Proteomic and In Silico Analyses Highlight Complement System’s Role in Bladder Cancer Immune Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Protein Extraction and Pool Formation
2.3. Enzymatic Digestion and LC-MS/MS Analysis
2.4. Functional Annotation and Pathway-Based Analysis of Immune-Associated Proteins
2.5. Immunological Analysis
2.6. Prognostic Analysis of Hub Genes
2.7. Verification of LC-MS/MS Analysis via Western Blot
3. Results
3.1. Study Cohort and Immune Correlated LC-MS/MS Data Analysis
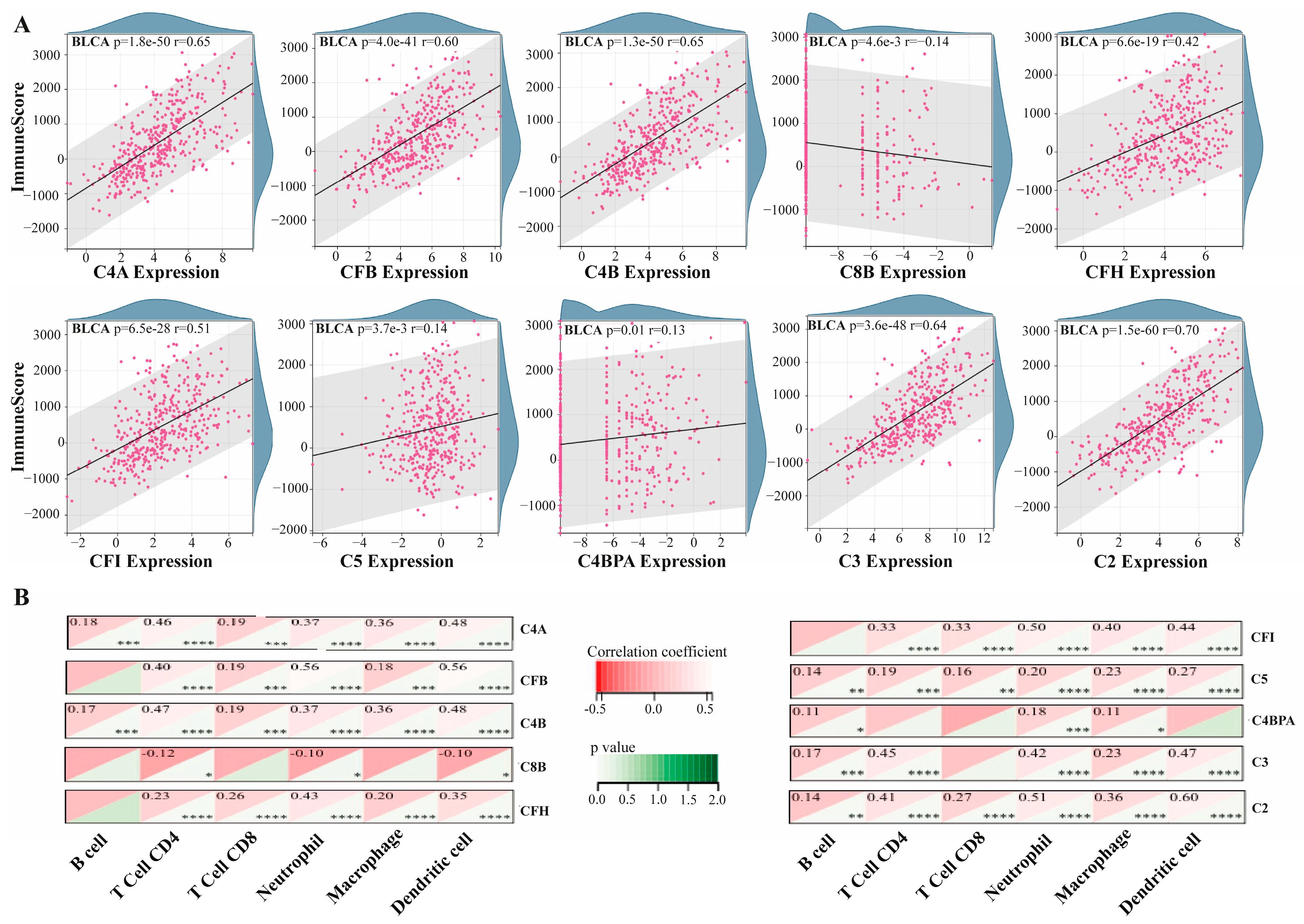
3.2. Association of Hub Proteins with Tumor Immune Microenvironment
3.3. Association Between Hub Gene Expression and ICP Markers
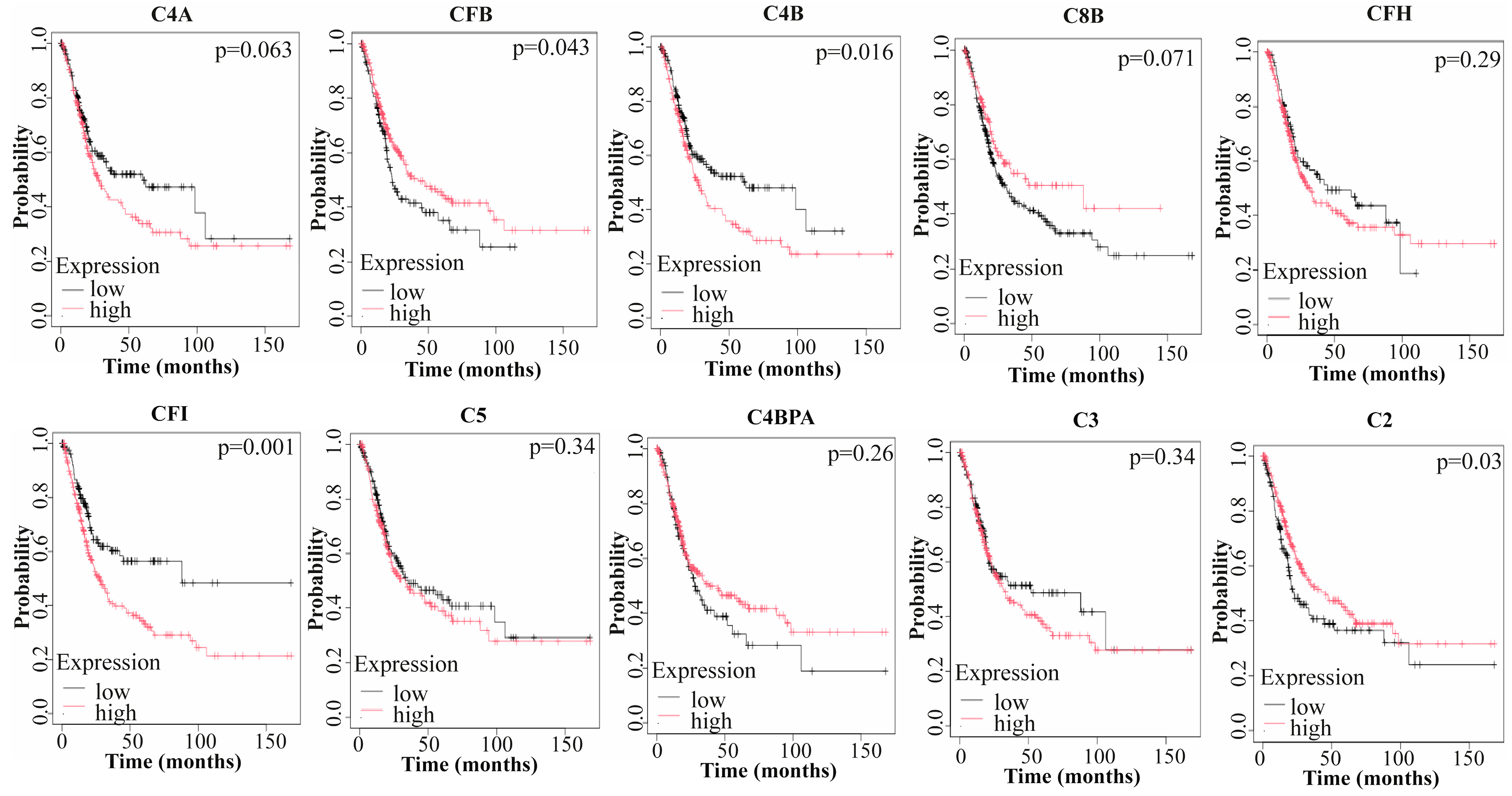
3.4. Prognostic Value of Hub Genes
3.5. Validation of Proteomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLCA | Bladder Cancer |
| LC-MS | Liquid Chromatography/Mass Spectrometry |
| BCG | Bacille Calmette–Guérin |
| IL | Interleukin |
| ICIs | Immune Checkpoint Inhibitors |
| TME | Tumor Microenvironment |
| TUR-P | Transurethral Resection of the Prostate |
| FDR | False Discovery Rate |
| DRPs | Differentially Regulated Proteins |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| PPI | Protein–Protein Interaction |
| ICPs | Immune Checkpoint Proteins |
| OS | Overall Survival |
| MCC | Maximal Clique Centrality |
| CF | Complement Factor |
| C4BPA | Complement 4 Binding Protein Alpha |
| DC | Dendritic Cell |
| MAC | Membrane Attack Complex |
| EMT | Epithelial–Mesenchymal Transition |
| sCC | Cutaneous Squamous Cell Carcinoma |
| MDSC | Myeloid-Derived Suppressor Cell |
| TH2 | T-Helper-2 |
| Tregs | Regulatory T-Cells |
| NET | Neutrophil Extracellular Trap |
| mCRPs | Membrane-Bound Complement Regulatory Proteins |
| LG | Low Grade |
| HG | High Grade |
| RCC | Renal Cell Carcinoma |
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Wéber, A.; Vignat, J.; Shah, R.; Morgan, E.; Laversanne, M.; Nagy, P.; Kenessey, I.; Znaor, A. Global Burden of Bladder Cancer Mortality in 2020 and 2040 According to GLOBOCAN Estimates. World J. Urol. 2024, 42, 237. [Google Scholar] [CrossRef] [PubMed]
