (2025). Retinal Microvascular Profile of Patients with Coronary Artery Disease. Medicina, 61(5), 834. https://doi.org/10.3390/medicina61050834






