Assessment of Decongestion Status Before Discharge in Acute Decompensated Heart Failure: A Review of Clinical, Biochemical, and Imaging Tools and Their Impact on Management Decisions
Abstract
1. Introduction
- intravascular congestion (increased fluid volume within the vascular system) which manifests as jugular venous distension (JVD) and hepatojugular reflux, directly reflecting elevated central venous pressure (CVP > 10 mmHg), and also as dyspnea, orthopnea, and bendopnea [7];
- tissue congestion (extravascular congestion) which presents as peripheral edema, pulmonary rales, and ascites, indicating fluid extravasation into interstitial spaces due to sustained capillary leakage [8].
2. Methods of Congestion Assessment and Their Role in Discharge Decision-Making
2.1. Methods of Congestion Assessment
2.1.1. Clinical Evaluation
2.1.2. Laboratory Assessment
- 1.
- Natriuretic Peptides (NPs)
- 2.
- Hemoconcentration
- 3.
- Renal function markers
- 4.
- Hepatic markers
- 5.
- Emerging biomarkers
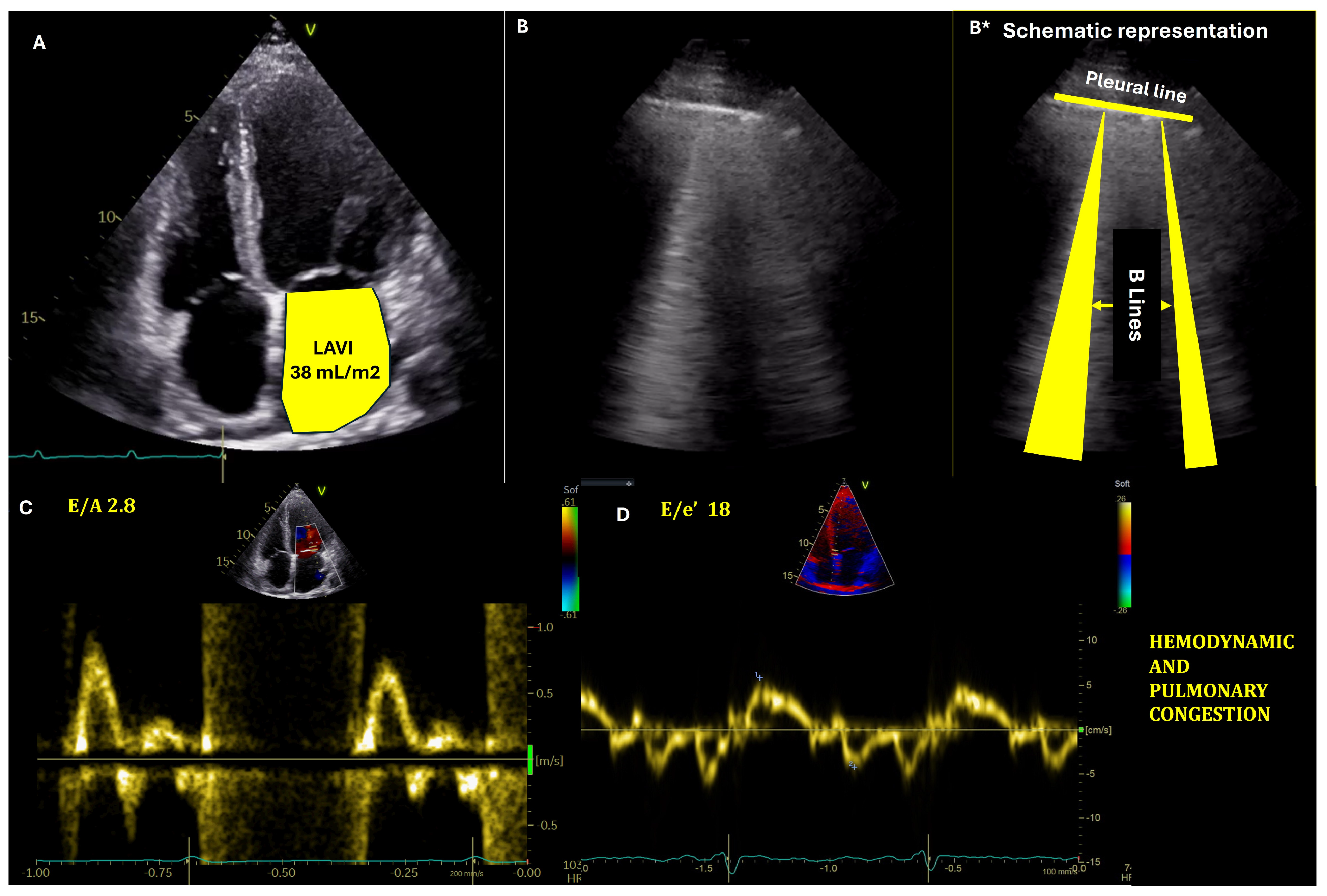
2.1.3. Imaging Tools
- A.
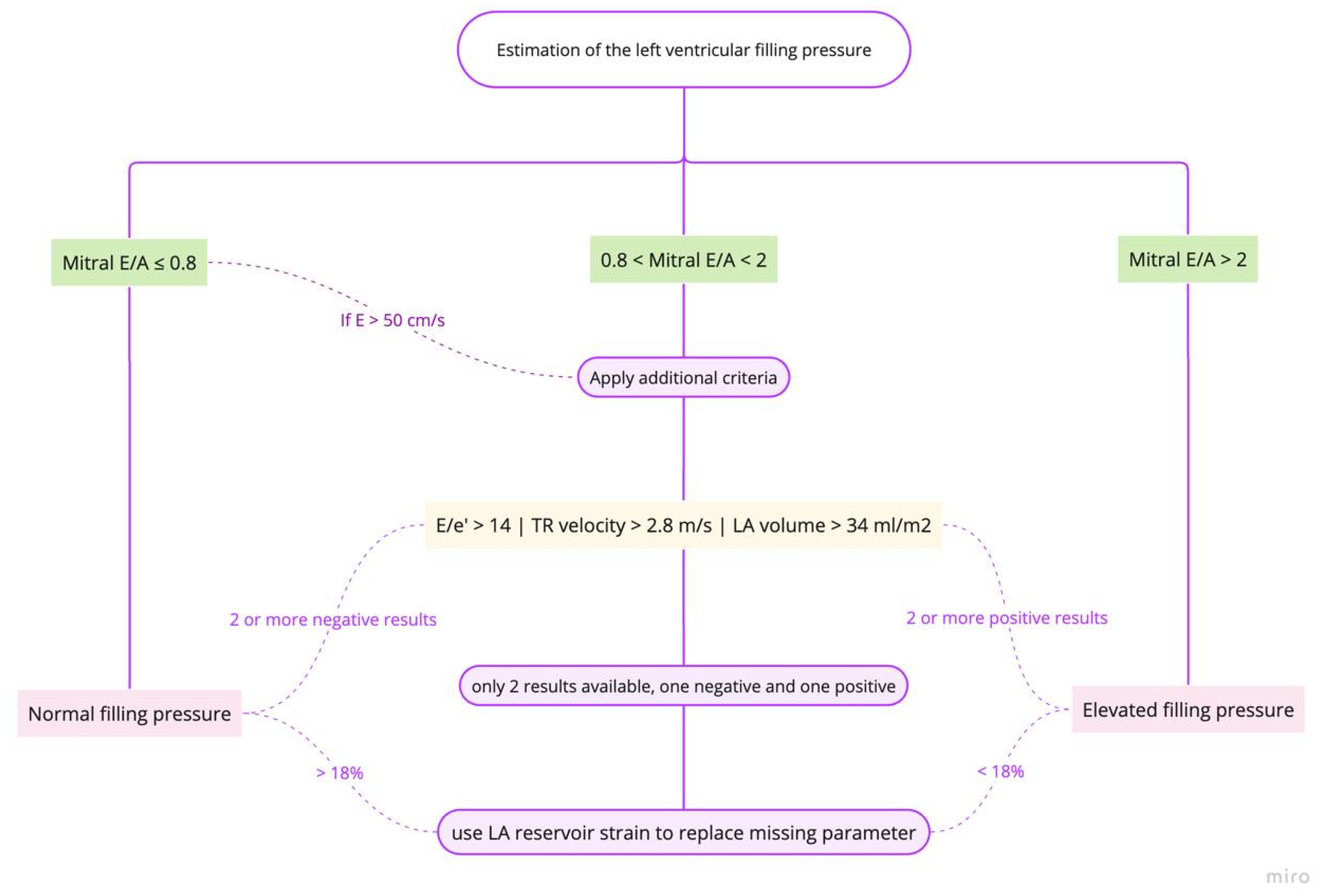
- Estimation of left ventricular (LV) filling pressures
- B.