- Wołącewicz, M.; Hrynkiewicz, R.; Grywalska, E.; Suchojad, T.; Leksowski, T.; Roliński, J.; Niedźwiedzka-Rystwej, P. Immunotherapy in Bladder Cancer: Current Methods and Future Perspectives. Cancers 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Jagodinsky, J.C.; Harari, P.M.; Morris, Z.S. The Promise of Combining Radiation Therapy with Immunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 6–16. [Google Scholar] [CrossRef]
- Yang, T.; Luo, W.; Yu, J.; Zhang, H.; Hu, M.; Tian, J. Bladder Cancer Immune-Related Markers: Diagnosis, Surveillance, and Prognosis. Front. Immunol. 2024, 15, 1481296. [Google Scholar] [CrossRef]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the Treatment of Bladder Cancer-Current Understanding and the Prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Parent, P.; Marcq, G.; Adeleke, S.; Turpin, A.; Boussios, S.; Rassy, E.; Penel, N. Predictive Biomarkers for Immune Checkpoint Inhibitor Response in Urothelial Cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231192402. [Google Scholar] [CrossRef]
- Aggen, D.H.; Drake, C.G. Biomarkers for Immunotherapy in Bladder Cancer: A Moving Target. J. Immunother. Cancer 2017, 5, 94. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Jo, H.-S.; Bae, S.; Seo, Y.; Song, P.; Song, M.; Yoon, J.H. Application of Proteomics in Cancer: Recent Trends and Approaches for Biomarkers Discovery. Front. Med. 2021, 8, 747333. [Google Scholar] [CrossRef]
- Cho, W.C.-S. Potentially Useful Biomarkers for the Diagnosis, Treatment and Prognosis of Lung Cancer. Biomed. Pharmacother. 2007, 61, 515–519. [Google Scholar] [PubMed]
- Wang, W.; Huang, G.; Lin, H.; Ren, L.; Fu, L.; Mao, X. Label-Free LC-MS/MS Proteomics Analyses Reveal CLIC1 as a Predictive Biomarker for Bladder Cancer Staging and Prognosis. Front. Oncol. 2023, 12, 1102392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xue, F.; Li, T.; Xue, J.; Yue, S.; Zhao, S.; Lu, H.; He, C. Exploration of Potential Biomarkers for Early Bladder Cancer Based on Urine Proteomics. Front. Oncol. 2024, 14, 1309842. [Google Scholar] [CrossRef] [PubMed]
