Factors Associated with Mortality Risk in Patients with Cardiogenic Shock Post-ST-Elevation Myocardial Infarction: Insights from a Regional Centre in Northwest Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Study Groups
2.3. Data Collection
2.4. Study Endpoints
2.5. Definitions and Variables
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Patients with STEMI and CS
3.2. Factors Associated with the Likelihood of In-Hospital Mortality of CS Patients
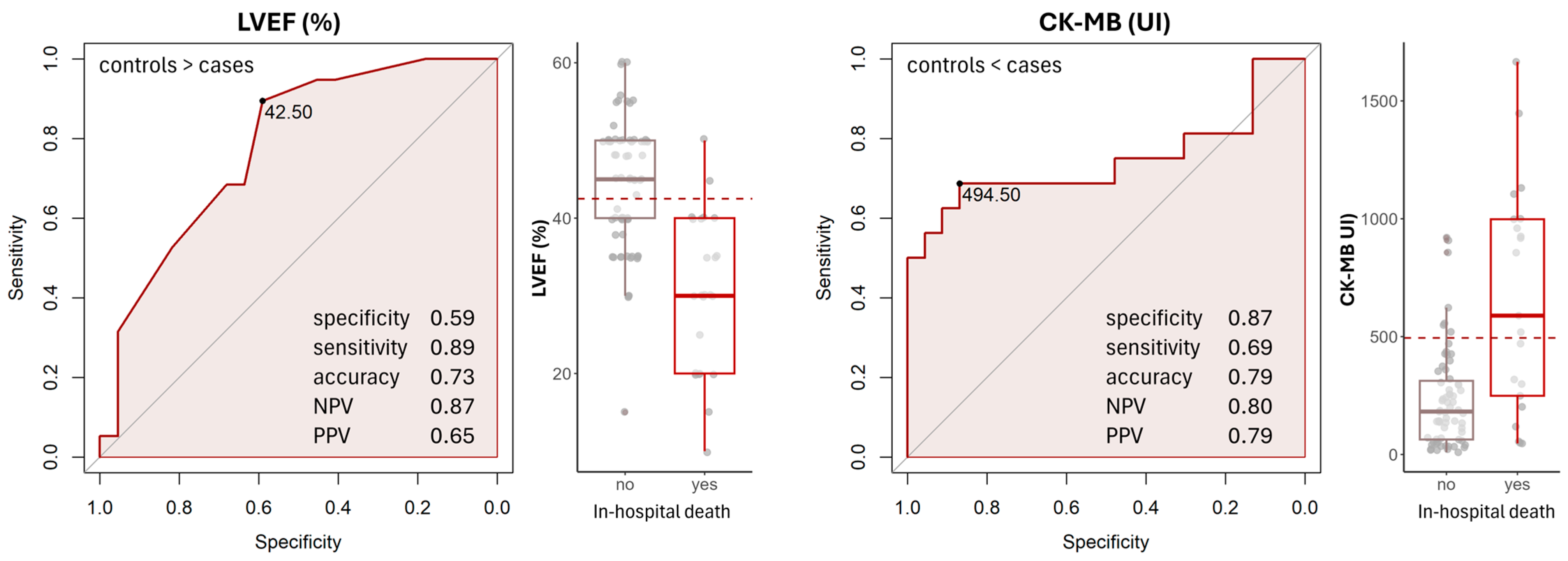
3.3. Defining the Cut-Off for Increased Risk of Mortality of Patients with STEMI and CS
4. Discussion
4.1. Results in the Context of Published Literature
4.2. Study Limitations
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elendu, C.; Amaechi, D.C.M.; Elendu, T.C.B.; Omeludike, E.K.M.; Alakwe-Ojimba, C.E.M.; Obidigbo, B.M.; Akpovona, O.L.M.; Sucari, Y.P.O.; Saggi, S.K.; Dang, K.; et al. Comprehensive review of ST-segment elevation myocardial infarction: Understanding pathophysiology, diagnostic strategies, and current treatment approaches. Medicine 2023, 102, e35687. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.; Butrymovich, V.; Kelbæk, H.; Wachtell, K.; Helqvist, S.; Kastrup, J.; Holmvang, L.; Clemmensen, P.; Engstrøm, T.; Grande, P.; et al. Short-and long-term cause of death in patients treated with primary PCI for STEMI. J. Am. Coll. Cardiol. 2014, 64, 2101–2108. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef]
- Hurtado, V.; Dangl, M.; Inestroza, K.; Hernandez, R.; Albosta, M.; Ergui, I.; Vergara, C.; Colombo, R. Cardiogenic Shock Following Acute Myocardial Infarction: Trends Of Incidence, Management, And Outcomes From The National Inpatient Sample Database Perspective. J. Card. Fail. 2023, 29, 636. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Psotka, M.A.; Rosner, C.; Singh, R.; Sinha, S.S.; Damluji, A.A.; Batchelor, W.B. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020, 8, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- De Luca, L.; Mistrulli, R.; Scirpa, R.; Thiele, H.; De Luca, G. Contemporary Management of Cardiogenic Shock Complicating Acute Myocardial Infarction. J. Clin. Med. 2023, 12, 2184. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; De Waha-Thiele, S.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Nordbeck, P.; Geisler, T.; Landmesser, U.; et al. One-year outcomes after PCI strategies in cardiogenic shock. N. Engl. J. Med. 2018, 379, 1699–1710. [Google Scholar] [CrossRef]
