Evolution of Untreated Moderate Mitral Regurgitation After Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Statistical Analysis
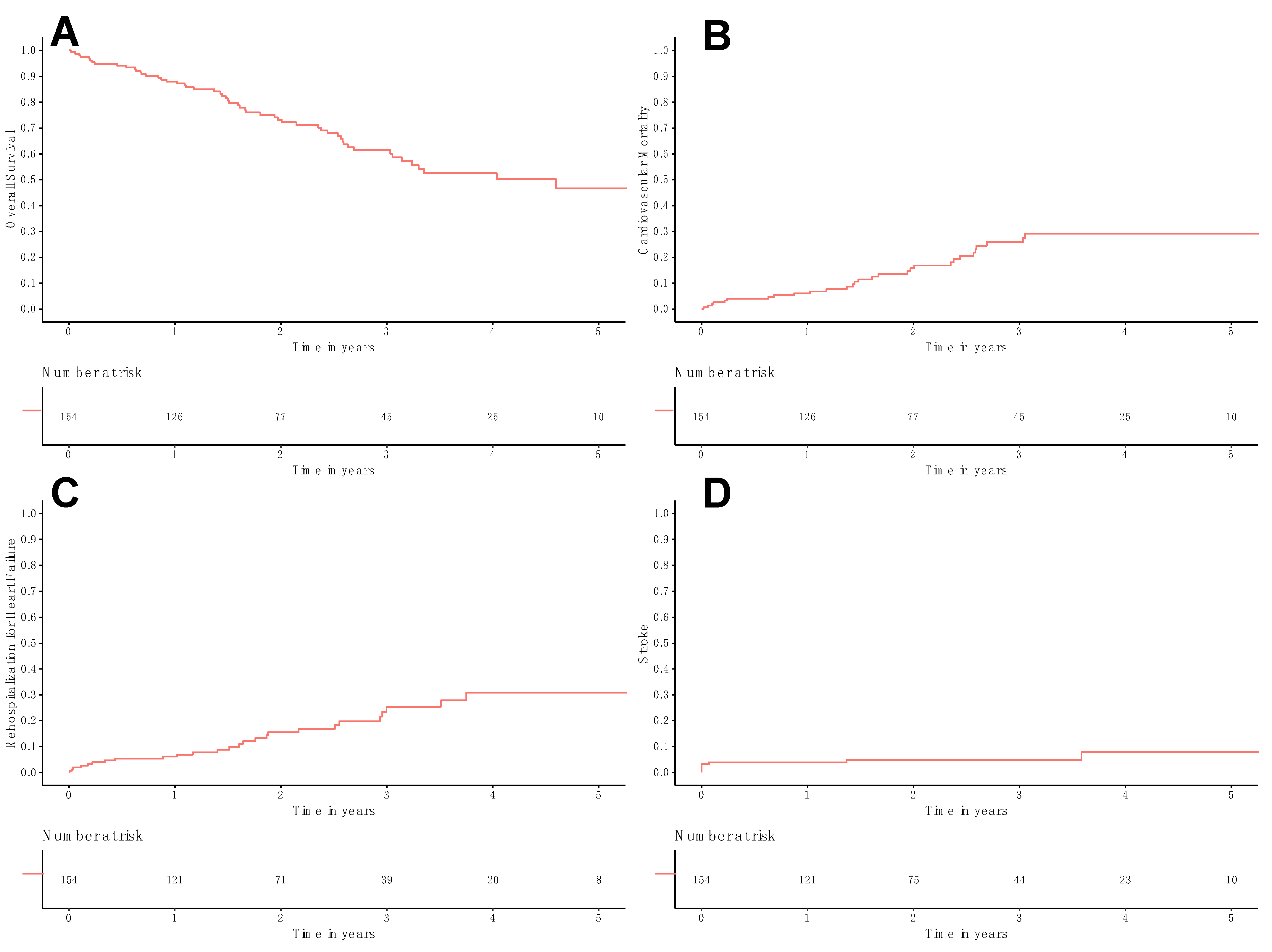
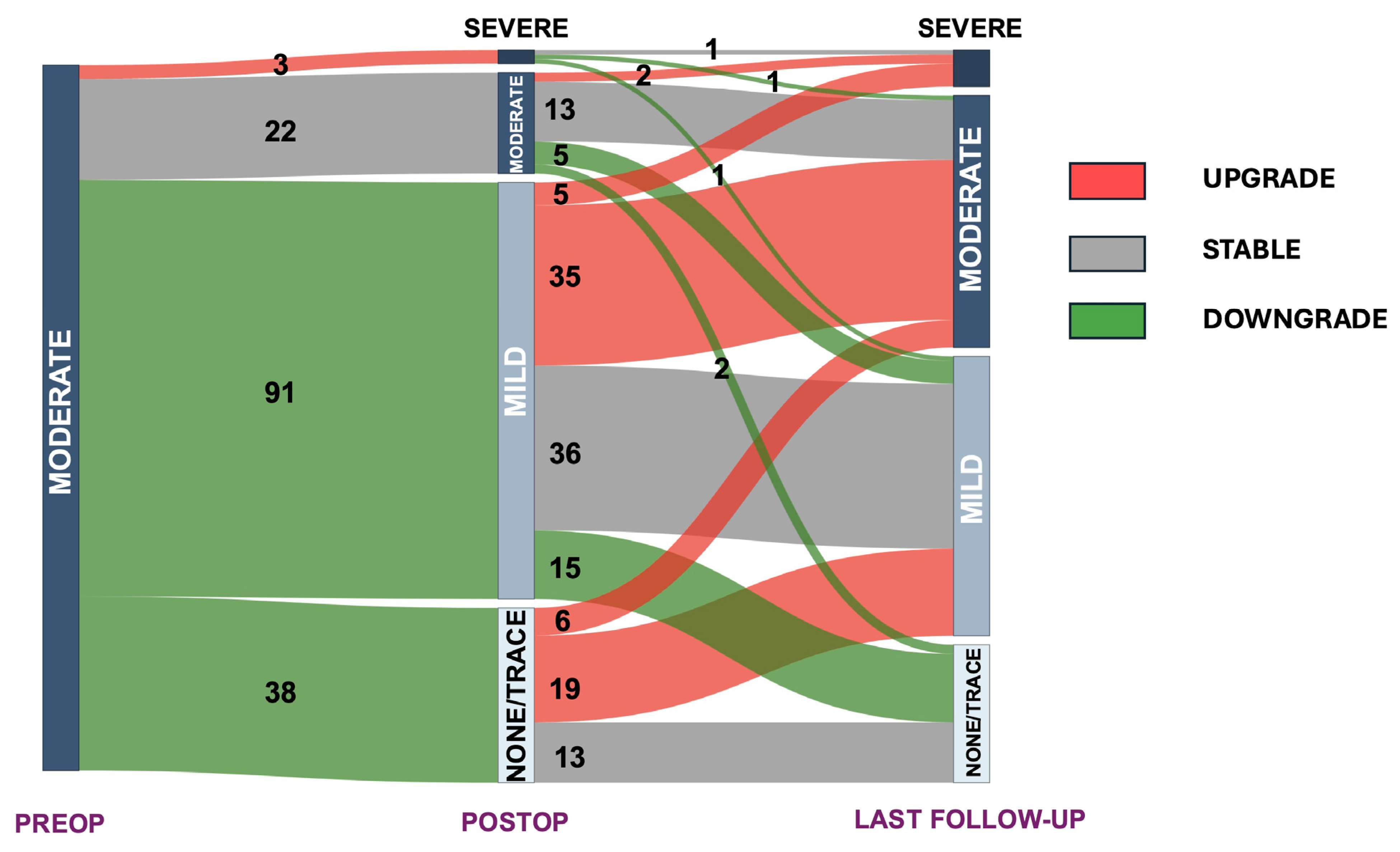
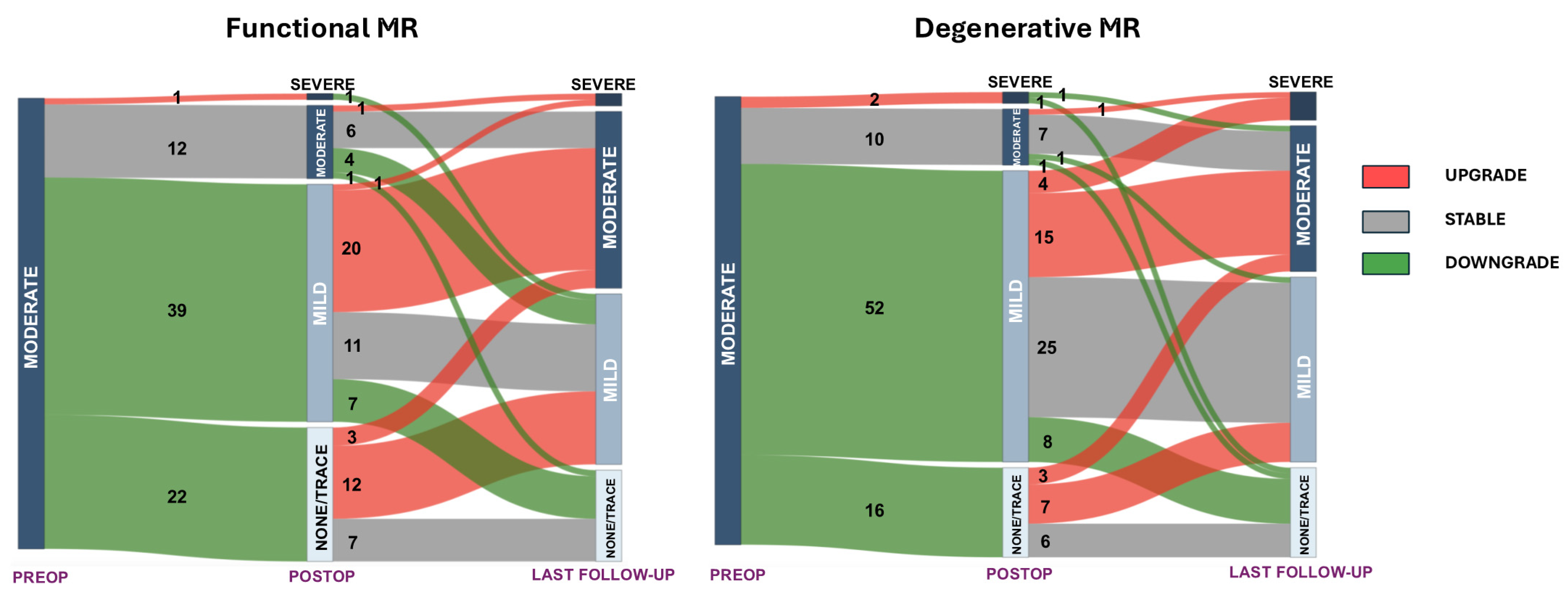
3. Results
4. Discussion
Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| dMR | Degenerative mitral regurgitation |
| fMR | Functional mitral regurgitation |
| MR | Mitral regurgitation |
| TAVI | Transcatheter aortic valve implantation |
References
- Nombela-Franco, L.; Eltchaninoff, H.; Zahn, R.; Testa, L.; Leon, M.B.; Trillo-Nouche, R.; D’Onofrio, A.; Smith, C.R.; Webb, J.; Bleiziffer, S.; et al. Clinical impact and evolution of mitral regurgitation following transcatheter aortic valve replacement: A meta-analysis. Heart Br. Card. Soc. 2015, 101, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; De Bonis, M.; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar] [CrossRef]
- Freitas-Ferraz, A.B.; Lerakis, S.; Barbosa Ribeiro, H.; Gilard, M.; Cavalcante, J.L.; Makkar, R.; Herrmann, H.C.; Windecker, S.; Enriquez-Sarano, M.; Cheema, A.N.; et al. Mitral Regurgitation in Low-Flow, Low-Gradient Aortic Stenosis Patients Undergoing TAVR: Insights From the TOPAS-TAVI Registry. JACC Cardiovasc. Interv. 2020, 13, 567–579. [Google Scholar] [CrossRef]
- Sannino, A.; Losi, M.A.; Schiattarella, G.G.; Gargiulo, G.; Perrino, C.; Stabile, E.; Toscano, E.; Giugliano, G.; Brevetti, L.; Franzone, A.; et al. Meta-analysis of mortality outcomes and mitral regurgitation evolution in 4,839 patients having transcatheter aortic valve implantation for severe aortic stenosis. Am. J. Cardiol. 2014, 114, 875–882. [Google Scholar] [CrossRef]