- Dressler, F.F.; Diedrichs, F.; Sabtan, D.; Hinrichs, S.; Krisp, C.; Gemoll, T.; Hennig, M.; Mackedanz, P.; Schlotfeldt, M.; Voß, H.; et al. Proteomic Analysis of the Urothelial Cancer Landscape. Nat. Commun. 2024, 15, 4513. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chung, T.; Wu, C.-C.; Ng, K.-F.; Yu, J.-S.; Tsai, C.-H.; Chang, Y.-S.; Liang, Y.; Tsui, K.-H.; Chen, Y.-T. Comparative Tissue Proteomics of Microdissected Specimens Reveals Novel Candidate Biomarkers of Bladder Cancer*. Mol. Cell. Proteom. 2015, 14, 2466–2478. [Google Scholar] [CrossRef]
- Yanar, S.; Albayrak, M.G.B.; Kasap, M.; Akpinar, G. From Androgen Dependence to Independence in Prostate Cancer: Unraveling Therapeutic Potential and Proteomic Landscape of Hydroxychloroquine as an Autophagy Inhibitor. OMICS A J. Integr. Biol. 2024, 28, 246–255. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Keşim, D.A.; Aşır, F.; Ayaz, H.; Korak, T. The Effects of Ellagic Acid on Experimental Corrosive Esophageal Burn Injury. Curr. Issues Mol. Biol. 2024, 46, 1579–1592. [Google Scholar] [CrossRef]
- Aşır, F.; Duran, S.Ç.; Afşin, M.; Duran, E.; Korak, T.; Şahin, F. Investigation of Vitamin D Levels in Men with Suspected Infertility. Life 2024, 14, 273. [Google Scholar] [CrossRef]
- Korak, T.; Albayrak, M.G.B.; Kasap, M.; Akpinar, G.; Yanar, S. Unlocking the Potential of RARRES1: A Pan-Cancer Analysis for Prognosis, Diagnosis, Tumor Immunity and Drug Sensitivity. J. Biol. Res.-Thessalon. 2024, 31, 1–17. [Google Scholar]
- Wang, L.; Huang, G.; Xiao, H.; Leng, X. A Pan-Cancer Analysis of the Association of METRN with Prognosis and Immune Infiltration in Human Tumors. Heliyon 2024, 10, e37213. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A Comprehensive, Interaction-friendly Clinical Bioinformatics Analysis Platform. iMeta 2022, 1, e36. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Integrated Analysis of Public Datasets for the Discovery and Validation of Survival-Associated Genes in Solid Tumors. Innovation 2024, 5, 100625. [Google Scholar] [CrossRef] [PubMed]
- Korak, T.; Albayrak, M.G.B.; Kasap, M.; Akpinar, G. Thymoquinone and Metabolic Reprogramming in Breast Cancer: A New Dimension from Proteomic Analysis. J. Biochem. Mol. Toxicol. 2025, 39, e70124. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.B.; Li, Y.; Su, W.H.; He, S.; Pan, S.P.; Xu, K.; Kou, W.H. Three Prognostic Biomarkers Correlate with Immune Checkpoint Blockade Response in Bladder Urothelial Carcinoma. Int. J. Genom. 2022, 2022, 3342666. [Google Scholar] [CrossRef]