- The role of Lung ultrasound in congestion assesment
- C.
- Estimation of right ventricular (RV) filling pressures
- 1.
- Inferior vena cava evaluation
- 2.
- Doppler Flow in the Hepatic Veins and the Portal veins
- 3.
- Ultrasonographic Evaluation of Intrarenal Venous Flow
- 4.
- Ultrasonographic evaluation of the internal jugular vein
- 5.
- Chest Radiography
- 6.
- Bioimpedance Vector Analysis (BIVA)
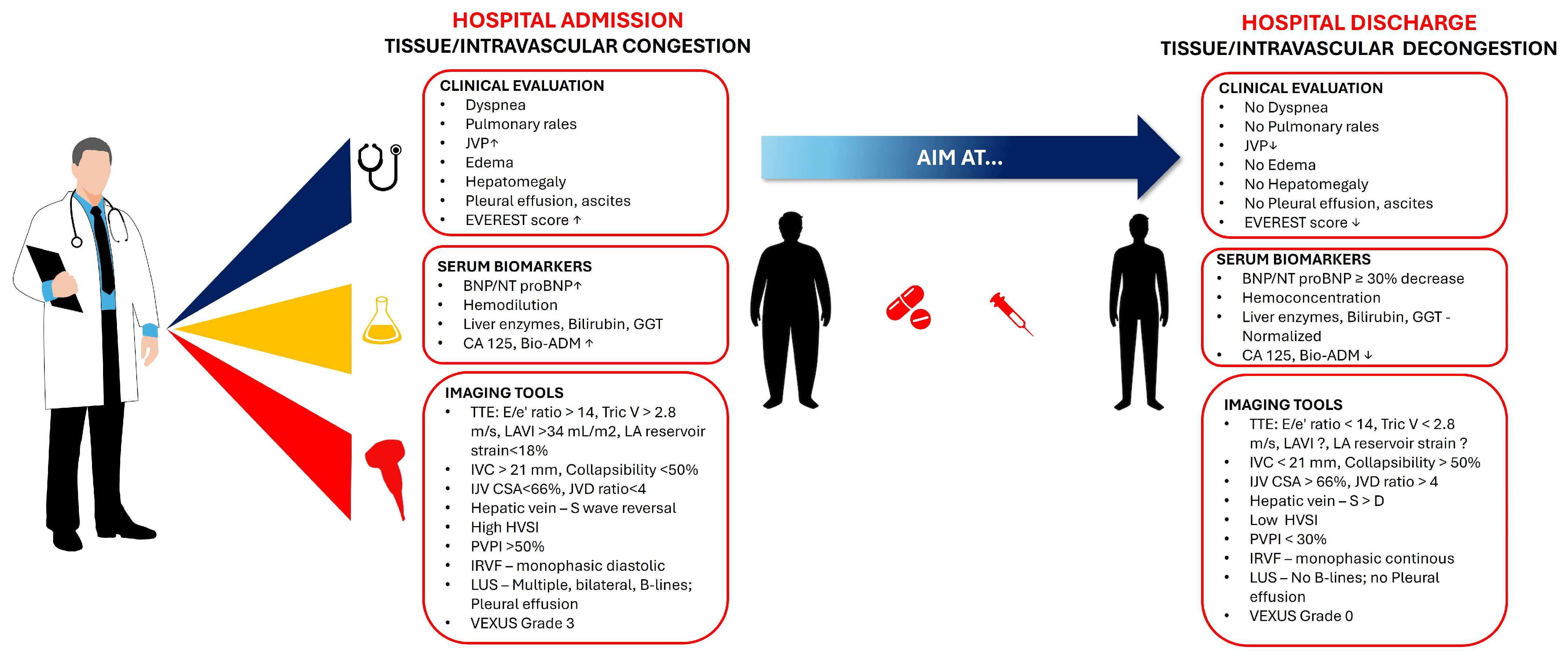
2.1.4. Integrated Assessment Tools
- clinical evaluation—to assess symptoms and signs of congestion: dyspnea, orthopnea, peripheral edema, and jugular venous distension;
- hemodynamic parameters—to evaluate vital signs (blood pressure, heart rate), and to estimate glomerular filtration rate;
- biomarkers—to monitor NPs levels (BNP or NT-proBNP)—A reduction of ≥30% in NT-proBNP from admission to discharge being associated with better outcomes;
- imaging—to utilize echocardiography and lung ultrasound to assess for residual congestion.
- symptoms and signs of congestion;
- blood pressure;
- heart rate;
- NT-proBNP values;
- potassium concentrations;
- estimated glomerular filtration rate.
Clinical Practice Scores
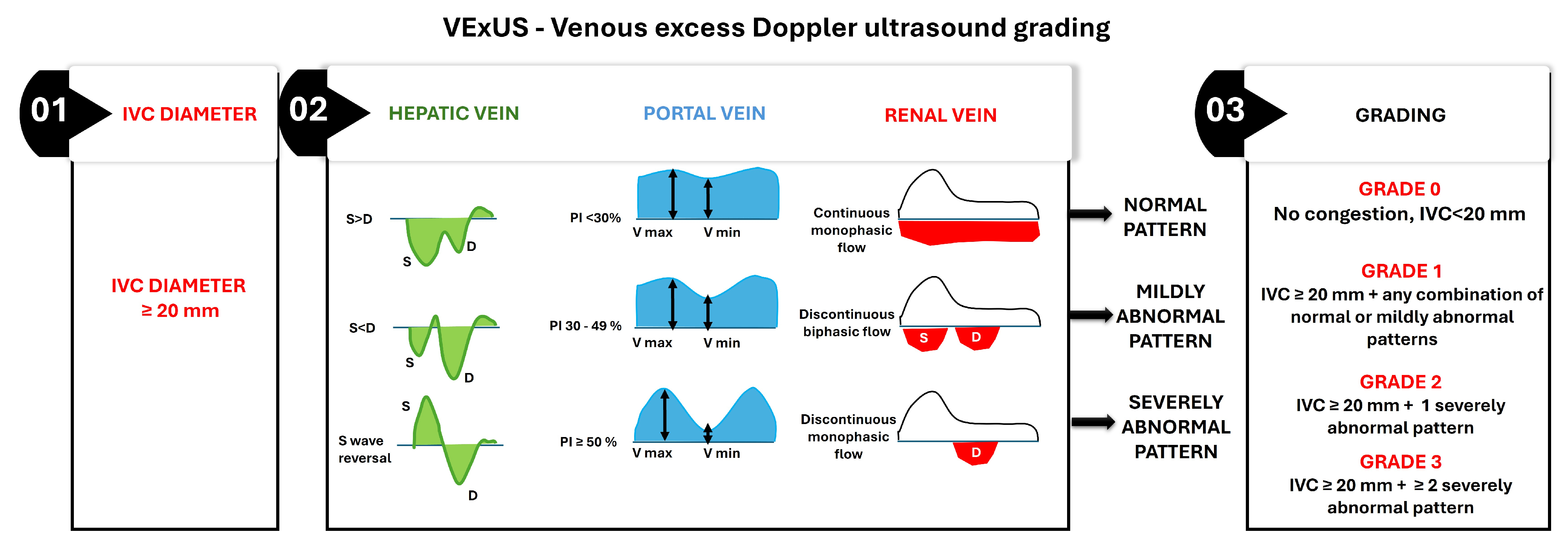
- IVC Diameter Assessment—The assessment begins with measuring the IVC diameter at its widest point during quiet respiration in the subcostal view. An IVC diameter ≥ 20 mm indicates elevated CVP, which is often associated with systemic venous congestion. This finding necessitates further evaluation of venous Doppler waveforms to confirm and grade the severity of congestion;
- Hepatic Vein Doppler Waveform Analysis—The hepatic vein Doppler waveform typically exhibits triphasic flow, with a dominant systolic (S) wave under normal conditions. As venous congestion worsens, the S wave diminishes relative to the diastolic (D) wave, and in severe cases, S wave reversal occurs;
- Portal Vein Pulsatility Index (PVPI)—The portal vein normally demonstrates continuous flow with minimal pulsatility. It increases with elevated CVP due to direct transmission of right atrial pressure, and a PVPI ≥ 50% indicates severe portal venous congestion;
- Renal Vein Doppler Waveform Analysis—The renal vein Doppler waveform is normally continuous and monophasic. With increasing venous congestion, the waveform becomes discontinuous or biphasic, and, in severe cases, it transitions to monophasic diastolic flow only;
- Grading system: The final VExUS grade is assigned based on the combination of IVC diameter and the severity of Doppler abnormalities in the hepatic, portal, and renal veins:
- Grade 0: IVC diameter < 20 mm, with no Doppler abnormalities, indicating no congestion;
- Grade 1: IVC diameter ≥ 20 mm with any combination of normal or mildly abnormal venous Doppler patterns;
- Grade 2: IVC diameter ≥ 20 mm with one severely abnormal venous Doppler pattern (S wave reversal in the hepatic vein, PVPI ≥ 50% in the portal vein, or discontinuous monophasic flow in the renal vein);
- Grade 3: IVC diameter ≥ 20 mm with two or more severely abnormal venous Doppler patterns.