- Witberg, G.; Codner, P.; Landes, U.; Schwartzenberg, S.; Barbanti, M.; Valvo, R.; De Backer, O.; Ooms, J.F.; Islas, F.; Marroquin, L.; et al. Effect of Transcatheter Aortic Valve Replacement on Concomitant Mitral Regurgitation and Its Impact on Mortality. JACC Cardiovasc. Interv. 2021, 14, 1181–1192. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, C.M.; Li, S.; Thourani, V.H.; Suri, R.M.; Williams, M.L.; Lee, R.; Rankin, J.S. Outcome characteristics of multiple-valve surgery: Comparison with single-valve procedures. Innov. Phila. Pa 2014, 9, 27–32. [Google Scholar] [CrossRef]
- Mauri, V.; Körber, M.I.; Kuhn, E.; Schmidt, T.; Frerker, C.; Wahlers, T.; Rudolph, T.K.; Baldus, S.; Adam, M.; Ten Freyhaus, H. Prognosis of persistent mitral regurgitation in patients undergoing transcatheter aortic valve replacement. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2020, 109, 1261–1270. [Google Scholar] [CrossRef]
- Mavromatis, K.; Thourani, V.H.; Stebbins, A.; Vemulapalli, S.; Devireddy, C.; Guyton, R.A.; Matsouaka, R.; Ghasemzadeh, N.; Block, P.C.; Leshnower, B.G.; et al. Transcatheter Aortic Valve Replacement in Patients With Aortic Stenosis and Mitral Regurgitation. Ann. Thorac. Surg. 2017, 104, 1977–1985. [Google Scholar] [CrossRef]
- Toggweiler, S.; Boone, R.H.; Rodés-Cabau, J.; Humphries, K.H.; Lee, M.; Nombela-Franco, L.; Bagur, R.; Willson, A.B.; Binder, R.K.; Gurvitch, R.; et al. Transcatheter aortic valve replacement: Outcomes of patients with moderate or severe mitral regurgitation. J. Am. Coll. Cardiol. 2012, 59, 2068–2074. [Google Scholar] [CrossRef]
- Chakravarty, T.; Van Belle, E.; Jilaihawi, H.; Noheria, A.; Testa, L.; Bedogni, F.; Rück, A.; Barbanti, M.; Toggweiler, S.; Thomas, M.; et al. Meta-analysis of the impact of mitral regurgitation on outcomes after transcatheter aortic valve implantation. Am. J. Cardiol. 2015, 115, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Webb, J.G.; Hahn, R.T.; Feldman, T.; Boone, R.H.; Smith, C.R.; Kodali, S.; Zajarias, A.; Thompson, C.R.; Green, P.; et al. Impact of preoperative moderate/severe mitral regurgitation on 2-year outcome after transcatheter and surgical aortic valve replacement: Insight from the Placement of Aortic Transcatheter Valve (PARTNER) Trial Cohort A. Circulation 2013, 128, 2776–2784. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Gasparetto, V.; Napodano, M.; Bianco, R.; Tarantini, G.; Renier, V.; Isabella, G.; Gerosa, G. Impact of preoperative mitral valve regurgitation on outcomes after transcatheter aortic valve implantation. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2012, 41, 1271–1276, discussion 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Cortés, C.; Amat-Santos, I.J.; Nombela-Franco, L.; Muñoz-Garcia, A.J.; Gutiérrez-Ibanes, E.; De La Torre Hernandez, J.M.; Córdoba-Soriano, J.G.; Jimenez-Quevedo, P.; Hernández-García, J.M.; Gonzalez-Mansilla, A.; et al. Mitral Regurgitation After Transcatheter Aortic Valve Replacement: Prognosis, Imaging Predictors, and Potential Management. JACC Cardiovasc. Interv. 2016, 9, 1603–1614. [Google Scholar] [CrossRef]
- Doldi, P.M.; Steffen, J.; Stolz, L.; Fischer, J.; Stocker, T.J.; Orban, M.; Theiss, H.; Rizas, K.; Sadoni, S.; Hagl, C.; et al. Impact of mitral regurgitation aetiology on the outcomes of transcatheter aortic valve implantation. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2023, 19, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Stankowski, T.; Aboul-Hassan, S.S.; Salem, M.; Rochor, K.; Schenk, S.; Erkenov, T.; Zinab, F.S.; Muehle, A.; Herwig, V.; Harnath, A.; et al. Prognostic Impact of Residual Moderate Mitral Regurgitation Following Valve-in-Valve Transcatheter Aortic Valve Implantation. Braz. J. Cardiovasc. Surg. 2023, 39, e20230012. [Google Scholar] [CrossRef]
- Twing, A.H.; Gokhale, S.; Slostad, B.; Meyer, J.; Simon, E.; Dickens, H.; Kaluzna, S.D.; Groo, V.; Kansal, M.; Shroff, A.; et al. Impact of Transcatheter Aortic Valve Implantation Among Patients With Co-existing Mild to Moderate Mitral Regurgitation. Am. J. Cardiol. 2022, 177, 84–89. [Google Scholar] [CrossRef]