- Afshar-Kharghan, V. The Role of the Complement System in Cancer. J. Clin. Investig. 2017, 127, 780–789. [Google Scholar] [CrossRef]
- Revel, M.; Daugan, M.V.; Sautés-Fridman, C.; Fridman, W.H.; Roumenina, L.T. Complement System: Promoter or Suppressor of Cancer Progression? Antibodies 2020, 9, 57. [Google Scholar] [CrossRef]
- Thi, T.N.; Thanh, H.D.; Nguyen, V.-T.; Kwon, S.-Y.; Moon, C.; Hwang, E.C.; Jung, C. Complement Regulatory Protein CD46 Promotes Bladder Cancer Metastasis through Activation of MMP9. Int. J. Oncol. 2024, 65, 71. [Google Scholar] [CrossRef]
- Zirakzadeh, A.A.; Sherif, A.; Rosenblatt, R.; Bergman, E.A.; Winerdal, M.; Yang, D.; Cederwall, J.; Jakobsson, V.; Hyllienmark, M.; Winqvist, O.; et al. Tumour-associated B Cells in Urothelial Urinary Bladder Cancer. Scand. J. Immunol. 2020, 91, e12830. [Google Scholar] [CrossRef]
- Ajona, D.; Cragg, M.S.; Pio, R. The Complement System in Clinical Oncology: Applications, Limitations and Challenges. Semin. Immunol. 2025, 77, 101921. [Google Scholar] [CrossRef]
- Lawal, B.; Tseng, S.-H.; Olugbodi, J.O.; Iamsaard, S.; Ilesanmi, O.B.; Mahmoud, M.H.; Ahmed, S.H.; Batiha, G.E.-S.; Wu, A.T.H. Pan-Cancer Analysis of Immune Complement Signature C3/C5/C3AR1/C5AR1 in Association with Tumor Immune Evasion and Therapy Resistance. Cancers 2021, 13, 4124. [Google Scholar] [CrossRef] [PubMed]
- Vanarsa, K.; Castillo, J.; Wang, L.; Lee, K.H.; Pedroza, C.; Lotan, Y.; Mohan, C. Comprehensive Proteomics and Platform Validation of Urinary Biomarkers for Bladder Cancer Diagnosis and Staging. BMC Med. 2023, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Rupaimoole, R.; Choi, H.-J.; Noh, K.; Chen, J.; Hu, Q.; Sood, A.K.; Afshar-Kharghan, V. Complement Component 3 Is Regulated by TWIST1 and Mediates Epithelial–Mesenchymal Transition. J. Immunol. 2016, 196, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Markiewski, M.M.; DeAngelis, R.A.; Benencia, F.; Ricklin-Lichtsteiner, S.K.; Koutoulaki, A.; Gerard, C.; Coukos, G.; Lambris, J.D. Modulation of the Antitumor Immune Response by Complement. Nat. Immunol. 2008, 9, 1225–1235. [Google Scholar] [CrossRef]
- Lin, K.; He, S.; He, L.; Chen, J.; Cheng, X.; Zhang, G.; Zhu, B. Complement Component 3 Is a Prognostic Factor of Non-Small Cell Lung Cancer. Mol. Med. Rep. 2014, 10, 811–817. [Google Scholar] [CrossRef]