Integrative, Multi-Parameter Approaches
Proposed Algorithm for Pre-Discharge Assessment
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHF | Acute decompensated heart failure |
| AHF | Acute heart failure |
| HF | Heart failure |
| CVP | Central venous pressure |
| LVFP | Left ventricular filling pressures |
| JVD | Jugular venous distention |
| PND | Paroxysmal nocturnal dyspnea |
| BNP | B-type natriuretic peptide |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| HR | Hazard ratio |
| CI | Confidence interval |
| ESC | European Society of Cardiology |
| eGFR | Estimated glomerular filtration rate |
| WRF | Worsening renal function |
| HC | Hemoconcentration |
| Hct | Hematocrit |
| Hgb | Hemoglobin |
| LFTs | Liver function tests |
| TBIL | Total bilirubin |
| ALP | Alkaline phosphatase |
| AST | Aspartate transaminase |
| ALT | Alanine transaminase |
| HFpEF | Heart failure with preserved ejection fraction |
| HFmrEF | Heart failure with mildly reduced ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| LV | Left ventricle |
| RV | Right ventricle |
| IVC | Inferior vena cava |
| LAVI | Left atrium volume index |
| LUS | Lung ultrasound |
| POCT | Point-of-Care Testing |
| IJV | Internal jugular vein |
| CXR | Chest X-ray |
| LUS | Lung ultrasound |
| BIVA | Bioimpedance vector analysis |
Appendix A
- IVC-CI is determined by the formula: (IVCmax − IVCmin)/IVCmax × 100;
- PVPR is calculated as the ratio of the difference between peak and minimum velocity to the peak velocity;
- PVPI is determined by the formula: (maximum velocity—minimum velocity)/maximum velocity × 100;
- The calculation of HVSI is the HV (hepatic vein) stasis flow time divided by the HV waveform cycle time, where HV stasis flow time is the duration of stasis in the hepatic vein flow, and the HV waveform cycle time is the total duration of one hepatic vein waveform cycle.
Appendix B
| Clinical Sign | Description | Clinical Relevance | Diagnostic Utility | Prognostic Value |
|---|---|---|---|---|
| Dyspnea | Cardinal symptom of pulmonary congestion. | Indicates pulmonary congestion. | Limited specificity; overlaps with respiratory conditions. | Resolution is not a reliable indicator of complete decongestion. |
| Orthopnea/PND | Symptoms associated with elevated LVFP. | Strongly indicative of HF; resolution suggests successful decongestion. | High specificity; absence does not exclude subclinical congestion. | Persistence indicates incomplete decongestion. |
| Jugular Venous Distension (JVD) | Reflects elevated central venous pressure. | Important for assessing right-sided filling pressures. | Sensitivity improves with corrected measurement. | Limited as a sole indicator. |
| Pulmonary Rales | Indicates fluid accumulation in alveoli. | Critical sign of pulmonary congestion; often emerges late. | Varies widely in sensitivity; specific to pulmonary congestion. | Presence correlates with severity and poor outcomes. |
| Pleural Effusions | Accumulation of fluid in the pleural space; often transudative in ADHF. | Frequent in ADHF; associated with elevated sPAP and NT-proBNP. | Clinical judgment alone has low sensitivity; ultrasound is more accurate. | Persistent PEs indicate residual congestion and increased risk of rehospitalization. |
| Peripheral Edema | Reflects fluid retention due to impaired cardiac function. | Not specific to HF; can be due to various conditions. | Not a reliable diagnostic marker. | Presence is associated with adverse outcomes. |
| Hepatomegaly | Indicates right-sided heart dysfunction. | Common in ADHF; persistence suggests residual congestion. | Not specific to HF; associated with liver function abnormalities. | Linked to increased risk of readmission and poor outcomes. |
| Ascites | Indicates severe fluid overload. | Less common than other signs; associated with poor outcomes. | Not specific to HF; requires noninvasive assessments. | Presence is associated with increased morbidity. |
| Weight Gain | Precedes visible edema; predictive of decompensation. | Important for early detection of fluid retention. | Predicts impending decompensation with high accuracy. | Early indicator of potential congestion. |
| Biochemical Tool | Description | Clinical Relevance | Diagnostic Utility | Prognostic Value |
|---|---|---|---|---|
| NT-proBNP | Biomarker for heart failure severity. | Elevated levels indicate increased ventricular wall stress. | Highly sensitive for HF diagnosis; useful in guiding treatment. | Prognostic value in predicting mortality and readmission. |
| BNP | Similar to NT-proBNP, used for HF diagnosis. | Useful in diagnosing HF, especially in acute dyspnea. | Highly sensitive but less specific than NT-proBNP. | Predicts outcomes similarly to NT-proBNP. |
| Hemoconcentration | Indicates intravascular volume reduction. | Useful in evaluating decongestion. | Associated with improved outcomes; limited by factors affecting blood volume. | Predicts reduced mortality and rehospitalization. |
| Renal Function Markers (eGFR) | Predict mortality in HF patients. | Important for assessing renal function and its impact on HF outcomes. | Independent predictor of mortality; not specific to congestion. | Each 10 mL/min decrease in eGFR increases mortality risk. |
| Hepatic Markers (AST, ALT, Albumin) | Reflect liver function and congestion. | Useful in assessing HF severity and prognosis. | Abnormalities predict worse outcomes; not specific to congestion. | Elevated levels correlate with increased mortality and rehospitalization. |
| Emerging Biomarkers (CA125, bio-ADM, sST2) | Indicate congestion and predict outcomes. | Useful in assessing fluid overload and systemic congestion. | Limited specificity and clinical experience; require further validation. | Elevated levels associated with increased mortality and readmission. |
| Imaging Tool | Description | Clinical Relevance | Diagnostic Utility | Prognostic Value |
|---|---|---|---|---|
| Echocardiography | Estimates LV filling pressures and assesses cardiac function. | Essential for managing HF; provides prognostic information. | Highly sensitive for assessing LVFP; useful in guiding therapy. | Predicts outcomes based on LVFP and cardiac function. |
| Lung Ultrasound | Detects pulmonary congestion through B-Lines. | Highly sensitive and specific for pulmonary congestion. | Useful in clinical decision-making; improves congestion evaluation. | Helps identify patients at risk for poor outcomes. |
| Chest Radiography (CXR) | Assesses pulmonary congestion and pleural effusions. | Useful in emergency settings; limited by interobserver variability. | Detects severe pulmonary congestion; less sensitive than LUS. | Predicts outcomes based on radiographic congestion score. |
| Inferior Vena Cava (IVC) Ultrasound | Evaluates right-sided filling pressures. | Useful in assessing volume status and guiding therapy. | Dilated IVC with reduced collapsibility indicates elevated CVP. | Predicts rehospitalization risk based on IVC diameter and collapsibility. |
| Hepatic Vein Ultrasound | Assesses right heart hemodynamics. | Provides insights into hepatic congestion and right atrial pressure. | Changes in waveform patterns correlate with congestion severity. | Predicts cardiac events based on hepatic venous stasis index. |