- Sethi, A.; Kodumuri, V.; Prasad, V.; Chaudhary, A.; Coromilas, J.; Kassotis, J. Does the Presence of Significant Mitral Regurgitation prior to Transcatheter Aortic Valve Implantation for Aortic Stenosis Impact Mortality?—Meta-Analysis and Systematic Review. Cardiology 2020, 145, 428–438. [Google Scholar] [CrossRef]
- Nombela-Franco, L.; Ribeiro, H.B.; Urena, M.; Allende, R.; Amat-Santos, I.; DeLarochellière, R.; Dumont, E.; Doyle, D.; DeLarochellière, H.; Laflamme, J.; et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: A comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J. Am. Coll. Cardiol. 2014, 63, 2643–2658. [Google Scholar] [CrossRef]
- Durst, R.; Avelar, E.; McCarty, D.; Poh, K.-K.; Friera, L.F.; Llano, M.F.; Chu, J.; Anumandla, A.K.R.; Rodriguez, L.L.; Mack, M.J.; et al. Outcome and improvement predictors of mitral regurgitation after transcatheter aortic valve implantation. J. Heart Valve Dis. 2011, 20, 272–281. [Google Scholar]
- Khan, F.; Okuno, T.; Malebranche, D.; Lanz, J.; Praz, F.; Stortecky, S.; Windecker, S.; Pilgrim, T. Transcatheter Aortic Valve Replacement in Patients With Multivalvular Heart Disease. JACC Cardiovasc. Interv. 2020, 13, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Kiramijyan, S.; Koifman, E.; Asch, F.M.; Magalhaes, M.A.; Didier, R.; Escarcega, R.O.; Negi, S.I.; Baker, N.C.; Jerusalem, Z.D.; Gai, J.; et al. Impact of Functional Versus Organic Baseline Mitral Regurgitation on Short- and Long-Term Outcomes After Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2016, 117, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Bianco, V.; Kliner, D.; Toma, C.; Serna-Gallegos, D.; West, D.; Makani, A.; Zhu, J.; Thoma, F.W.; Brown, J.A.; et al. Outcomes of Transcatheter Aortic Valve Replacement in Patients With Concomitant Aortic Regurgitation. Ann. Thorac. Surg. 2023, 116, 728–734. [Google Scholar] [CrossRef]
