Risk Factors and Outcomes of Hemorrhagic Transformation in Acute Ischemic Stroke Following Thrombolysis: Analysis of a Single-Center Experience and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Hemorrhagic Transformation and Patient Outcomes
3.2. Age Distribution and Comorbidities
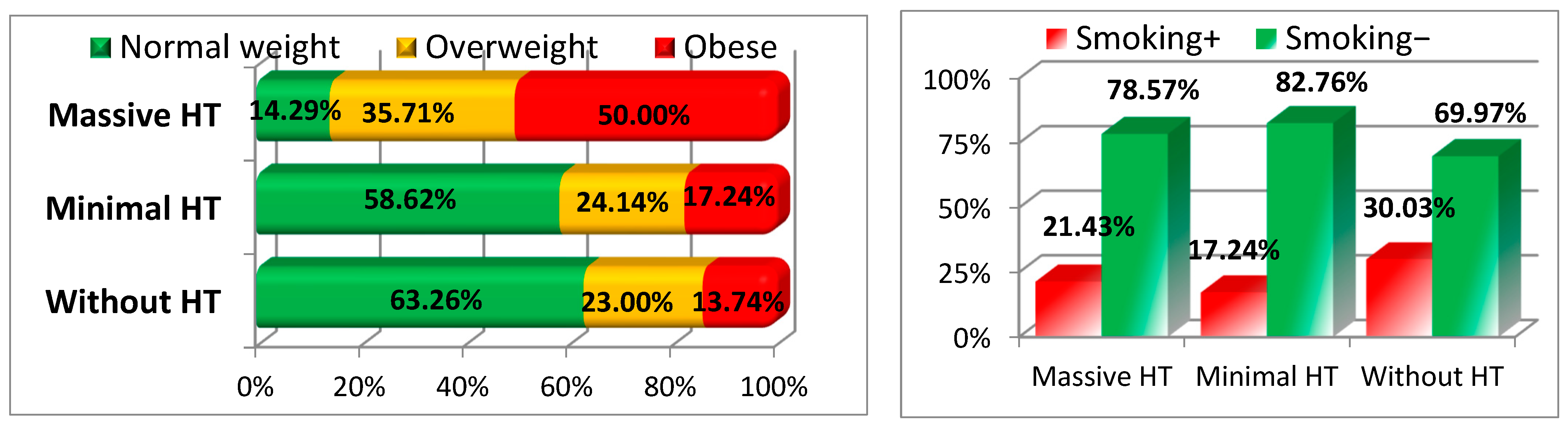
3.3. Nutritional Status and Risk Factors
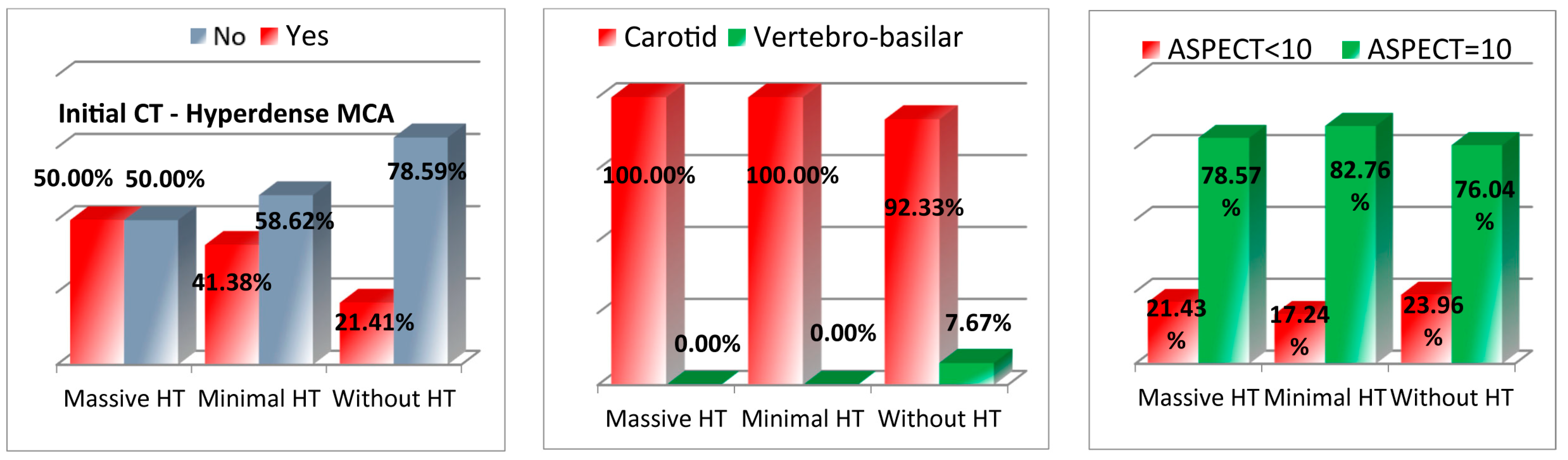
3.4. Imaging Findings
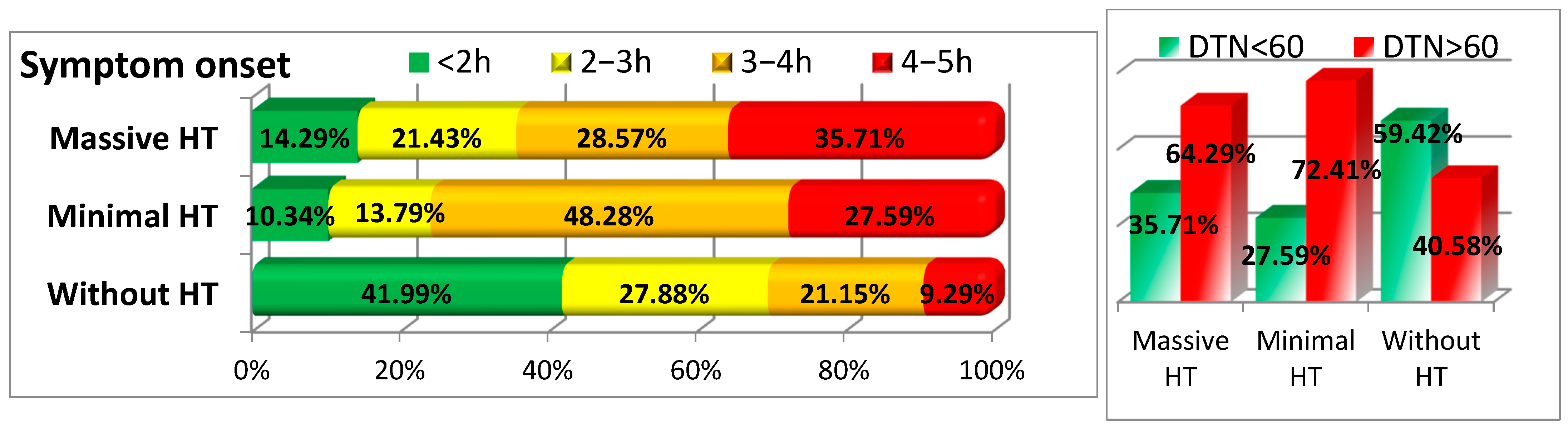
3.5. Timing of Symptom Onset and Treatment
3.6. Laboratory and Clinical Parameters by Type of Hemorrhagic Transformation
3.6.1. QT Interval
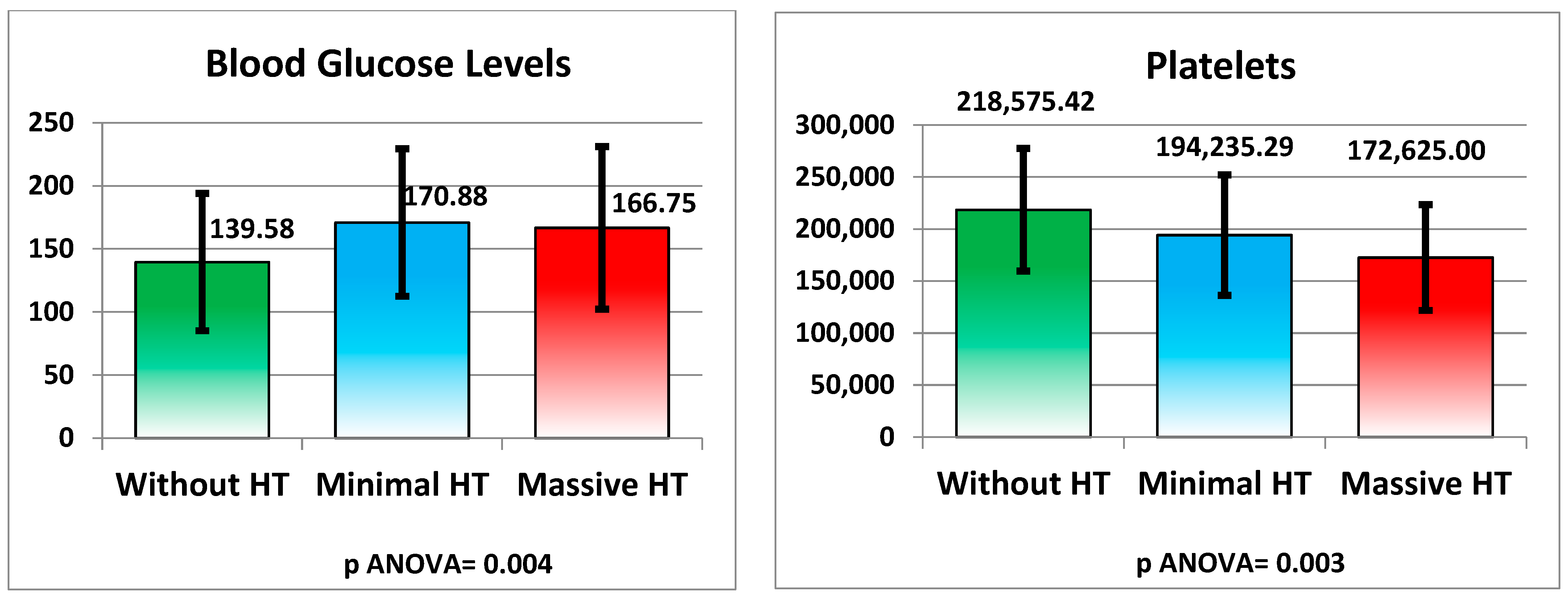
3.6.2. Metabolic and Hematological Changes
3.6.3. Liver Function and Lipid Profile
3.6.4. Kidney Function and Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Widimsky, P.; Snyder, K.; Sulzenko, J.; Hopkins, L.N.; Stetkarova, I. Acute Ischaemic Stroke: Recent Advances in Reperfusion Treatment. Eur. Heart J. 2023, 44, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Walter, K. What is Acute Ischemic Stroke? JAMA 2022, 327, 885. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhang, Y.; Ma, W.; Liu, X.; Cheng, Y.; Zhao, Z.; Hu, W.; Zhang, C.; Wang, J.; Yang, Y. Signaling Pathways Involved in Ischemic Stroke: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Smajlović, D. Strokes in Young Adults: Epidemiology and Prevention. Vasc. Health Risk Manag. 2015, 11, 157–164. [Google Scholar] [CrossRef]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and Clinical Features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Zhang, W.; Guo, L.; Sun, Y.; Zhuang, J.; Xu, T.; Zhang, L.; Zhang, Z.; Li, S. Current Status of Intravenous Tissue Plasminogen Activator Dosage for Acute Ischaemic Stroke: An Updated Systematic Review. Stroke Vasc. Neurol. 2018, 3, 28–33. [Google Scholar] [CrossRef]
- Ho, J.P.; Powers, W.J. Contemporary Management of Acute Ischemic Stroke. Annu. Rev. Med. 2025, 76, 417–429. [Google Scholar] [CrossRef]
- Spronk, E.; Otte, W.M.; de Groot, J.M.; Dippel, D.W.J.; van der Lugt, A.; van der Worp, H.B.; de Haan, G.G.; Vroomen, P.C. Hemorrhagic Transformation in Ischemic Stroke and the Role of Inflammation. Front. Neurol. 2021, 12, 661955. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, D.S.; Kim, M.; Choi, Y.J.; Kim, Y.S.; Park, J.H.; Cho, Y.S.; Lee, K.H.; Lee, Y.H. Hemorrhagic Transformation After Ischemic Stroke: Mechanisms and Management. Front. Neurol. 2021, 12, 703258. [Google Scholar] [CrossRef]