| Portal Vein Ultrasound | Evaluates systemic venous congestion. | Useful in assessing portal vein pulsatility and congestion severity. | PVPI ≥ 50% indicates severe systemic congestion. | Predicts outcomes based on changes in PVPI. |
| Renal Vein Ultrasound | Assesses renal hemodynamics and venous congestion. | Useful in identifying patients at risk of complications. | Discontinuous flow patterns indicate severe congestion. | Predicts outcomes based on improvement in renal venous Doppler parameters. |
| Internal Jugular Vein (IJV) Ultrasound | Estimates CVP and guides discharge decisions. | Useful in predicting readmission risk based on IJV compliance. | Normalization of IJV compliance predicts reduced readmission risk. | Predicts 30-day readmission risk based on IJV CSA change. |
| BIVA | Assesses hydration status and body composition. | Useful in detecting subclinical congestion. | Superior to BNP in detecting peripheral congestion; requires specialized equipment. | Helps identify patients at risk for poor outcomes; useful in guiding discharge decisions. |
References
- Stienen, S.; Salah, K.; Moons, A.H.; Bakx, A.L.; van Pol, P.; Kortz, R.A.M.; Ferreira, J.P.; Marques, I.; Schroeder-Tanka, J.M.; Keijer, J.T.; et al. NT-proBNP (N-Terminal pro-B-Type Natriuretic Peptide)-Guided Therapy in Acute Decompensated Heart Failure: PRIMA II Randomized Controlled Trial. Circulation 2018, 137, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B. Successful Decongestion as a Clinical Target, Performance Indicator, and as a Study Endpoint in Hospitalized Heart Failure Patients. J. Am. Coll. Cardiol. Heart Fail. 2023, 11, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Kociol, R.D.; McNulty, S.E.; Hernandez, A.F.; Felker, G.M.; Eapen, Z.J.; Piña, I.L.; Adams, K.F., Jr.; Gattis Stough, W.; O’Connor, C.M.; Lee, K.L.; et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ. Heart Fail. 2013, 6, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Girerd, N.; Seronde, M.F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative assessment of congestion in heart failure throughout the patient journey. J. Am. Coll. Cardiol. Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef]
- Bozkurt, B. Targets for success in treatment of patients hospitalized for heart failure. J. Am. Coll. Cardiol. Heart Fail. 2022, 10, 782–784. [Google Scholar] [CrossRef]
- Karauzum, K.; Karauzum, I.; Kilic, T.; Sahin, T.; Baydemir, C.; Argun, S.B.; Celikyurt, U.; Bildirici, U.; Agir, A. Bendopnea and Its Clinical Importance in Outpatient Patients with Pulmonary Arterial Hypertension. Acta Cardiol. Sin. 2018, 34, 518–525. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Narang, N.; Chung, B.; Nguyen, A.; Kalathiya, R.J.; Laffin, L.J.; Holzhauser, L.; Ebong, I.A.; Besser, S.A.; Imamura, T.; Smith, B.A.; et al. Discordance between clinical assessment and invasive hemodynamics in patients with advanced heart failure. J. Card. Fail. 2020, 26, 128–135. [Google Scholar] [CrossRef]
- Greene, S.J.; Velazquez, E.J.; Anstrom, K.J.; Mentz, R.J.; Schulte, P.J.; Dunning, A.; Ambrosy, A.P.; McNulty, S.E.; Felker, G.M.; Hernandez, A.F.; et al. Pragmatic design of randomized clinical trials for heart failure: Rationale and design of the TRANSFORM-HF Trial. J. Am. Coll. Cardiol. Heart Fail. 2021, 9, 325–335. [Google Scholar]
- Lindenfeld, J.; Zile, M.R.; Desai, A.S.; Bhatt, K.; Ducharme, A.; Horstmanshof, D.; Jarolim, P.; McCague, K.; Melynk, M.; Miller, A.B.; et al. Haemodynamic-guided management of heart failure (GUIDE-HF): A randomised controlled trial. Lancet 2021, 398, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Follath, F.; Ponikowski, P.; Barsuk, J.H.; Blair, J.E.; Cleland, J.G.; Dickstein, K.; Drazner, M.H.; Fonarow, G.C.; Jaarsma, T.; et al. Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology. Eur. J. Heart Fail. 2010, 12, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Evangelista, I.; Beltrami, M.; Pirrotta, F.; Tavera, M.C.; Gennari, L.; Ruocco, G. Clinical, Laboratory and Lung Ultrasound Assessment of Congestion in Patients with Acute Heart Failure. J. Clin. Med. 2022, 11, 1642. [Google Scholar] [CrossRef] [PubMed]
- Kayama, K.; Kikuchi, S.; Sugimoto, T.; Seo, Y. Association of renal circulation at acute phase with decongestion level at discharge in patients admitted for acute decompensated heart failure. Eur. Heart J. 2024, 45, ehae666.1016. [Google Scholar] [CrossRef]
- Liang, B.; Li, R.; Bai, J.Y.; Gu, N. Bioimpedance Vector Analysis for Heart Failure: Should We Put It on the Agenda? Front. Cardiovasc. Med. 2021, 8, 744243. [Google Scholar] [CrossRef]
- Gargani, L.; Ferre, R.M.; Pang, P.S. B-lines in heart failure: Will comets guide us? Eur. J. Heart Fail. 2019, 21, 1616–1618. [Google Scholar] [CrossRef]
- Testani, J.M.; Chen, J.; McCauley, B.D.; Kimmel, S.E.; Shannon, R.P. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010, 122, 265–272. [Google Scholar] [CrossRef]
- Testani, J.M.; Brisco, M.A.; Chen, J.; McCauley, B.D.; Parikh, C.R.; Tang, W.H.W. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: Importance of sustained decongestion. J. Am. Coll. Cardiol. 2013, 62, 516–524. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Pang, P.S.; Ambrosy, A.P.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure. Heart Fail. Rev. 2012, 17, 485–509. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Gimpelewicz, C.; et al. Effects of Serelaxin in Patients with Acute Heart Failure. N. Engl. J. Med. 2019, 381, 716–726. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Yagishita-Tagawa, Y.; Yumino, D.; Takagi, A.; Serizawa, N.; Hagiwara, N. Association between sleep apnea and overnight hemodynamic changes in hospitalized heart failure patients with and without paroxysmal nocturnal dyspnea. J. Cardiol. 2013, 61, 348–353. [Google Scholar] [CrossRef][Green Version]
- Mocan, D.; Lala, R.I.; Puschita, M.; Pilat, L.; Darabantiu, D.A.; Pop-Moldovan, A. The Congestion “Pandemic” in Acute Heart Failure Patients. Biomedicines 2024, 12, 951. [Google Scholar] [CrossRef]
- Espinosa, B.; Gil, V.; Jacob, J. Diagnóstico clínico de la insuficiencia cardíaca aguda. CorSalud 2022, 14, 380–388. [Google Scholar]
- Istrail, L.; Stepanova, M. Novel point-of-care ultrasound (POCUS) technique to modernize the JVP exam and rule out elevated right atrial pressures. medRxiv 2021. [Google Scholar] [CrossRef]
- Melenovsky, V.; Andersen, M.J.; Andress, K.; Reddy, Y.N.; Borlaug, B.A. Lung congestion in chronic heart failure: Haemodynamic, clinical, and prognostic implications. Eur. J. Heart Fail. 2015, 17, 1161–1171. [Google Scholar] [CrossRef]
- Morales-Rull, J.; Bielsa, S.; Conde-Martel, A.; Aramburu-Bodas, O.; Llacer, P.; Quesada, M.; Suárez-Pedreira, I.; Manzano, L.; Montero-Pérez-Barquero, M.; Porcel, J. Pleural effusions in acute decompensated heart failure: Prevalence and prognostic implications. Eur. J. Intern. Med. 2018, 52, 49–53. [Google Scholar] [CrossRef]
- Gómez-Mesa, J.E.; Gutiérrez-Posso, J.L.; Escalante-Forero, M.; Cárdenas-Marín, P.; Perna, E.; Valle-Ramos, M.; Giraldo-Gonzalez, G.; Flórez-Alarcón, N.; Rodríguez-Caballero, I.; Núñez-Carrizo, C.; et al. American Registry of Ambulatory or acutely decompensated heart failure (AMERICCAASS Registry): First 1000 patients. Clin. Cardiol. 2023, 47, e24182. [Google Scholar] [CrossRef]