- Bedogni, F.; Latib, A.; De Marco, F.; Agnifili, M.; Oreglia, J.; Pizzocri, S.; Latini, R.A.; Lanotte, S.; Petronio, A.S.; De Carlo, M.; et al. Interplay between mitral regurgitation and transcatheter aortic valve replacement with the CoreValve Revalving System: A multicenter registry. Circulation 2013, 128, 2145–2153. [Google Scholar] [CrossRef]
- Sultan, I.; Fukui, M.; Bianco, V.; Brown, J.A.; Kliner, D.E.; Hickey, G.; Thoma, F.W.; Lee, J.S.; Schindler, J.T.; Kilic, A.; et al. Impact of Combined Pre and Postcapillary Pulmonary Hypertension on Survival after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 131, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Arnold, S.V.; Herrmann, H.C.; Holmes, D.R.; Szeto, W.Y.; Allen, K.B.; Chhatriwalla, A.K.; Vemulapali, S.; O’Brien, S.; Dai, D.; et al. Impact of Ejection Fraction and Aortic Valve Gradient on Outcomes of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2349–2358. [Google Scholar] [CrossRef]
- Scisło, P.; Grodecki, K.; Rymuza, B.; Zbroński, K.; Kochman, J.; Wilimski, R.; Huczek, Z. Impact of transcatheter aortic valve implantation on coexistent mitral regurgitation parameters. Kardiol. Pol. 2021, 79, 179–184. [Google Scholar] [CrossRef]
- Tzikas, A.; Piazza, N.; van Dalen, B.M.; Schultz, C.; Geleijnse, M.L.; van Geuns, R.-J.; Galema, T.W.; Nuis, R.-J.; Otten, A.; Gutierrez-Chico, J.-L.; et al. Changes in mitral regurgitation after transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2010, 75, 43–49. [Google Scholar] [CrossRef]
- Onishi, H.; Naganuma, T.; Izumo, M.; Ouchi, T.; Yuki, H.; Mitomo, S.; Nakamura, S. Prognostic relevance of B-type natriuretic peptide in patients with moderate mixed aortic valve disease. ESC Heart Fail. 2022, 9, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n = 154 |
|---|---|
| Female sex, n (%) | 74 (48.1) |
| Age, years, mean (SD) | 81.4 (7.8) |
| BSA, m2, mean (SD) | 1.84 (0.23) |
| BMI, kg/m2, median [IQR] | 25.31 [22.54, 29.26] |
| NYHA functional class, n (%) | |
| Class I | 5 (3.2) |