- Charbonnier, G.; Bonnet, L.; Biondi, A.; Moulin, T.; Duhamel, A.; Lemoine, N.; Roussel, M.; Boudot, C.; Ménager, E.; Dubois, C. Intracranial Bleeding After Reperfusion Therapy in Acute Ischemic Stroke. Front. Neurol. 2020, 11, 629920. [Google Scholar] [CrossRef]
- Wong, Y.S.; Lee, K.; Ho, P.; Choi, R.; Huang, Z. The Impact of Loading Dose on Outcome in Stroke Patients Receiving Low-Dose Tissue Plasminogen Activator Thrombolytic Therapy. Drug Des. Devel. Ther. 2020, 14, 257–263. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, J.; Yang, W.; Tan, H.; Lee, W. High Neutrophil-to-Lymphocyte Ratio Predicts Hemorrhagic Transformation in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Int. J. Hypertens. 2020, 2020, 5980261. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Pan, Z.; Kong, L.; Du, G. Neuroinflammation in Hemorrhagic Transformation after Tissue Plasminogen Activator Thrombolysis: Potential Mechanisms, Targets, Therapeutic Drugs and Biomarkers. Int. Immunopharmacol. 2021, 90, 107216. [Google Scholar] [CrossRef] [PubMed]
- Kassner, A.; Merali, Z. Assessment of Blood-Brain Barrier Disruption in Stroke. Stroke 2015, 46, 3310–3315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Wu, Y.; Song, Q.; Li, S. The Risk Factors of Early Hemorrhage after Emergency Intravenous Thrombolysis in Patients with Acute Ischemic Stroke. Ann. Palliat. Med. 2021, 10, 5706–5713. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zeng, J.; Wang, F.; Zhuang, X.; Chen, X.; Miao, J. Risk Factors of Hemorrhagic Transformation after Intravenous Thrombolysis with rt-PA in Acute Cerebral Infarction. QJM 2019, 112, 323–326. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Age-Associated Changes in the Immune System and Blood-Brain Barrier Functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Nguyen, H.M.; McCullough, L.D.; Zuloaga, K.L. Aging Alters the Immunological Response to Ischemic Stroke. Acta Neuropathol. 2018, 136, 89–110. [Google Scholar] [CrossRef]