- Arrigo, M.; Price, S.; Baran, D.A.; Pöss, J.; Aissaoui, N.; Bayes-Genis, A.; Bonello, L.; François, B.; Gayat, E.; Gilard, M.; et al. Optimising clinical trials in acute myocardial infarction complicated by cardiogenic shock: A statement from the 2020 Critical Care Clinical Trialists Workshop. Lancet Respir. Med. 2021, 9, 1192–1202. [Google Scholar] [CrossRef]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef]
- Kammler, J.; Kypta, A.; Hofmann, R.; Kerschner, K.; Grund, M.; Sihorsch, K.; Steinwender, C.; Lambert, T.; Helml, W.; Leisch, F. TIMI 3 flow after primary angioplasty is an important predictor for outcome in patients with acute myocardial infarction. Clin. Res. Cardiol. 2009, 98, 165–170. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Akin, I.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2023, 389, 1286–1297. [Google Scholar] [CrossRef]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef]
- Clinical Outcome and Cost-effectiveness of Reduced Noradrenaline by Using a Lower Blood Pressure Target in Patients With Cardiogenic Shock From Acute Myocardial Infarction (NORSHOCK). ClinicalTrials.gov ID NCT05168462. Available online: https://clinicaltrials.gov/ (accessed on 4 April 2025).
- Evaluation of Unloading the Heart in Patients with Cardiogenic Shock Treated with Mechanical Circulatory Support Devices. ISRCTN82431978. Available online: https://www.isrctn.com/ISRCTN82431978 (accessed on 4 April 2025).
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.; Lassus, J.; Sionis, A.; Køber, L.; Tarvasmäki, T.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur. J. Heart Fail. 2015, 17, 501–509. [Google Scholar] [CrossRef]
- Pöss, J.; Köster, J.; Fuernau, G.; Eitel, I.; de Waha, S.; Ouarrak, T.; Lassus, J.; Harjola, V.-P.; Zeymer, U.; Thiele, H.; et al. Risk Stratification for Patients in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Ducas, R.; Ariyarajah, V.; Philipp, R.; Ducas, J.; Elliott, J.; Jassal, D.; Tam, J.; Garber, P.; Shaikh, N.; Hussain, F. The presence of ST-elevation in lead aVR predicts significant left main coronary artery stenosis in cardiogenic shock resulting from myocardial infarction: The Manitoba cardiogenic shock registry. Int. J. Cardiol. 2013, 166, 465–468. [Google Scholar] [CrossRef]
- ESC. Atlas of Cardiology, Country Profile Romania, RSC Survey, Survey on Romanian Society of Cardiology; European Society of Cardiology: Antipolis, France, 2015. [Google Scholar]
- Predescu, L.M.; Udroiu, C.A. The workload of the interventional cardiology centers in Romania: A 2022 overview and 2014–2022 trends. Romanian J. Cardiol. 2024, 34, 31–41. [Google Scholar] [CrossRef]