| Class II | 54 (35.1) |
| Class III | 73 (47.4) |
| Class IV | 22 (14.3) |
| BNP, pg/mL, median [IQR] | 465 [231, 998.5] |
| Urgent, n (%) | 22 (14.3) |
| STS PROM, median [IQR] | 3.60 [2.64, 5.90] |
| Hypertension, n (%) | 138 (89.6) |
| Dyslipidemia, n (%) | 137 (89.0) |
| Diabetes mellitus, n (%) | 54 (35.1) |
| Liver disease, n (%) | 5 (3.2) |
| eGFR, mL/min/1.73 m2, mean (SD) | 56.79 (21.99) |
| Dialysis, n (%) | 5 (3.2) |
| Smoke status, n (%) | |
| Active | 8 (5.2) |
| Former | 32 (20.8) |
| Never | 114 (74.0) |
| Chronic lung disease, n (%) | 22 (14.3) |
| Prior CVA, n (%) | 17 (11.0) |
| PAD, n (%) | 40 (26.0) |
| Prior MI, n (%) | 31 (20.1) |
| Prior CABG, n (%) | 28 (18.2) |
| Prior PCI, n (%) | 57 (37.0) |
| Atrial fibrillation, n (%) | |
| No | 84 (54.5) |
| Paroxysmal | 43 (27.9) |
| Persistent | 26 (16.9) |
| Long-standing Persistent | 1 (0.6) |
| Prior pacemaker, n (%) | 21 (13.6) |
| Prior ICD, n (%) | 6 (3.9) |
| MDCT | |
| SoV diameter, mm, mean (SD) | 33.1 (4.0) |
| STJ diameter, mm, mean (SD) | 27.1 (4.0) |
| Annulus diameter, mm, mean (SD) | 25.1 (3.1) |
| Area, mm2, mean (SD) | 502 (107) |
| Perimeter, mm, mean (SD) | 81.0 (8.8) |
| Echocardiography | |
| Ejection fraction, %, median [IQR] | 58.0 [40.8, 65.0] |
| AVA, cm, mean (SD) | 0.71 (0.18) |
| Indexed AVA, cm/m2, mean (SD) | 0.39 (0.11) |
| Mean gradient, mmHg, mean (SD) | 41.4 (13.2) |
| Peak velocity, m/s, mean (SD) | 4.10 (0.66) |
| Outcome | n = 154 |
|---|---|
| Transfemoral approach, n (%) | 141 (91.6) |
| Pre dilatation, n (%) | 49 (31.8) |
| Post dilatation, n (%) | 10 (6.5) |
| Device, n (%) | |
| Evolut FX | 10 (6.5) |
| Evolut PRO | 38 (24.7) |
| Evolut PRO Plus | 25 (16.2) |
| AcurateNeo 2 | 1 (0.6) |
| SAPIEN 3 | 55 (35.7) |
| SAPIEN 3 Ultra | 25 (16.2) |
| Device size, n (%) | |
| 23 | 18 (11.7) |
| 26 | 61 (39.6) |
| 29 | 63 (40.9) |
| 34 | 11 (7.1) |
| XL | 1 (0.6) |
| Surgical conversion, n (%) | 1 (0.6) |