- Glargaard, S.; Deis, T.; Abild-Nielsen, A.G.; Stark, A.; Thomsen, J.H.; Kristensen, S.L.; Gustafsson, F.; Køber, L.; Rossing, K.; Thune, J.J.; et al. Pleural Effusion and Invasive Hemodynamic Measurements in Advanced Heart Failure. Circ. Heart Fail. 2024, 17, e011253. [Google Scholar] [CrossRef]
- Glargaard, S.; Thomsen, J.H.; Løgstrup, B.B.; Schou, M.; Iversen, K.K.; Tuxen, C.; Nielsen, O.W.; Bang, C.A.; Lindholm, M.G.; Seven, E.; et al. Thoracentesis to alleviate pleural effusion in acute heart failure: Study protocol for the multicentre, open-label, randomised controlled TAP-IT trial. BMJ Open 2024, 14, e078155. [Google Scholar] [CrossRef]
- Abassi, Z.; Khoury, E.; Karram, T.; Aronson, D. Edema formation in congestive heart failure and the underlying mechanisms. Front. Cardiovasc. Med. 2022, 9, 933215. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar] [PubMed]
- Mangini, S.; Pires, P.V.; Braga, F.G.; Bacal, F. Decompensated heart failure. Einstein 2013, 11, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Fang, J.C. Gastrointestinal and Liver Issues in Heart Failure. Circulation 2016, 133, 1696–1703. [Google Scholar] [CrossRef]
- Scholfield, M.; Schabath, M.B.; Guglin, M. Longitudinal trends, hemodynamic profiles, and prognostic value of abnormal liver function tests in patients with acute decompensated heart failure: An analysis of the ESCAPE trial. J. Card. Fail. 2014, 20, 476–484. [Google Scholar] [CrossRef]
- Al-Refaie, N.; Taylor, L. Daily weight and fluid balance assessment in patients admitted with acute heart failure. Heart 2021, 107, A113–A114. [Google Scholar]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Smith, P.K.; Sugarman, J.E.; Miller, L.W.; et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 2005, 294, 1625–1633. [Google Scholar]
- Suri, S.S.; Pamboukian, S.V. Optimal diuretic strategies in heart failure. Ann. Transl. Med. 2021, 9, 517. [Google Scholar] [CrossRef]
- Agarwal, R.; Weir, M.R. Dry-weight: A concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1255–1260. [Google Scholar] [CrossRef]
- Goh, Z.N.L.; Teo, R.Y.L.; Chung, B.K.; Wong, A.C.; Seak, C.J. At the heart of the problem: Congestive cardiac failure as a cause of ascites: A narrative review. Medicine 2022, 101, e29951. [Google Scholar] [CrossRef] [PubMed]
- Hill, L. Producing an effective care plan in advanced heart failure. Eur. Heart J. Suppl. 2019, 21, M61–M63. [Google Scholar] [CrossRef] [PubMed]
- Ilieșiu, A.M.; Hodorogea, A.S.; Balahura, A.M.; Bădilă, E. Non-Invasive Assessment of Congestion by Cardiovascular and Pulmonary Ultrasound and Biomarkers in Heart Failure. Diagnostics 2022, 12, 962. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Herrmann, H.C.; Steg, P.G.; Duc, P.; Westheim, A.; Omland, T.; Knudsen, C.W.; et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: Analysis from Breathing Not Properly (BNP) Multinational Study. Circulation 2002, 106, 416–422. [Google Scholar] [CrossRef]
- Wettersten, N. Biomarkers in Acute Heart Failure: Diagnosis, Prognosis, and Treatment. Int. J. Heart Fail. 2021, 3, 81–105. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; Chae, C.U.; et al. The N-terminal pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef]
- Salah, K.; Stienen, S.; Pinto, Y.M.; Eurlings, L.W.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Tijssen, J.G.P.; Kok, W.E.; van Veldhuisen, D.J.; et al. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019, 105, 1182–1189. [Google Scholar] [CrossRef]
- Kociol, R.D.; Horton, J.R.; Fonarow, G.C.; Yancy, C.W.; Heidenreich, P.A.; Peterson, E.D.; Albert, N.M.; Curtis, L.H.; Hernandez, A.F.; Mehta, R.H.; et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: Data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ. Heart Fail. 2011, 4, 628–636. [Google Scholar] [CrossRef]
- Logeart, D.; Thabut, G.; Jourdain, P.; Chavelas, C.; Beyne, P.; Beauvais, F.; Slama, M.; Logeart, I.; Leblanc, M.H.; Solal, A.C.; et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J. Am. Coll. Cardiol. 2004, 43, 635–641. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.S.; Filippatos, G.; Ruschitzka, F.; Laroche, C.; Piepoli, M.F.; Crespo-Leiro, M.G.; Seferovic, P.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef]
- Tripoliti, E.E.; Ioannidou, P.; Toumpaniaris, P.; Rammos, A.; Pacitto, D.; Lourme, J.-C.; Goletsis, Y.; Naka, K.K.; Errachid, A.; Fotiadis, D.I.I. Point-of-Care Testing Devices for Heart Failure Analyzing Blood and Saliva Samples. IEEE Rev. Biomed. Eng. 2020, 13, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Laule-Kilian, K.; Schindler, C.; Klima, T.; Frana, B.; Rodriguez, D.; Scholer, A.; Christ, M.; Perruchoud, A.P. Cost-effectiveness of B-Type Natriuretic Peptide Testing in Patients with Acute Dyspnea. Arch. Intern. Med. 2006, 166, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.M. Biomarkers in Acute Heart Failure-Cardiac And Kidney. Card. Fail. Rev. 2015, 1, 107. [Google Scholar] [CrossRef]
- Mallick, A.; Januzzi, J.L., Jr. Biomarkers in acute heart failure. Rev. Esp. Cardiol. Engl. Ed. 2015, 68, 514–525. [Google Scholar] [CrossRef]
- Georges, G.; Fudim, M.; Burkhoff, D.; Leon, M.B.; Généreux, P. Patient Selection and End Point Definitions for Decongestion Studies in Acute Decompensated Heart Failure: Part 1. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101060. [Google Scholar]
- van der Meer, P.; Postmus, D.; Ponikowski, P.; Valente, M.A.E.; Voors, A.A.; Cotter, G.; Metra, M.; Cleland, J.G.F.; O’Connor, C.M.; Teerlink, J.R.; et al. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J. Am. Coll. Cardiol. 2013, 61, 1973–1981. [Google Scholar] [CrossRef]
- Darawsha, W.; Chirmicci, S.; Solomonica, A.; Wattad, M.; Kaplan, M.; Makhoul, B.F.; Abassi, Z.A.; Azzam, Z.S.; Aronson, D. Discordance between hemoconcentration and clinical assessment of decongestion in acute heart failure. J. Card. Fail. 2016, 22, 680–688. [Google Scholar] [CrossRef]
- Udani, S.M.; Koyner, J.L. The effects of heart failure on renal function. Cardiol. Clin. 2010, 28, 453–465. [Google Scholar] [CrossRef]
- Blair, J.E.A.; Pang, P.S.; Schrier, R.W.; Metra, M.; Traver, B.; Cook, T.; Campia, U.; Ambrosy, A.; Burnett, J.C., Jr.; Grinfeld, L.; et al. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur. Heart J. 2011, 32, 2563–2572. [Google Scholar] [CrossRef]
- Abraham, W.T.; Psotka, M.A.; Fiuzat, M.; O’Connor, C.M.; Fonarow, G.C.; Gheorghiade, M.; Greenberg, B.H.; Januzzi, J.L.; Lindenfeld, J.; Massie, B.M.; et al. Standardized definitions for evaluation of heart failure therapies: Scientific expert panel from the Heart Failure Collaboratory and Academic Research Consortium. J. Am. Coll. Cardiol. Heart Fail. 2020, 8, 961–972. [Google Scholar]