- Bouwens, T.A.M.; Trouw, L.A.; Veerhuis, R.; Dirven, C.M.F.; Lamfers, M.L.M.; Al-Khawaja, H. Complement Activation in Glioblastoma Multiforme Pathophysiology: Evidence from Serum Levels and Presence of Complement Activation Products in Tumor Tissue. J. Neuroimmunol. 2015, 278, 271–276. [Google Scholar] [CrossRef]
- Nunez-Cruz, S.; Gimotty, P.A.; Guerra, M.W.; Connolly, D.C.; Wu, Y.-Q.; DeAngelis, R.A.; Lambris, J.D.; Coukos, G.; Scholler, N. Genetic and Pharmacologic Inhibition of Complement Impairs Endothelial Cell Function and Ablates Ovarian Cancer Neovascularization. Neoplasia 2012, 14, 994–1004,IN1. [Google Scholar] [CrossRef]
- Nitta, H.; Wada, Y.; Kawano, Y.; Murakami, Y.; Irie, A.; Taniguchi, K.; Kikuchi, K.; Yamada, G.; Suzuki, K.; Honda, J.; et al. Enhancement of Human Cancer Cell Motility and Invasiveness by Anaphylatoxin C5a via Aberrantly Expressed C5a Receptor (CD88). Clin. Cancer Res. 2013, 19, 2004–2013. [Google Scholar] [CrossRef]
- Riihilä, P.M.; Nissinen, L.M.; Ala-aho, R.; Kallajoki, M.; Grénman, R.; Meri, S.; Peltonen, S.; Peltonen, J.; Kähäri, V.-M. Complement Factor H: A Biomarker for Progression of Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2014, 134, 498–506. [Google Scholar] [CrossRef]
- Davidson, S.; Efremova, M.; Riedel, A.; Mahata, B.; Pramanik, J.; Huuhtanen, J.; Kar, G.; Vento-Tormo, R.; Hagai, T.; Chen, X.; et al. Single-Cell RNA Sequencing Reveals a Dynamic Stromal Niche That Supports Tumor Growth. Cell Rep. 2020, 31, 107628. [Google Scholar] [CrossRef]
- Bao, D.; Zhang, C.; Li, L.; Wang, H.; Li, Q.; Ni, L.; Lin, Y.; Huang, R.; Yang, Z.; Zhang, Y.; et al. Integrative Analysis of Complement System to Prognosis and Immune Infiltrating in Colon Cancer and Gastric Cancer. Front. Oncol. 2021, 10, 553297. [Google Scholar] [CrossRef] [PubMed]
- Kolev, M.; Das, M.; Gerber, M.; Baver, S.; Deschatelets, P.; Markiewski, M.M. Inside-Out of Complement in Cancer. Front. Immunol. 2022, 13, 931273. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, D.M.; Ghouse, S.M.; Karbowniczek, M.M.; Markiewski, M.M. Complementing Cancer Metastasis. Front. Immunol. 