- Gąsecki, D.; Kwarciany, M.; Kowalczyk, K.; Narkiewicz, K.; Karaszewski, B. Blood Pressure Management in Acute Ischemic Stroke. Curr. Hypertens. Rep. 2020, 23, 3. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, Z.; Fang, Z.; Ma, F.; Lv, M.; Zhang, J. Risk Factors for Thrombolysis-Related Intracranial Hemorrhage: A Systematic Review and Meta-Analysis. Thromb. J. 2023, 21, 27. [Google Scholar] [CrossRef]
- Asberg, S.; Fridriksson, A.; Olsson, T.; Svensson, M.; Eriksson, M. Ischemic Stroke and Secondary Prevention in Clinical Practice: A Cohort Study of 14,529 Patients in the Swedish Stroke Register. Stroke 2010, 41, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Dorňák, T.; Böhm, A.; Tkáčová, N.; Chmelová, M.; Antal, P. Predictors for Intracranial Hemorrhage Following Intravenous Thrombolysis in Posterior Circulation Stroke. Transl. Stroke Res. 2018, 9, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Barba, R.; Cervera, A.; Valle, M.; Alonso, J.; Toledo, E.; Rivas, M. The Obesity Paradox in Stroke: Impact on Mortality and Short-Term Readmission. J. Stroke Cerebrovasc. Dis. 2015, 24, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, C.K.; Jung, S.; Yoon, B.W.; Lee, S.H. Obesity-Stroke Paradox and Initial Neurological Severity. J. Neurol. Neurosurg. Psychiatry 2015, 86, 743–747. [Google Scholar] [CrossRef]
- Kim, C.K.; Ryu, W.S.; Kim, B.J.; Lee, S.H. Paradoxical Effect of Obesity on Hemorrhagic Transformation after Acute Ischemic Stroke. BMC Neurol. 2013, 13, 123. [Google Scholar] [CrossRef]
- Dehlendorff, C.; Andersen, K.K.; Olsen, T.S. Body Mass Index and Death by Stroke: No Obesity Paradox. JAMA Neurol. 2014, 71, 978–984. [Google Scholar] [CrossRef]
- Branscheidt, M.; Nimjee, S.M.; Swaminathan, S.; Kumar, V. No Impact of Body Mass Index on Outcome in Stroke Patients Treated with IV Thrombolysis. PLoS ONE 2016, 11, e0164413. [Google Scholar] [CrossRef]
- Seet, R.C.; Zhang, Y.; Wijdicks, E.F.; Rabinstein, A.A. Thrombolysis Outcomes Among Obese and Overweight Stroke Patients: An Age- and NIHSS-Matched Comparison. J. Stroke Cerebrovasc. Dis. 2014, 23, 1–6. [Google Scholar] [CrossRef]