- Palazzuoli, A.; Crescenzi, F.; Luschi, L.; Brazzi, A.; Feola, M.; Rossi, A.; Pagliaro, A.; Ghionzoli, N.; Ruocco, G. Different Renal Function Patterns in Patients With Acute Heart Failure: Relationship With Outcome and Congestion. Front. Cardiovasc. Med. 2022, 9, 779828. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.P.; Dahlström, U.; Filippatos, G.; Chioncel, O.; Crespo-Leiro, M.G.; Drozdz, J.; Erglis, A.; Fazlibegovic, E.; Fonseca, C.; Hellmich, M.; et al. EURObservational Research Programme: Regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur. J. Heart Fail. 2013, 15, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Odajima, S.; Fujimoto, W.; Kuroda, K.; Yamashita, S.; Imanishi, J.; Iwasaki, M.; Todoroki, T.; Okuda, M.; Hayashi, T.; Konishi, A.; et al. Association of congestion with worsening renal function in acute decompensated heart failure according to age. ESC Heart Fail. 2022, 9, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Ezekowitz, J.; Filippatos, G.; Laroche, C.; McDonagh, T.; et al. Circulating heart failure biomarkers beyond natriuretic peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef]
- Biegus, J.; Hillege, H.L.; Postmus, D.; Valente, M.A.; Bloomfield, D.M.; Cleland, J.G.; Cotter, G.; Damman, K.; Davison, B.A.; Filippatos, G.; et al. Abnormal liver function tests in acute heart failure: Relationship with clinical characteristics and outcome in the PROTECT study. Eur. J. Heart Fail. 2016, 18, 830–839. [Google Scholar] [CrossRef]
- Liang, W.; He, X.; Wu, D.; Xue, R.; Dong, B.; Owusu-Agyeman, M.; Zhao, L.; Cai, Y.; You, S.; Dong, Y.; et al. Prognostic Implication of Liver Function Tests in Heart Failure With Preserved Ejection Fraction Without Chronic Hepatic Diseases: Insight From TOPCAT Trial. Front. Cardiovasc. Med. 2021, 8, 618816. [Google Scholar] [CrossRef]
- Alvarez, A.M.; Mukherjee, D. Liver abnormalities in cardiac diseases and heart failure. Int. J. Angiol. 2011, 20, 135–142. [Google Scholar] [CrossRef]
- Li, K.H.C.; Gong, M.; Li, G.; Baranchuk, A.; Liu, T.; Wong, M.C.S.; Jesuthasan, A.; Lai, R.W.C.; Lai, J.C.L.; Lee, A.P.W.; et al. Cancer antigen-125 and outcomes in acute heart failure: A systematic review and meta-analysis. Heart Asia 2018, 10, e011044. [Google Scholar] [CrossRef]
- Egerstedt, A.; Czuba, T.; Bronton, K.; Lejonberg, C.; Ruge, T.; Wessman, T.; Rådegran, G.; Schulte, J.; Hartmann, O.; Melander, O.; et al. Bioactive adrenomedullin for assessment of venous congestion in heart failure. ESC Heart Fail. 2022, 9, 3543–3555. [Google Scholar] [CrossRef]
- Pandhi, P.; Ter Maaten, J.M.; Emmens, J.E.; Struck, J.; Bergmann, A.; Cleland, J.G.; Givertz, M.M.; Metra, M.; O’Connor, C.M.; Teerlink, J.R.; et al. Clinical value of pre-discharge bio-adrenomedullin as a marker of residual congestion and high risk of heart failure hospital readmission. Eur. J. Heart Fail. 2020, 22, 683–691. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L., Jr. Established and Emerging Roles of Biomarkers in Heart Failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating Left Ventricular Filling Pressure by Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Claggett, B.; Inciardi, R.M.; Santos, A.B.S.; Shah, S.J.; Zile, M.R.; Pfeffer, M.A.; Shah, A.M.; Solomon, S.D. Prognostic Value of Minimal Left Atrial Volume in Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e019545. [Google Scholar] [CrossRef]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Nagumo, S.; Ebato, M.; Tsujiuchi, M.; Mizukami, T.; Maezawa, H.; Omura, A.; Kubota, M.; Ohmi, M.; Numajiri, Y.; Kitai, H.; et al. Prognostic value of left atrial reverse remodelling in patients hospitalized with acute decompensated heart failure. ESC Heart Fail. 2024, 11, 4285–4295. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, e34–e61. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Kosugi, S.; Ozaki, T.; Mishima, T.; Date, M.; Ueda, Y.; Uematsu, M.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic impact of echocardiographic congestion grade in HFpEF with and without atrial fibrillation. JACC Asia 2022, 2, 73–84. [Google Scholar] [CrossRef]
- Lancellotti, P.; Galderisi, M.; Edvardsen, T.; Donal, E.; Goliasch, G.; Cardim, N.; Magne, J.; Laginha, S.; Hagendorff, A.; Haland, T.F.; et al. Echo-Doppler estimation of left ventricular filling pressure: Results of the multicentre EACVI Euro-Filling study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 961–968. [Google Scholar] [CrossRef]
- Tanaka, H.; Nabeshima, Y.; Kitano, T.; Nagumo, S.; Tsujiuchi, M.; Ebato, M.; Sugiura, T.; Fukuda, Y.; Sakamoto, K.; Matsumoto, K.; et al. Optimal timing of echocardiography for heart failure inpatients in Japanese institutions: OPTIMAL Study. ESC Heart Fail. 2020, 7, 4213–4221. [Google Scholar] [CrossRef]
- Hoshida, S.; Tachibana, K.; Shinoda, Y.; Minamisaka, T.; Yamada, T.; Higuchi, Y.; Nishikawa, R.; Nishida, T.; Okada, Y.; Kato, T.; et al. Left atrial pressure overload and prognosis in elderly patients with heart failure and preserved ejection fraction: A prospective multicenter observational study. BMJ Open 2021, 11, e044605. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Mondillo, S.; Padeletti, M.; Ballo, P.; Tsioulpas, C.; Bernazzali, S.; Maccherini, M.; Colella, A.; Pierli, C.; et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc. Ultrasound 2010, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Buessler, A.; Chouihed, T.; Duarte, K.; Bassand, A.; Huot-Marchand, M.; Gottwalles, Y.; Jaeger, D.; Moustafa, F.; Sadoune, M.; Prochowski, V.; et al. Accuracy of several lung ultrasound methods for the diagnosis of acute heart failure in the ED: A multicenter prospective study. Chest 2020, 157, 99–110. [Google Scholar] [CrossRef]
- Gargani, L.; Girerd, N.; Platz, E.; Pellicori, P.; Stankovic, I.; Palazzuoli, A.; Miglioranza, M.H.; Mandoli, G.E.; Ancona, R.; Dini, F.L.; et al. Lung ultrasound in acute and chronic heart failure: A clinical consensus statement of the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1569–1582. [Google Scholar] [CrossRef]
- Maw, A.M.; Hassanin, A.; Ho, P.M.; McInnes, M.D.F.; Moss, A.; Juarez-Colunga, E.; Anstrom, K.J.; Davidson, K.W.; Daugherty, S.L.; Glasgow, R.E.; et al. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults With Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e190703. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Ruocco, G.; Beltrami, M.; Nuti, R.; Cleland, J.G. Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin. Res. Cardiol. 2018, 107, 586–596. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Maestro, A.; Fernández-Martínez, J.; López-López, L.; Solé-González, E.; Vives-Borrás, M.; Álvarez-García, J.; Mirabet, S.; Mesado, N.; Sobrino, J.M.; et al. Prevalence and prognostic impact of subclinical pulmonary congestion at discharge in patients with acute heart failure. ESC Heart Fail. 2020, 7, 2621–2628. [Google Scholar] [CrossRef]
- Cohen, A.; Li, T.; Maybaum, S.; Fridman, D.; Gordon, M.; Shi, D.; Nelson, M.; Stevens, G.R. Pulmonary Congestion on Lung Ultrasound Predicts Increased Risk of 30-Day Readmission in Heart Failure Patients. J. Ultrasound Med. 2023, 42, 1809–1818. [Google Scholar] [CrossRef]
- Platz, E.; Merz, A.A.; Jhund, P.S.; Vazir, A.; Campbell, R.; McMurray, J.J. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: A systematic review. Eur. J. Heart Fail. 2017, 19, 1154–1163. [Google Scholar] [CrossRef]