2018, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.; Hassan, M.A.; Huynh, T.; Feng, X.; Wang, H. Shedding Light on the Role of Complement C4 Activation in Cancer. Hum. Immunol. 2025, 86, 111226. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, F.; Zheng, X.; Chen, D.; Li, Z.; Bi, Q.; Qiu, X.; Sun, Z.; Wang, W. Identification of Bladder Cancer Subtypes and Predictive Signature for Prognosis, Immune Features, and Immunotherapy Based on Immune Checkpoint Genes. Sci. Rep. 2024, 14, 14431. [Google Scholar] [CrossRef]
- Lubbers, R.; van Essen, M.F.; van Kooten, C.; Trouw, L.A. Production of Complement Components by Cells of the Immune System. Clin. Exp. Immunol. 2017, 188, 183–194. [Google Scholar] [CrossRef]
- Cai, X.; Qiu, W.; Qian, M.; Feng, S.; Peng, C.; Zhang, J.; Wang, Y.; Wang, Y. A Candidate Prognostic Biomarker Complement Factor I Promotes Malignant Progression in Glioma. Front. Cell Dev. Biol. 2021, 8, 615970. [Google Scholar] [CrossRef]
- Zafar, G.I.; Grimm, E.A.; Wei, W.; Johnson, M.M.; Ellerhorst, J.A. Genetic Deficiency of Complement Isoforms C4A or C4B Predicts Improved Survival of Metastatic Renal Cell Carcinoma. J. Urol. 2009, 181, 1028–1034. [Google Scholar] [CrossRef]
- Zhang, G.; Li, S.; Xiao, W.; Zhang, C.; Li, T.; Liao, Z.; Liu, H.; Xing, R.; Yao, W.; Yang, J. Tumoral C2 Regulates the Tumor Microenvironment by Increasing the Ratio of M1/M2 Macrophages and Tertiary Lymphoid Structures to Improve Prognosis in Melanoma. Cancers 2024, 16, 908. [Google Scholar] [CrossRef]
- He, C.; Wang, X.; Jiang, B.; Zhu, M.; Zhang, H.; Duan, Y.; Li, Y. Complement Factor B (CFB) Inhibits the Malignant Progression of Lung Adenocarcinoma by Downregulating the Ras/MAPK Signaling Pathway. Arch. Biochem. Biophys. 2024, 760, 110130. [Google Scholar] [CrossRef]
- Shimazaki, R.; Takano, S.; Satoh, M.; Takada, M.; Miyahara, Y.; Sasaki, K.; Yoshitomi, H.; Kagawa, S.; Furukawa, K.; Takayashiki, T.; et al. Complement Factor B Regulates Cellular Senescence and Is Associated with Poor Prognosis in Pancreatic Cancer. Cell. Oncol. 2021, 44, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Roumenina, L.T.; Daugan, M.V.; Petitprez, F.; Sautès-Fridman, C.; Fridman, W.H. Context-Dependent Roles of Complement in Cancer. Nat. Rev. Cancer 2019, 19, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Bulla, R.; Tripodo, C.; Rami, D.; Ling, G.S.; Agostinis, C.; Guarnotta, C.; Zorzet, S.; Durigutto, P.; Botto, M.; Tedesco, F. C1q Acts in the Tumour Microenvironment as a Cancer-Promoting Factor Independently of Complement Activation. Nat. Commun. 2016, 7, 10346. [Google Scholar] [CrossRef] [PubMed]