- Tatu-Chitoiu, G.; Arafat, R.; Deleanu, D.; Vinereanu, D.; Udroiu, C.; Petris, A. Impact of the Romanian national programme for interventional therapy in ST-elevation myocardial infarction. EuroIntervention 2012, 8, 126–132. [Google Scholar] [CrossRef]
- Cretu, D.E.; Udroiu, C.A.; Stoicescu, C.I.; Tatu-Chitoiu, G.; Vinereanu, D. Predictors of in-Hospital Mortality of ST-Segment Elevation Myocardial Infarction Patients Undergoing Interventional Treatment. An Analysis of Data from the RO-STEMI Registry. Maedica 2015, 10, 295–303. [Google Scholar]
- Chioncel, O.; Vinereanu, D.; Datcu, M.; Ionescu, D.D.; Capalneanu, R.; Brukner, I.; Dorobantu, M.; Ambrosy, A.; Macarie, C.; Gheorghiade, M. The Romanian Acute Heart Failure Syndromes (RO-AHFS) Registry. Am. Heart J. 2011, 162, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Institutul Național de Statistică. Rezultate definitive RPL 2021—Recensamantul Populatiei si Locuintelor. Available online: https://www.recensamantromania.ro/rezultate-rpl-2021/rezultate-definitive/ (accessed on 8 February 2025).
- Waksman, R.; Pahuja, M.; Van Diepen, S.; Proudfoot, A.G.; Morrow, D.; Spitzer, E.; Nichol, G.; Weisfeldt, M.L.; Moscucci, M.; Lawler, P.R.; et al. Standardized definitions for cardiogenic shock research and mechanical circulatory support devices: Scientific expert panel from the shock academic research consortium (SHARC). Circulation 2023, 148, 1113–1126. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF). Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 13, 305–338. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Levitov, A.; Frankel, H.L.; Blaivas, M.; Kirkpatrick, A.W.; Su, E.; Evans, D.; Summerfield, D.T.; Slonim, A.; Breitkreutz, R.; Price, S.; et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—Part II: Cardiac ultrasonography. Crit. Care Med. 2016, 44, 1206–1227. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Shibayama, K.; Iwano, H.; Izumo, M.; Kagiyama, N.; Kurosawa, K.; Mihara, H.; Oe, H.; Onishi, T.; Onishi, T.; et al. Reduced variability of visual left ventricular ejection fraction assessment with reference images: The Japanese Association of Young Echocardiography Fellows multicenter study. J. Cardiol. 2018, 72, 74–80. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 11 December 2023).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Ben-Shachar, M.S.; Lüdecke, D.; Makowski, D. effectsize: Estimation of Effect Size Indices and Standardized Parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Champely, S.; Ekstrom, C.; Dalgaard, P.; Gill, J. R package, version 1.3-0, Pwr: Basic Functions for Power Analysis; GitHub: San Francisco, CA, USA, 2020; Available online: https://github.com/heliosdrm/pwr (accessed on 4 April 2025).