- Bas, D.F.; Ozdemir, A.O. The Effect of Metabolic Syndrome and Obesity on Outcomes of Acute Ischemic Stroke Patients Treated with Systemic Thrombolysis. J. Neurol. Sci. 2017, 383, 1–4. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, H.J.; Lee, W.; Lee, Y.H.; Kim, S. Prognostic Significance of Prolonged Corrected QT Interval in Acute Ischemic Stroke. Front. Neurol. 2021, 12, 759822. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Lu, Z.; Zhang, F.; Li, Q. QT Interval Prolongation in Patients with Acute Ischemic Stroke: A Report in Northwest China. J. Int. Med. Res. 2019, 47, 5986–5995. [Google Scholar] [CrossRef] [PubMed]
- Balch, M.H.H.; Nimjee, S.M.; Rink, C.; Hannawi, Y. Beyond the Brain: The Systemic Pathophysiological Response to Acute Ischemic Stroke. J. Stroke 2020, 22, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Ackerman, M.J. The Long QT Syndrome: A Transatlantic Clinical Approach to Diagnosis and Therapy. Eur. Heart J. 2013, 34, 3109–3116. [Google Scholar] [CrossRef]
- de Jesus, M.; Maheshwary, A.; Kumar, M.; da Cunha Godoy, L.; Kuo, C.L.; Grover, P. Association of Electrocardiographic and Echocardiographic Variables with Neurological Outcomes after Ischemic Stroke. Am. Heart J. Plus 2023, 34, 100313. [Google Scholar] [CrossRef]
- Hromádka, M.; Beranek, M.; Holeček, J.; Langová, K.; Kubešová, B. Prolonged Corrected QT Interval as a Predictor of Clinical Outcome in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2911–2917. [Google Scholar] [CrossRef]
- Johnston, K.C.; Xie, J.; Luna, J.; Shahar, E.; Adelman, E.E.; Maguire, M.B. Intensive vs Standard Treatment of Hyperglycemia and Functional Outcome in Patients with Acute Ischemic Stroke: The SHINE Randomized Clinical Trial. JAMA 2019, 322, 326–335. [Google Scholar] [CrossRef]
- Desilles, J.P.; Jacquin, J.P.; Heitz, A.; Duhamel, A.; Touboul, P.J.; Giroud, M. Diabetes Mellitus, Admission Glucose, and Outcomes After Stroke Thrombolysis: A Registry and Systematic Review. Stroke 2013, 44, 1915–1923. [Google Scholar] [CrossRef]
- Wei, X.; Xing, Z.; Huang, T.; Zhang, M.; Song, J.; Zhao, Y. Hyperglycemia Disrupted the Integrity of the Blood-Brain Barrier Following Diffuse Axonal Injury through the sEH/NF-κB Pathway. Immun. Inflamm. Dis. 2023, 11, e1105. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Z.; Zhu, Y.; Liu, W.; Li, L. Post-Acute Ischemic Stroke Hyperglycemia Aggravates Destruction of the Blood-Brain Barrier. Neural Regen. Res. 2024, 19, 1344–1350. [Google Scholar] [CrossRef]