- Harrison, N.E.; Ehrman, R.; Collins, S.; Desai, A.A.; Duggan, N.M.; Ferre, R.; Gargani, L.; Goldsmith, A.; Kapur, T.; Lane, K.; et al. The prognostic value of improving congestion on lung ultrasound during treatment for acute heart failure differs based on patient characteristics at admission. J. Cardiol. 2024, 83, 121–129. [Google Scholar] [CrossRef]
- Pang, P.S.; Russell, F.M.; Ehrman, R.; Ferre, R.; Gargani, L.; Levy, P.D.; Noble, V.; Lane, K.A.; Li, X.; Collins, S.P.; et al. Lung ultrasound-guided emergency department management of acute heart failure (BLUSHED-AHF) a randomized controlled pilot trial. Heart Fail. 2021, 9, 638–648. [Google Scholar]
- Cogliati, C.; Ceriani, E.; Gambassi, G.; De Matteis, G.; Perlini, S.; Perrone, T.; Muiesan, M.L.; Salvetti, M.; Leidi, F.; Ferrara, F.; et al. Phenotyping congestion in patients with acutely decompensated heart failure with preserved and reduced ejection fraction: The Decongestion duRing therapY for acute decOmpensated heart failure in HFpEF vs HFrEF-DRY-OFF study. Eur. J. Intern. Med. 2022, 97, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, Ø.; Claggett, B.; Lewis, E.F.; Groarke, J.D.; Swamy, V.; Lindner, M.; Solomon, S.D.; Platz, E. A-lines and B-lines in patients with acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Burgos, L.M.; Baro Vila, R.; Goyeneche, A.; Muñoz, F.; Spaccavento, A.; Fasan, M.A.; Ballari, F.; Vivas, M.; Riznyk, L.; Ghibaudo, S.; et al. Design and rationale of the inferior vena CAVA and Lung UltraSound-guided therapy in Acute Heart Failure (CAVAL US-AHF Study): A randomised controlled trial. Open Heart 2022, 9, e002105. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European Society of cardiology, and the Canadian Society of echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Sampath-Kumar, R.; Ben-Yehuda, O. Inferior vena cava diameter and risk of acute decompensated heart failure rehospitalisations. Open Heart 2023, 10, e002331. [Google Scholar] [CrossRef]
- Pellicori, P.; Carubelli, V.; Zhang, J.; Castiello, T.; Sherwi, N.; Clark, A.L.; Cleland, J.G. IVC diameter in patients with chronic heart failure: Relationships and prognostic significance. JACC Cardiovasc. Imaging 2013, 6, 16–28. [Google Scholar] [CrossRef]
- Cubo-Romano, P.; Torres-Macho, J.; Soni, N.J.; Reyes, L.F.; Rodríguez-Almodóvar, A.; Fernández-Alonso, J.M.; González-Davia, R.; Casas-Rojo, J.M.; Restrepo, M.I.; de Casasola, G.G. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J. Hosp. Med. 2016, 11, 778–784. [Google Scholar] [CrossRef]
- Scheinfeld, M.; Bilali, A.; Koenigsberg, M. Understanding the Spectral Doppler Waveform of the Hepatic Veins in Health and Disease. Radiographics 2009, 29, 2081–2098. [Google Scholar] [CrossRef]
- Ohara, H.; Yoshihisa, A.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Sugawara, Y.; Ichijo, Y.; Sato, Y.; Misaka, T.; Sato, T.; et al. Hepatic Venous Stasis Index Reflects Hepatic Congestion and Predicts Adverse Outcomes in Patients With Heart Failure. J. Am. Heart Assoc. 2023, 12, e029857. [Google Scholar] [CrossRef]
- Sugawara, Y.; Yoshihisa, A.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Ohara, H.; Ichijo, Y.; Watanabe, K.; Hotsuki, Y.; Anzai, F.; et al. Liver Congestion Assessed by Hepatic Vein Waveforms in Patients With Heart Failure. CJC Open 2021, 3, 778–786. [Google Scholar] [CrossRef]
- Landi, I.; Guerritore, L.; Iannaccone, A.; Ricotti, A.; Rola, P.; Garrone, M. Assessment of venous congestion with venous excess ultrasound score in the prognosis of acute heart failure in the emergency department: A prospective study. Eur. Heart J. Open 2024, 4, oeae050. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Koratala, A. Utility of Doppler ultrasound derived hepatic and portal venous waveforms in the management of heart failure exacerbation. Clin. Case Rep. 2020, 8, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Kobalava, Z.D.; Vladimirovna, T.V.; Kanatbekovich, S.B.; Aslanova, R.S.; Alekseevich, L.A.; Sergeevich, N.I.; Pavlovich, S.I.; Vatsik-Gorodetskaya, M.V.; Tabatabaei, G.A.; Al Zakwani, I.; et al. Prognostic Role of Ultrasound Diagnostic Methods in Patients with Acute Decompensated Heart Failure. Oman Med. J. 2024, 39, e625. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.; Grigore, A.M.; Ilieșiu, A.M. Portal Vein Pulsatility: A Valuable Approach for Monitoring Venous Congestion and Prognostic Evaluation in Acute Decompensated Heart Failure. Diagnostics 2024, 14, 2029. [Google Scholar] [CrossRef]
- Kuwahara, N.; Honjo, T.; Sone, N.; Imanishi, J.; Nakayama, K.; Kamemura, K.; Iwahashi, M.; Ohta, S.; Kaihotsu, K. Clinical impact of portal vein pulsatility on the prognosis of hospitalized patients with acute heart failure. World J. Cardiol. 2023, 15, 599–608. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Beaubien-Souligny, W.; Oussaïd, E.; Henri, C.; Racine, N.; Denault, A.Y.; Rouleau, J.L. Assessing Splanchnic Compartment Using Portal Venous Doppler and Impact of Adding It to the EVEREST Score for Risk Assessment in Heart Failure. CJC Open 2020, 2, 311–320. [Google Scholar] [CrossRef]
- Rinaldi, P.M.; Rihl, M.F.; Boniatti, M.M. VExUS Score at Discharge as a Predictor of Readmission in Patients with Acute Decompensated Heart Failure: A Cohort Study. Arq. Bras. Cardiol. 2024, 121, e20230745. [Google Scholar] [CrossRef]
- Sovetova, S.; Charaya, K.; Erdniev, T.; Shchekochikhin, D.; Bogdanova, A.; Panov, S.; Plaksina, N.; Mutalieva, E.; Ananicheva, N.; Fomin, V.; et al. Venous Excess Ultrasound Score Is Associated with Worsening Renal Function and Reduced Natriuretic Response in Patients with Acute Heart Failure. J. Clin. Med. 2024, 13, 6272. [Google Scholar] [CrossRef]
- Koratala, A.; Romero-González, G.; Soliman-Aboumarie, H.; Kazory, A. Unlocking the potential of VExUS in assessing venous congestion: The art of doing it right. Cardiorenal Med. 2024, 14, 350–374. [Google Scholar] [CrossRef]
- Ohara, H.; Yoshihisa, A.; Horikoshi, Y.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Sugawara, Y.; Ichijo, Y.; Hotsuki, Y.; Watanabe, K.; et al. Renal Venous Stasis Index Reflects Renal Congestion and Predicts Adverse Outcomes in Patients With Heart Failure. Front. Cardiovasc. Med. 2022, 9, 772466. [Google Scholar] [CrossRef]
- Iida, N.; Seo, Y.; Sai, S.; Machino-Ohtsuka, T.; Yamamoto, M.; Ishizu, T.; Kawakami, Y.; Aonuma, K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016, 4, 674–682. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Wallbach, M.; Valentova, M.; Schroeter, M.R.; Alkabariti, A.; Iraki, I.; Leha, A.; Tampe, D.; Hasenfuß, G.; Zeisberg, M.; Hellenkamp, K.; et al. Intrarenal Doppler ultrasonography in patients with HFrEF and acute decompensated heart failure undergoing recompensation. Clin. Res. Cardiol. 2023, 112, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Sukhi, A.; Simon, M.A.; Villanueva, F.S.; Pacella, J.J. Role of Internal Jugular Venous Ultrasound in suspected or confirmed Heart Failure: A Systematic Review. J. Card. Fail. 2022, 28, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Platz, E.; Dauw, J.; Ter Maaten, J.M.; Martens, P.; Pivetta, E.; Cleland, J.G.F.; McMurray, J.J.V.; Mullens, W.; Solomon, S.D.; et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2021, 23, 703–712. [Google Scholar] [CrossRef]