- Dekker, L.R.; Bezzina, C.R.; Henriques, J.P.; Tanck, M.W.; Koch, K.T.; Alings, M.W.; Arnold, A.E.; de Boer, M.J.; Gorgels, A.P.; Michels, H.R.; et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: A case-control study in acute myocardial infarction patients. Circulation 2006, 114, 1140–1145. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Zhang, R.; Liu, Y.; Liu, H.; Yu, J.; Zhou, X.; Du, Y.; Cong, H. Risk factors for cardiac rupture after acute ST-segment elevation myocardial infarction during the percutaneous coronary intervention era: A retrospective case-control study. J. Thorac. Dis. 2022, 14, 1256–1266. [Google Scholar] [CrossRef]
- Kalayci, A.; Oduncu, V.; Geçmen, Ç.; Topcu, S.; Karabay, C.Y.; İZGİ, İ.A.; Kirma, C. A simple risk score in acute ST-elevation myocardial infarction: Modified ACEF(age, creatinine, and ejection fraction) score. Turk. J. Med Sci. 2016, 46, 1688–1693. [Google Scholar] [CrossRef]
- Margolis, G.; Khoury, S.; Ben-Shoshan, J.; Letourneau-Shesaf, S.; Flint, N.; Keren, G.; Shacham, Y. Prognostic Implications of Mid-Range Left Ventricular Ejection Fraction on Patients Presenting With ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2017, 120, 186–190. [Google Scholar] [CrossRef]
- Bengtson, J.R.; Kaplan, A.J.; Pieper, K.S.; Wildermann, N.M.; Mark, D.B.; Pryor, D.B.; Phillips, H.R.; Califf, R.M. Prognosis in cardiogenic shock after acute myocardial infarction in the intervencional era. Circulation 1992, 20, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Johnson, S.; Altwail, M.; Brackney, A.; Xiao, J.; Price, J.; Shotkin, P.; Chen, N.-W. Left Ventricular Ejection Fraction Assessment by Emergency Physician-Performed Bedside Echocardiography: A Prospective Comparative Evaluation of Multiple Modalities. J. Emerg. Med. 2021, 61, 711–719. [Google Scholar] [CrossRef]
- Ünlüer, E.; Karagöz, A.; Akoğlu, H.; Bayata, S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J. Emerg. Med. 2014, 15, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, P.; Rydberg, E.; Winter, R.; Willenheimer, R. Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int. J. Cardiol. 2005, 101, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Abdel-Wahab, M.; Robinson, D.; Richardt, G. Predictors of mortality in patients with cardiogenic shock treated with primary percutaneous coronary intervention and intra-aortic balloon counterpulsation. Med. Klin—Intensiv. und Notfallmedizin 2015, 111, 715–722. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Hsieh, M.-J.; Chen, C.-C.; Wu, V.C.-C.; Lan, W.-C.; Huang, Y.-T.; Hsieh, I.-C.; Chang, S.-H. Prognosis of patients with cardiogenic shock following acute myocardial infarction: The difference between ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Medicine 2022, 101, e30426. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; de Waha, A.; Richardt, G.; Hennersdorf, M.; Empen, K.; et al. Intraaortic Balloon Pump in cardiogenic shock II (IABP-SHOCK II) trial investigators. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): Final 12 month results of a randomised, open-label trial. Lancet 2013, 382, 1638–1645. [Google Scholar] [CrossRef]