- Gensicke, H.; Meyers, M.S.; Kuriakose, A.; Lindner, S. The Intravenous thrombolysis and platelet count. Neurology 2018, 90, e690–e697. [Google Scholar] [CrossRef]
- Prodan, C.I.; Stoner, J.A.; Cowan, L.D.; Dale, G.L. Lower Coated-Platelet Levels Are Associated with Early Hemorrhagic Transformation in Patients with Non-Lacunar Brain Infarction. J. Thromb. Haemost. 2010, 8, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Prodan, C.I.; Stoner, J.A.; Gordon, D.L.; Dale, G.L. Cerebral Microbleeds in Nonlacunar Brain Infarction Are Associated with Lower Coated-Platelet Levels. J. Stroke Cerebrovasc. Dis. 2014, 23, e325–e330. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, Y.; Zhu, Y.; Li, W.; Wang, H.; Zeng, S.; Li, M.; Wu, X. Platelet Volume Indices for the Prognosis of Acute Ischemic Stroke Patients with Intravenous Thrombolysis. Int. J. Neurosci. 2019, 129, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Xian, W.; Liang, J.; Yang, H.; Weng, B. Early Changes in Fibrinogen after Administration of Alteplase Are Associated with the Short-Term Efficacy of Thrombolysis. Medicine 2018, 97, e0241. [Google Scholar] [CrossRef] [PubMed]
- Machlus, K.R.; Cardenas, J.C.; Church, F.C.; Wolberg, A.S. Causal Relationship between Hyperfibrinogenemia, Thrombosis, and Resistance to Thrombolysis in Mice. Blood 2011, 117, 4953–4963. [Google Scholar] [CrossRef]
- Di Napoli, M.; Papa, F.; Bocola, V. Prognostic Influence of Increased C-Reactive Protein and Fibrinogen Levels in Ischemic Stroke. Stroke 2001, 32, 133–138. [Google Scholar] [CrossRef]
- Bang, O.Y.; Saver, J.L.; Lee, K.H.; Kim, S.J.; Ovbiagele, B.; Lee, M.J. Cholesterol Level and Symptomatic Hemorrhagic Transformation after Ischemic Stroke Thrombolysis. Neurology 2007, 68, 737–742. [Google Scholar] [CrossRef]
- Lin, S.F.; Tsai, W.C.; Hsu, P.C.; Chao, T.H.; Lin, Y.C.; Chang, C.C. Low Cholesterol Levels Increase Symptomatic Intracranial Hemorrhage Rates After Intravenous Thrombolysis: A Multicenter Cohort Validation Study. J. Atheroscler. Thromb. 2019, 26, 513–527. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, W.; Ma, X.; Li, Y.; Yang, W.; Liu, Y. Low Level of Low-Density Lipoprotein Cholesterol Is Related with Increased Hemorrhagic Transformation after Acute Ischemic Cerebral Infarction. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 673–678. [Google Scholar]