- Riihilä, P.; Nissinen, L.; Farshchian, M.; Kallajoki, M.; Kivisaari, A.; Meri, S.; Grénman, R.; Peltonen, S.; Peltonen, J.; Pihlajaniemi, T.; et al. Complement Component C3 and Complement Factor B Promote Growth of Cutaneous Squamous Cell Carcinoma. Am. J. Pathol. 2017, 187, 1186–1197. [Google Scholar] [CrossRef]
- Boire, A.; Zou, Y.; Shieh, J.; Macalinao, D.G.; Pentsova, E.; Massagué, J. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell 2017, 168, 1101–1113.e13. [Google Scholar] [CrossRef]
- Merle, N.S.; Roumenina, L.T. The Complement System as a Target in Cancer Immunotherapy. Eur. J. Immunol. 2024, 54, e2350820. [Google Scholar] [CrossRef]
- Russo, F.; Esposito, S.; Tripodi, L.; Pandolfo, S.D.; Aveta, A.; Amato, F.; Nardelli, C.; Imbimbo, C.; Pastore, L.; Castaldo, G. Insights into Porphyromonas Somerae in Bladder Cancer Patients: Urinary Detection by DdPCR. Microorganisms 2024, 12, 2049. [Google Scholar] [CrossRef]
- Huang, X.; Nepovimova, E.; Adam, V.; Sivak, L.; Heger, Z.; Valko, M.; Wu, Q.; Kuca, K. Neutrophils in Cancer Immunotherapy: Friends or Foes? Mol. Cancer 2024, 23, 107. [Google Scholar] [CrossRef]
- Joseph, M.; Enting, D. Immune Responses in Bladder Cancer-Role of Immune Cell Populations, Prognostic Factors and Therapeutic Implications. Front. Oncol. 2019, 9, 1270. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, B.; Chen, J.; Sun, X.; Yang, W.; Yang, T.; Yu, H.; Chen, P.; Chen, K.; Huang, X.; et al. Type III Interferon Inhibits Bladder Cancer Progression by Reprogramming Macrophage-Mediated Phagocytosis and Orchestrating Effective Immune Responses. J. Immunother. Cancer 2024, 12, e007808. [Google Scholar] [CrossRef]
- Adyns, L.; Proost, P.; Struyf, S. Role of Defensins in Tumor Biology. Int. J. Mol. Sci. 2023, 24, 5268. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among Smoking, Oxidative Stress, Inflammation, Macromolecular Damage, and Cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.M.; Duan, F.; Srivastava, P.K. Smoking-Induced Immune Deviation Contributes to Progression of Bladder and Other Cancers. OncoImmunology 2015, 4, e1019199. [Google Scholar] [CrossRef] [PubMed]