- Mandawat, A.; Rao, S.V. Percutaneous mechanical circulatory support devices in cardiogenic shock. Circ. Cardiovasc. Interv. 2017, 10, 5. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; White, H.D.; Dzavik, V.; Wong, S.C.; Menon, V.; Webb, J.G.; Steingart, R.; Picard, M.H.; Menegus, M.A.; et al. One-Year Survival Following Early Revascularization for Cardiogenic Shock. JAMA 2001, 285, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Assanelli, E.; Campodonico, J.; De Metrio, M.; Lauri, G.; Marana, I.; Moltrasio, M.; Rubino, M.; Veglia, F.; Montorsi, P.; et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit. Care Med. 2010, 38, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Tarvasmäki, T.; Haapio, M.; Mebazaa, A.; Sionis, A.; Silva-Cardoso, J.; Tolppanen, H.; Lindholm, M.G.; Pulkki, K.; Parissis, J.; Harjola, V.; et al. Acute kidney injury in cardiogenic shock: Definitions, incidence, haemodynamic alterations, and mortality. Eur. J. Heart Fail. 2017, 20, 572–581. [Google Scholar] [CrossRef]
- Rebora, P.; Centola, M.; Morici, N.; Sacco, A.; Occhino, G.; Viola, G.; Oreglia, J.; Castini, D.; Persampieri, S.; Sabatelli, L.; et al. Uric acid associated with acute heart failure presentation in Acute Coronary Syndrome patients. Eur. J. Intern. Med. 2022, 99, 30–37. [Google Scholar] [CrossRef]
- Akpek, M.; Kaya, M.G.; Uyarel, H.; Yarlioglues, M.; Kalay, N.; Gunebakmaz, O.; Dogdu, O.; Ardic, I.; Elcik, D.; Sahin, O.; et al. The association of serum uric acid levels on coronary flow in patients with STEMI undergoing primary PCI. Atherosclerosis 2011, 219, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Mandurino-Mirizzi, A.; Crimi, G.; Raineri, C.; Pica, S.; Ruffinazzi, M.; Gianni, U.; Repetto, A.; Ferlini, M.; Marinoni, B.; Leonardi, S.; et al. Elevated serum uric acid affects myocardial reperfusion and infarct size in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. J. Cardiovasc. Med. 2018, 19, 240–246. [Google Scholar] [CrossRef]
- Fuernau, G.; Poenisch, C.; Eitel, I.; Desch, S.; De Waha, S.; Schuler, G.; Adams, V.; Werdan, K.; Zeymer, U.; Thiele, H. Renal failure in myocardial infarction with cardiogenic shock—Comparison of established and novel biomarkers—A biomarker substudy of the IABP-SHOCK II-trial. Eur. Heart J. 2013, 34, 4409. [Google Scholar] [CrossRef]
- Wong, C.-K.; Gao, W.; Stewart, R.A.; Benatar, J.; French, J.K.; Aylward, P.E.; White, H.D. aVR ST elevation: An important but neglected sign in ST elevation acute myocardial infarction. Eur. Heart J. 2010, 31, 1845–1853. [Google Scholar] [CrossRef]
- Wang, A.; Singh, V.; Duan, Y.; Su, X.; Su, H.; Zhang, M.; Cao, Y. Prognostic implications of ST-segment elevation in lead aVR in patients with acute coronary syndrome: A meta-analysis. Ann. Noninvasive Electrocardiol. 2021, 26, e12811. [Google Scholar] [CrossRef]
- Sousa, P.J.; Teles, R.C.; Brito, J.; Abecasis, J.; Gonçalves, P.d.A.; Calé, R.; Leal, S.; Dourado, R.; Raposo, L.; Silva, A.; et al. Primary PCI in ST-elevation myocardial infarction: Mode of referral and time to PCI. Rev. Port. Cardiol. Engl. Ed. 2012, 31, 641–646. [Google Scholar] [CrossRef]
- Dumitraşcu, S.; Cîrjan, A.; Bartoş, D.; Chioncel, O.; Ştefan, M.; Deleanu, D. Critical Appraisal of Medical System Performance for STEMI Management—A Comprehensive Analysis of Time Efficiency. J. Cardiovasc. Emergencies 2024, 10, 27–37. [Google Scholar] [CrossRef]