- Escudero-Martínez, I.; Sánchez-González, J.; Díez-Tejedor, E.; Cuadrado-Godia, E.; Romero, S.; Rodríguez, J.; Muñoz-Pereira, M. Association of Cholesterol Levels with Hemorrhagic Transformation and Cerebral Edema after Reperfusion Therapies. Eur. Stroke J. 2023, 8, 294–300. [Google Scholar] [CrossRef]
- D’Amelio, M.; Terruso, V.; Famoso, G.; Ragonese, P.; Aridon, P.; Savettieri, G. Cholesterol Levels and Risk of Hemorrhagic Transformation after Acute Ischemic Stroke. Cerebrovasc. Dis. 2011, 32, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Nardi, K.; Rost, N.S.; McFadden, E.; Green, M.; Lindholm, P.; Christensen, S.; Madsen, M. Influence of Lipid Profiles on the Risk of Hemorrhagic Transformation after Ischemic Stroke: Systematic Review. Cerebrovasc. Dis. Extra 2011, 1, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Sohal, A.S.; Gangji, A.S.; Crowther, M.A.; Treleaven, D. Uremic Bleeding: Pathophysiology and Clinical Risk Factors. Thromb. Res. 2006, 118, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Molshatzki, N.; Goldstein, L.B.; Dabbah, S.; Koton, S.; Schwartz, D.; Tanne, D. Chronic Kidney Disease in Patients with Acute Intracerebral Hemorrhage: Association with Large Hematoma Volume and Poor Outcome. Cerebrovasc. Dis. 2011, 31, 271–277. [Google Scholar] [CrossRef]
- Lee, J.G.; Heo, J.H.; Lee, M.H.; Kim, Y.S.; Cho, Y.S.; Park, J.K. Low Glomerular Filtration Rate Increases Hemorrhagic Transformation in Acute Ischemic Stroke. Cerebrovasc. Dis. 2013, 35, 53–59. [Google Scholar] [CrossRef]
- Kudo, K.; Kubo, M.; Mori, M.; Ueda, K.; Saito, K.; Shiroto, H.; Watanabe, Y.; Yamagata, Z. Relationship Between Kidney Damage and Stroke Types in Japanese Patients. Clin. Exp. Nephrol. 2012, 16, 564–569. [Google Scholar] [CrossRef]





| Variable | Without HT (n = 313) | Minimal HT (n = 29) | Massive HT (n = 14) | p-Value |
|---|---|---|---|---|
| Hemorrhagic Transformation | 313 (87.92%) | 29 (8.15%) | 14 (3.93%) | - |
| Deceased | 42 (13.42%) | 12 (41.38%) | 10 (71.43%) | <0.001 |
| Alive | 271 (86.58%) | 17 (58.62%) | 4 (28.57%) | |
| Age < 60 | 56 (17.89%) | 0 (0%) | 0 (0%) | <0.001 |
| Age 60–69 | 86 (27.48%) | 14 (48.28%) | 2 (14.29%) | |