| BLCA Patients | |
|---|---|
| Gender, n (%) | |
| Male | 15 (55.6) |
| Female | 12 (44.4) |
| Age (mean ± SD), years | 68 ± 10.5 |
| Tumor diameter (mean ± SD), cm | 3.07 ± 1.8 |
| Recurrence, n (%) | |
| Observed | 9 (33.3) |
| Not observed | 18 (66.7) |
| Smoking, n(%) | |
| Present | 25 (92.6) |
| Absent | 2 (7.4) |
| Concurrent malignancy, n (%) | |
| Present | 0 (0) |
| Absent | 27 (100) |
| Healthy Controls | |
| Gender, n (%) | |
| Male | 16 (59.3) |
| Female | 11 (40.7) |
| Age (mean ± SD), years | 63.4 ± 8.5 |
| Smoking, n (%) | |
| Present | 19 (70.4) |
| Absent | 8 (29.6) |
| Concurrent malignancy, n (%) | |
| Present | 0 (0) |
| Absent | 27 (100) |
| Gene Name | Uniprot ID | Expression Fold Change |
|---|---|---|
| C4A | P0C0L4 | 4.9 |
| CFB | P00751 | 3.7 |
| C4B | P0C0L5 | 6.2 |
| C8B | P07358 | 3.4 |
| CFH | P08603 | 10.1 |
| CFI | P05156 | 3.1 |
| C5 | P01031 | 2.8 |
| C4BPA | P04003 | 5.2 |
| C3 | P01024 | 5.5 |
| C2 | P06681 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korak, T.; Baloğlu, İ.H.; Kasap, M.; Arisan, E.D.; Akpinar, G.; Arisan, S. Proteomic and In Silico Analyses Highlight Complement System’s Role in Bladder Cancer Immune Regulation. Medicina 2025, 61, 735. https://doi.org/10.3390/medicina61040735
Korak T, Baloğlu İH, Kasap M, Arisan ED, Akpinar G, Arisan S. Proteomic and In Silico Analyses Highlight Complement System’s Role in Bladder Cancer Immune Regulation. Medicina. 2025; 61(4):735. https://doi.org/10.3390/medicina61040735
Chicago/Turabian StyleKorak, Tuğcan, İbrahim Halil Baloğlu, Murat Kasap, Elif Damla Arisan, Gurler Akpinar, and Serdar Arisan. 2025. "Proteomic and In Silico Analyses Highlight Complement System’s Role in Bladder Cancer Immune Regulation" Medicina 61, no. 4: 735. https://doi.org/10.3390/medicina61040735
APA StyleKorak, T., Baloğlu, İ. H., Kasap, M., Arisan, E. D., Akpinar, G., & Arisan, S. (2025). Proteomic and In Silico Analyses Highlight Complement System’s Role in Bladder Cancer Immune Regulation. Medicina, 61(4), 735. https://doi.org/10.3390/medicina61040735