- Żurowska-Wolak, M.; Piekos, P.; Jąkała, J.; Mikos, M. The effects of prehospital system delays on the treatment efficacy of STEMI patients. Scand. J. Trauma, Resusc. Emerg. Med. 2019, 27, 39. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ms.ro/ro/transparenta-decizionala/acte-normative-in-transparenta/hot%C4%83r%C3%A2re-de-guvern-pentru-aprobarea-strategiei-na%C8%9Bionale-pentru-combaterea-bolilor-cardiovasculare-%C8%99i-cerebrovasculare-2024-2030/ (accessed on 4 April 2025).
| Variable | Patients with STEMI N = 51 | Patients with STEMI and CS N = 50 | p-Value | |
|---|---|---|---|---|
| Age at presentation, median (IQR) | 65 (51.5; 73) | 68.5 (58; 75.75) | 0.115 | |
| Sex, N (%) | Female | 18 (35.29) | 19 (38) | 0.778 |
| Male | 33 (64.71) | 31 (62) | ||
| Smoker status, N (%) | 17 (33.33) | 18 (42.86) | 0.345 | |
| Arterial hypertension, N (%) | 27 (52.94) | 31 (64.58) | 0.240 | |
| Diabetes mellitus, N (%) | 16 (31.37) | 10 (20.83) | 0.234 | |
| Number of affected coronary vessels, N (%) | 1 | 16 (34.04) | 14 (31.82) | 0.505 |
| 2 | 11 (23.4) | 15 (34.09) | ||
| 3 | 20 (42.55) | 15 (34.09) | ||
| History of ischemic cardiomyopathy, N (%) | 3 (6.38) | 9 (20.45) | 0.047 | |
| Time from symptom debut (h), N (%) | <12 | 42 (84) | 32 (76.19) | 0.347 |
| >12 | 8 (16) | 10 (23.81) | ||
| Transferred from another hospital, N (%) | 29 (56.86) | 26 (53.06) | 0.702 | |
| Thrombolysis, N (%) | 19 (38) | 13 (26.53) | 0.222 | |
| TIMI, N (%) | ≤2 | 9 (20) | 12 (27.27) | 0.419 |
| 3 | 36 (80) | 32 (72.73) | ||
| aVR ↑ST ≥1 mm, N (%) | 10 (19.6) | 10 (20) | 0.960 | |
| MI complications | ||||
| Mechanical complications, N (%) | 1 (1.96) | 0 (0) | 1 | |
| Arrhythmia, N (%) | 3 (5.88) | 24 (48.98) | <0.001 | |
| Heart block, N (%) | 5 (9.8) | 10 (20) | 0.150 | |
| In-hospital death, N (%) | 6 (11.76) | 26 (52) | <0.001 | |
| Paraclinical investigations | ||||
| Haemoglobin (g/dL), median (IQR) | 14 (12.8; 15) | 13.1 (11.6; 14.3) | 0.051 | |
| WBC (/μL), median (IQR) | 10,770 (9400; 13,350) | 14,700 (10,700; 18,675) | 0.001 | |
| CRP (mg/L), median (IQR) | 17 (7.3; 75) | 45.17 (13.77; 143.5) | 0.123 | |
| hs-cTnI (ng/L), median (IQR) | 7000 (431; 37,433.5) | 18,434 (189.5; 32,437.5) | 0.729 | |
| CK (U/L), median (IQR) | 943.5 (398; 2443) | 2033 (1211; 4275) | 0.004 | |
| CK-MB (U/L), median (IQR) | 142 (58; 295) | 320 (192; 739.5) | 0.001 | |
| Glycemia (mg/dL), median (IQR) | 109 (96; 159) | 126 (107; 194) | 0.406 | |
| LDL (mg/dL), median (IQR) | 97.6 (80.5; 139.6) | 80.2 (61; 110) | 0.025 | |
| Creatinine (mg/dL), median (IQR) | 0.91 (0.76; 1.13) | 1.47 (1; 2.07) | <0.001 | |
| Uric acid (mg/dL), median (IQR) | 6.95 (5.5; 7.88) | 8.2 (6.4; 10.3) | 0.001 | |
| LVEF (%), median (IQR) | 45 (40; 50) | 38 (30; 48) | 0.014 | |
| Variable | Survivors N = 24 | In-Hospital Death N = 26 | p-Value | |
|---|---|---|---|---|
| Age, median (IQR) | 72 (61.75; 75.75) | 63 (56.25; 74.75) | 0.127 | |
| Sex, N (%) | Female | 12 (50) | 7 (26.92) | 0.093 |
| Male | 12 (50) | 19 (73.08) | ||
| Smoker status, N (%) | 8 (38.1) | 10 (47.62) | 0.533 | |
| Arterial hypertension, N (%) | 16 (69.57) | 15 (60) | 0.489 | |
| Diabetes mellitus, N (%) | 5 (20.83) | 5 (20.83) | 1 | |
| Obesity, N (%) | 4 (18.18) | 3 (13.04) | 0.699 | |
| Dyslipidemia, N (%) | 12 (66.67) | 9 (50) | 0.310 | |
| CKD, N (%) | 5 (25) | 2 (10.53) | 0.407 | |
| Number of affected coronary vessels, N (%) | 1 | 6 (26.09) | 8 (38.1) | 0.381 |