| Age 70–79 | 124 (39.62%) | 10 (34.48%) | 5 (35.71%) | |
| Age 80–89 | 47 (15.02%) | 5 (17.24%) | 7 (50%) | |
| Hypertension+ | 168 (53.67%) | 22 (75.86%) | 12 (85.71%) | 0.005 |
| Hypertension− | 145 (46.33%) | 7 (24.14%) | 2 (14.29%) | |
| Diabetes Mellitus+ | 67 (21.41%) | 8 (27.59%) | 8 (57.14%) | 0.007 |
| Diabetes Mellitus− | 246 (78.59%) | 21 (72.41%) | 6 (42.86%) | |
| Atrial Fibrillation+ | 79 (25.2%) | 12 (41.38%) | 9 (64.29%) | 0.001 |
| Atrial Fibrillation− | 234 (74.8%) | 17 (58.62%) | 5 (35.71%) | |
| NHSS increased at 2 h/24 h | 11 (3.51%) | 5 (17.24%) | 3 (21.43%) | <0.001 |
| NHSS decreased at 2 h/24 h | 302 (96.49%) | 24 (82.76%) | 11 (78.57%) | |
| Nutritional Status—Normal | 198 (63.26%) | 17 (58.62%) | 2 (14.29%) | 0.001 |
| Nutritional Status—Overweight | 84 (23.00%) | 7 (24.14%) | 5 (35.71%) | |
| Nutritional Status—Obese | 55 (13.74%) | 5 (17.24%) | 7 (50.00%) | |
| Smoker+ | 94 (30.03%) | 5 (17.24%) | 3 (21.43%) | 0.287 |
| Smoker− | 219 (69.97%) | 24 (82.75%) | 11 (78.57%) | |
| Dense CT MCA+ | 67 (21.4%) | 12 (41.37%) | 7 (50%) | 0.003 |
| Dense CT MCA− | 246 (78.6%) | 17 (58.62%) | 7 (50%) | |
| Carotid | 289 (92.3%) | 29 (100%) | 14 (100%) | 0.17 |
| Vertebro-basilar | 24 (7.7%) | 0 (0%) | 0 (0%) | |
| ASPECT < 10 | 75 (23.9%) | 5 (17.24%) | 3 (21.43%) | 0.70 |
| ASPECT = 10 | 238 (76.1%) | 24 (82.76%) | 11 (78.57%) | |
| Symptom Onset < 2 h | 131 (41.99%) | 3 (10.34%) | 2 (14.29%) | <0.001 |
| Symptom Onset 2–3 h | 87 (27.88%) | 4 (13.79%) | 3 (21.43%) | |
| Symptom Onset 3–4 h | 66 (21.15%) | 14 (48.28%) | 4 (28.57%) | |
| Symptom Onset 4–5 h | 29 (9.29%) | 8 (27.59%) | 5 (35.71%) | |
| DTN < 60 min | 186 (59.42%) | 8 (27.59%) | 5 (35.71%) | <0.001 |
| DTN > 60 min | 127 (40.58%) | 21 (72.41%) | 9 (64.29%) |
| Variable | Without HT (Mean ± SD) | Minimal HT (Mean ± SD) | Massive HT (Mean ± SD) | p-Value |
|---|---|---|---|---|
| QT Interval (ms) | 449.87 ± 47.04 | 467.47 ± 25.85 | 483.75 ± 21.24 | 0.004 |
| Blood Glucose (mg/dL) | 139.58 ± 54.46 | 170.88 ± 58.43 | 166.75 ± 64.43 | 0.004 |
| Platelets (cells/μL) | 218,575.42 ± 58,885.04 | 194,235.29 ± 57,919.70 | 172,625.00 ± 50,793.52 | 0.003 |
| INR (International Normalized Ratio) | 1.12 ± 0.18 | 1.11 ± 0.10 | 1.20 ± 0.10 | 0.186 |