- Simon, M.A.; Schnatz, R.G.; Romeo, J.D.; Pacella, J.J. Bedside Ultrasound Assessment of Jugular Venous Compliance as a Potential Point-of-Care Method to Predict Acute Decompensated Heart Failure 30-Day Readmission. J. Am. Heart Assoc. 2018, 7, e008184. [Google Scholar] [CrossRef]
- Ammirati, E.; Marchetti, D.; Colombo, G.; Pellicori, P.; Gentile, P.; D’Angelo, L.; Garascia, A.; Cipriani, M.; Gagliardi, C.; Manes, M.; et al. Estimation of right atrial pressure by ultrasound-assessed jugular vein distensibility in patients with heart failure. Circ. Heart Fail. 2024, 17, e010973. [Google Scholar] [CrossRef]
- Kobayashi, M.; Douair, A.; Duarte, K.; Jaeger, D.; Giacomin, G.; Bassand, A.; Jeangeorges, V.; Abensur Vuillaume, L.; Preud’homme, G.; Huttin, O.; et al. Diagnostic performance of congestion score index evaluated from chest radiography for acute heart failure in the emergency department: A retrospective analysis from the PARADISE cohort. PLoS Med. 2020, 17, e1003419. [Google Scholar] [CrossRef]
- Pan, D.; Pellicori, P.; Dobbs, K.; Bulemfu, J.; Sokoreli, I.; Urbinati, A.; Brown, O.; Sze, S.; Rigby, A.S.; Kazmi, S.; et al. Prognostic value of the chest X-ray in patients hospitalised for heart failure. Clin. Res. Cardiol. 2021, 110, 1743–1756. [Google Scholar] [CrossRef]
- Collins, S.P.; Lindsell, C.; Storrow, A.B.; Abraham, W.T. Prevalence of Negative Chest Radiography Results in the Emergency Department Patient with Decompensated Heart Failure. Ann. Emerg. Med. 2006, 47, 13–18. [Google Scholar] [CrossRef]
- Pirrotta, F.; Mazza, B.; Gennari, L.; Palazzuoli, A. Pulmonary Congestion Assessment in Heart Failure: Traditional and New Tools. Diagnostics 2021, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Siotto, M.; Cocco, C.; Lanza, G.; Lombardi, F.; Carassiti, M.; Bianchi, M.; D’Angelo, C.; Di Lazzaro, V.; Calabrò, R.S.; et al. Usefulness of body composition assessment by bioelectrical impedance vector analysis in subacute post-stroke patients in rehabilitation. Sci. Rep. 2025, 15, 1774. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Passantino, A.; Guida, P.; Sanasi, M.; Piscopo, A.; Romito, R.; Valle, R.; Caldarola, P.; et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J. Cardiol. 2020, 75, 47–52. [Google Scholar] [CrossRef] [PubMed]
- La Porta, E.; Faragli, A.; Herrmann, A.; Lo Muzio, F.P.; Estienne, L.; Nigra, S.G.; Bellasi, A.; Deferrari, G.; Ricevuti, G.; Di Somma, S.; et al. Bioimpedance Analysis in CKD and HF Patients: A Critical Review of Benefits, Limitations, and Future Directions. J. Clin. Med. 2024, 13, 6502. [Google Scholar] [CrossRef]
- Santarelli, S.; Russo, V.; D’Onofrio, A.; Ammirati, F.; Ammirati, A.L.; Boccanelli, A.; Cacciatore, G.; Cianfrocca, C.; De Luca, L.; Di Lenarda, A.; et al. Prognostic value of decreased peripheral congestion detected by Bioelectrical Impedance Vector Analysis (BIVA) in patients hospitalized for acute heart failure: BIVA prognostic value in acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 339–347. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Bernal-Ceballos, F.; Castillo-Martínez, L.; Reyes-Paz, Y.; Villanueva-Juárez, J.L.; Hernández-Gilsoul, T. Clinical application of phase angle and BIVA Z-score analyses in patients admitted to an emergency department with acute heart failure. J. Vis. Exp. 2023, 196, e65660. [Google Scholar] [CrossRef]
- Thanapholsart, J.; Khan, E.; Lee, G.A. A Current Review of the Uses of Bioelectrical Impedance Analysis and Bioelectrical Impedance Vector Analysis in Acute and Chronic Heart Failure Patients: An Under-valued Resource? Biol. Res. Nurs. 2023, 25, 240–249. [Google Scholar] [CrossRef]
- Pagnesi, M.; Staal, L.; Ter Maaten, J.M.; Beldhuis, I.E.; Cotter, G.; Davison, B.A.; Jongs, N.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; et al. Decongestion and Outcomes in Patients Hospitalized for Acute Heart Failure: Insights From the RELAX-AHF-2 Trial. JACC Heart Fail. 2024, 13, 414–429. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.P.; Martindale, J.L.; Storrow, A.B.; Levy, P.D. Acute Heart Failure: Wet and Sent Home. JACC Heart Fail. 2025, 13, 123–135. [Google Scholar] [CrossRef]
- Santarelli, S.; Russo, V.; D’Onofrio, A.; Ammirati, F.; Ammirati, A.L.; Boccanelli, A.; Cacciatore, G.; Cianfrocca, C.; De Luca, L.; Di Lenarda, A.; et al. Efficacy of modern therapies for heart failure with reduced ejection fraction in specific population subgroups: A systematic review and network meta-analysis. Cardiorenal Med. 2024, 14, 570–580. [Google Scholar]
- Stergiopoulos, G.M.; Elayadi, A.N.; Chen, E.S.; Galiatsatos, P. The effect of telemedicine employing telemonitoring instruments on readmissions of patients with heart failure and/or COPD: A systematic review. Front. Digit. Health 2024, 6, 1441334. [Google Scholar] [CrossRef]
- Chiem, A.T.; Lim, G.W.; Tabibnia, A.P.; Takemoto, A.S.; Weingrow, D.M.; Shibata, J.E. Feasibility of patient-performed lung ultrasound self-exams (Patient-PLUS) as a potential approach to telemedicine in heart failure. ESC Heart Fail. 2021, 8, 3997–4006. [Google Scholar] [CrossRef]
- Scholte, N.T.; van Ravensberg, A.E.; Shakoor, A.; Boersma, E.; Ronner, E.; de Boer, R.A.; Brugts, J.J.; Bruining, N.; van der Boon, R.M. A scoping review on advancements in noninvasive wearable technology for heart failure management. npj Digit. Med. 2024, 7, 279. [Google Scholar] [CrossRef]
- Bourazana, A.; Katsi, V.; Papageorgiou, N.; Papadopoulos, C.; Gkoufa, A.; Vrettou, A.R.; Papazoglou, A.S.; Gialernios, T.; Kallistratos, M.S.; Tousoulis, D.; et al. Artificial intelligence in heart failure: Friend or foe? Life 2024, 14, 145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pena, D.-L.; Ilieșiu, A.-M.; Aurelian, J.; Grigore, M.; Hodorogea, A.-S.; Ciobanu, A.; Weiss, E.; Badilă, E.; Balahura, A.-M. Assessment of Decongestion Status Before Discharge in Acute Decompensated Heart Failure: A Review of Clinical, Biochemical, and Imaging Tools and Their Impact on Management Decisions. Medicina 2025, 61, 816. https://doi.org/10.3390/medicina61050816
Pena D-L, Ilieșiu A-M, Aurelian J, Grigore M, Hodorogea A-S, Ciobanu A, Weiss E, Badilă E, Balahura A-M. Assessment of Decongestion Status Before Discharge in Acute Decompensated Heart Failure: A Review of Clinical, Biochemical, and Imaging Tools and Their Impact on Management Decisions. Medicina. 2025; 61(5):816. https://doi.org/10.3390/medicina61050816
Chicago/Turabian StylePena, Diana-Ligia, Adriana-Mihaela Ilieșiu, Justin Aurelian, Mihai Grigore, Andreea-Simona Hodorogea, Ana Ciobanu, Emma Weiss, Elisabeta Badilă, and Ana-Maria Balahura. 2025. "Assessment of Decongestion Status Before Discharge in Acute Decompensated Heart Failure: A Review of Clinical, Biochemical, and Imaging Tools and Their Impact on Management Decisions" Medicina 61, no. 5: 816. https://doi.org/10.3390/medicina61050816
APA StylePena, D.-L., Ilieșiu, A.-M., Aurelian, J., Grigore, M., Hodorogea, A.-S., Ciobanu, A., Weiss, E., Badilă, E., & Balahura, A.-M. (2025). Assessment of Decongestion Status Before Discharge in Acute Decompensated Heart Failure: A Review of Clinical, Biochemical, and Imaging Tools and Their Impact on Management Decisions. Medicina, 61(5), 816. https://doi.org/10.3390/medicina61050816