| 2 | 7 (30.43) | 8 (38.1) | ||
| 3 | 10 (43.48) | 5 (23.81) | ||
| History of ischemic cardiomyopathy, N (%) | 5 (21.74) | 4 (19.05) | 1 | |
| Time from symptom debut (h), N (%) | <12 | 18 (81.82) | 14 (70) | 0.477 |
| >12 | 4 (18.18) | 6 (30) | ||
| Transferred from another hospital, N (%) | no | 9 (37.5) | 14 (56) | 0.195 |
| yes | 15 (62.5) | 11 (44) | ||
| Thrombolysis, N (%) | no | 19 (79.17) | 17 (68) | 0.376 |
| yes | 5 (20.83) | 8 (32) | ||
| TIMI, N (%) | ≤2 | 5 (21.74) | 7 (33.33) | 0.388 |
| 3 | 18 (78.26) | 14 (66.67) | ||
| aVR ↑ST ≥1 mm, N (%) | 5 (20.8) | 5 (19.2) | 0.880 | |
| MI complications | ||||
| Arrhythmia, N (%) | 9 (37.5) | 15 (60) | 0.115 | |
| Heart block, N (%) | 7 (29.17) | 3 (11.54) | 0.119 | |
| Paraclinical investigations | ||||
| Haemoglobin (g/dL), median (IQR) | 12.8 (11.45;13.93) | 14 (11.8; 14.3) | 0.096 | |
| WBC (/μL), median (IQR) | 13,650 (10,450; 15,820) | 17,000 (11,675; 23,420) | 0.240 | |
| CRP (mg/L), median (IQR) | 33.91 (13.24; 121.88) | 47.67 (19.35; 207.25) | 0.286 | |
| hs-cTnI (ng/L), median (IQR) | 18,434 (254.5; 30,125) | 12,689.5 (429.5; 32,437.5) | 0.887 | |
| CK (U/L), median (IQR) | 1814 (691.5; 2056.25) | 5165 (2252; 6360) | <0.001 | |
| CK-MB (U/L), median (IQR) | 274 (157.5; 386) | 887 (237.25; 1024.75) | 0.009 | |
| Glycemia (mg/dL), median (IQR) | 111 (98.5; 167.5) | 159.5 (122.25; 195) | 0.035 | |
| LDL (mg/dL), median (IQR) | 79.1 (54.35; 105.5) | 87 (70; 122) | 0.462 | |
| Creatinine (mg/DL), median (IQR) | 1.22 (0.9; 2.08) | 1.9 (1.27; 2.06) | 0.097 | |
| Uric acid (mg/dL), median (IQR) | 7.95 (6.4; 10.3) | 8.2 (7.2; 9.8) | 0.723 | |
| LVEF (%), median (IQR) | 45 (35; 50) | 30 (22.5; 40) | 0.002 | |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| LVEF (%) | 0.90 (0.84; 0.97) | 0.005 | 0.89 (0.81; 0.98) | 0.018 |
| CK-MB | 1 (1; 1.01) | 0.005 | 1 (1; 1.01) | 0.014 |
| Glycemia | 1.01 (0.99; 1.02) | 0.148 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, C.F.; Coadă, C.A.; Lupu, M.; Ferenț, I.F.; Hodas, R.I.; Pintilie, A.; Ursu, M.-Ş. Factors Associated with Mortality Risk in Patients with Cardiogenic Shock Post-ST-Elevation Myocardial Infarction: Insights from a Regional Centre in Northwest Romania. Medicina 2025, 61, 725. https://doi.org/10.3390/medicina61040725
Pop CF, Coadă CA, Lupu M, Ferenț IF, Hodas RI, Pintilie A, Ursu M-Ş. Factors Associated with Mortality Risk in Patients with Cardiogenic Shock Post-ST-Elevation Myocardial Infarction: Insights from a Regional Centre in Northwest Romania. Medicina. 2025; 61(4):725. https://doi.org/10.3390/medicina61040725
Chicago/Turabian StylePop, Călin Florin, Camelia Alexandra Coadă, Mihai Lupu, Ioan Florin Ferenț, Roxana Ioana Hodas, Andreea Pintilie, and Mădălina-Ştefana Ursu. 2025. "Factors Associated with Mortality Risk in Patients with Cardiogenic Shock Post-ST-Elevation Myocardial Infarction: Insights from a Regional Centre in Northwest Romania" Medicina 61, no. 4: 725. https://doi.org/10.3390/medicina61040725
APA StylePop, C. F., Coadă, C. A., Lupu, M., Ferenț, I. F., Hodas, R. I., Pintilie, A., & Ursu, M.-Ş. (2025). Factors Associated with Mortality Risk in Patients with Cardiogenic Shock Post-ST-Elevation Myocardial Infarction: Insights from a Regional Centre in Northwest Romania. Medicina, 61(4), 725. https://doi.org/10.3390/medicina61040725