| GOT (AST) (U/L) | 25.65 ± 8.46 | 21.34 ± 8.66 | 32.63 ± 13.45 | <0.001 |
| GPT (ALT) (U/L) | 27.10 ± 7.83 | 29.41 ± 7.95 | 34.50 ± 15.87 | 0.002 |
| Fibrinogen (mg/dL) | 295.45 ± 51.79 | 273.39 ± 47.68 | 260.14 ± 44.53 | 0.005 |
| Total Cholesterol (mg/dL) | 204.04 ± 55.94 | 165.00 ± 29.78 | 208.80 ± 45.74 | 0.001 |
| Triglycerides (mg/dL) | 136.09 ± 64.97 | 85.31 ± 33.39 | 157.40 ± 75.88 | <0.001 |
| LDL Cholesterol (mg/dL) | 133.14 ± 50.13 | 93.80 ± 18.44 | 140.00 ± 38.72 | <0.001 |
| HDL Cholesterol (mg/dL) | 47.00 ± 27.00 | 53.31 ± 21.06 | 38.40 ± 20.23 | 0.210 |
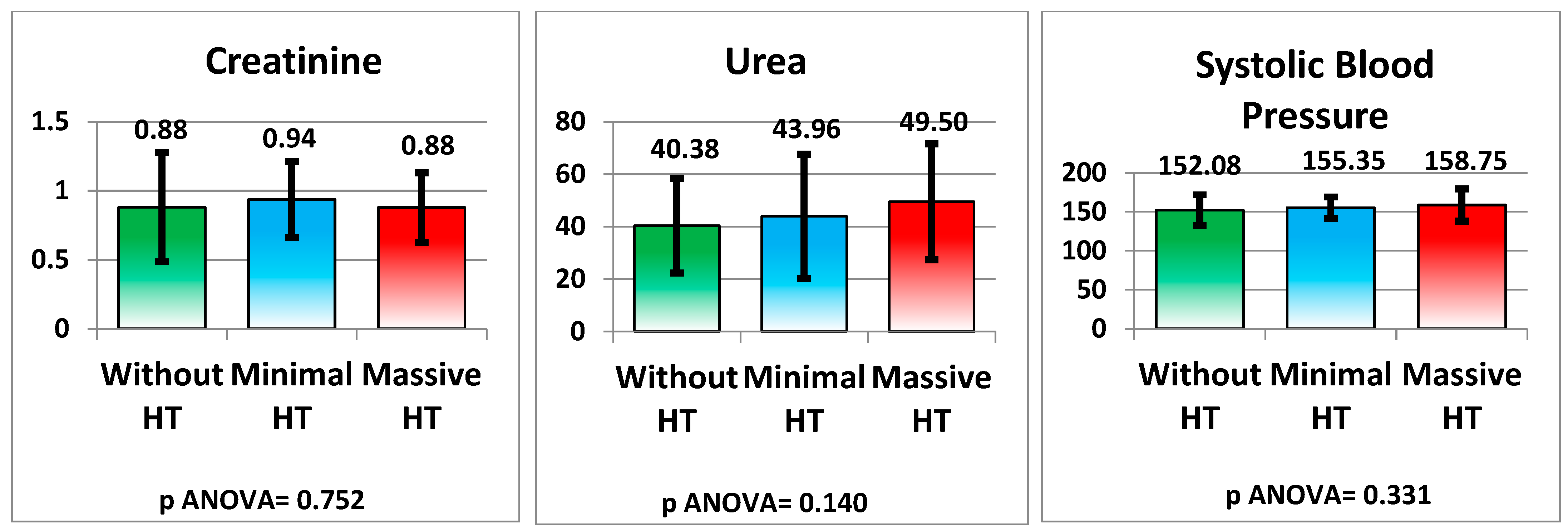
| Creatinine (mg/dL) | 0.88 ± 0.40 | 0.94 ± 0.28 | 0.88 ± 0.25 | 0.752 |
| Urea (mg/dL) | 40.38 ± 18.05 | 43.96 ± 23.69 | 49.50 ± 22.12 | 0.140 |
| Systolic Blood Pressure (mmHg) | 152.08 ± 19.78 | 155.35 ± 13.74 | 158.75 ± 20.72 | 0.331 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neacă, I.; Negroiu, C.E.; Tudorașcu, I.; Dănoiu, R.; Moise, C.G.; Toader, D.M.; Dănoiu, S. Risk Factors and Outcomes of Hemorrhagic Transformation in Acute Ischemic Stroke Following Thrombolysis: Analysis of a Single-Center Experience and Review of the Literature. Medicina 2025, 61, 722. https://doi.org/10.3390/medicina61040722
Neacă I, Negroiu CE, Tudorașcu I, Dănoiu R, Moise CG, Toader DM, Dănoiu S. Risk Factors and Outcomes of Hemorrhagic Transformation in Acute Ischemic Stroke Following Thrombolysis: Analysis of a Single-Center Experience and Review of the Literature. Medicina. 2025; 61(4):722. https://doi.org/10.3390/medicina61040722
Chicago/Turabian StyleNeacă, Ileana, Cristina Elena Negroiu, Iulia Tudorașcu, Raluca Dănoiu, Cristiana Gianina Moise, Despina Manuela Toader, and Suzana Dănoiu. 2025. "Risk Factors and Outcomes of Hemorrhagic Transformation in Acute Ischemic Stroke Following Thrombolysis: Analysis of a Single-Center Experience and Review of the Literature" Medicina 61, no. 4: 722. https://doi.org/10.3390/medicina61040722
APA StyleNeacă, I., Negroiu, C. E., Tudorașcu, I., Dănoiu, R., Moise, C. G., Toader, D. M., & Dănoiu, S. (2025). Risk Factors and Outcomes of Hemorrhagic Transformation in Acute Ischemic Stroke Following Thrombolysis: Analysis of a Single-Center Experience and Review of the Literature. Medicina, 61(4), 722. https://doi.org/10.3390/medicina61040722