| Intraoperative transfusion, n (%) | 8 (5.2) |
| Valve malposition, n (%) | 0 (0.0) |
| Second valve, n (%) | 0 (0.0) |
| Annulus rupture, n (%) | 1 (0.6) |
| Cardiac tamponade, n (%) | 1 (0.6) |
| Coronary obstruction, n (%) | 0 (0.0) |
| Overt bleeding, n (%) | 4 (2.6) |
| Major vascular complication, n (%) | 3 (1.9) |
| Minor vascular complication, n (%) | 7 (4.5) |
| Stroke, n (%) | 5 (3.2) |
| TIA, n (%) | 1 (0.6) |
| Myocardial infarction, n (%) | 1 (0.6) |
| AKI >stage 2, n (%) | 4 (2.6) |
| New dialysis, n (%) | 1 (0.6) |
| New atrial fibrillation, n (%) | 7 (4.5) |
| New pacemaker, n (%) | 21 (13.6) |
| New LBBB, n (%) | 29 (18.8) |
| Postop transfusion, n (%) | 15 (9.7) |
| Length of stay, days, median [IQR] | 1.0 [1.0, 3.0] |
| 30-day mortality, n (%) | 3 (1.9) |
| Echocardiography | |
| Ejection fraction, %, mean (SD) | 57.35 (13.93) |
| EOA, cm2, median [IQR] | 2.13 (0.52) |
| Indexed EOA, cm2, median [IQR] | 1.18 (0.33) |
| Mean gradient, mmHg, mean (SD) | 9.22 (4.17) |
| Peak velocity, m/s, mean (SD) | 1.98 (0.46) |
| Mitral regurgitation, n (%) | |
| None/Trace | 38 (24.7) |
| Mild | 91 (59.1) |
| Moderate | 22 (14.3) |
| Severe | 3 (1.9) |
| Follow-Up Echocardiography * | n = 154 |
|---|---|
| Ejection fraction, %, mean (SD) | 57.5 (12.5) |
| Mitral regurgitation, n (%) | |
| None/Trace | 31 (20.1) |
| Mild | 61 (39.6) |
| Moderate | 55 (35.7) |
| Severe | 7 (4.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudo, M.; Sicouri, S.; Cabrucci, F.; Yamashita, Y.; Magouliotis, D.E.; Carnila, S.M.; Abramson, S.V.; Hawthorne, K.M.; Jarrett, H.; Rodriguez, R.; et al. Evolution of Untreated Moderate Mitral Regurgitation After Transcatheter Aortic Valve Implantation. Medicina 2025, 61, 686. https://doi.org/10.3390/medicina61040686
Baudo M, Sicouri S, Cabrucci F, Yamashita Y, Magouliotis DE, Carnila SM, Abramson SV, Hawthorne KM, Jarrett H, Rodriguez R, et al. Evolution of Untreated Moderate Mitral Regurgitation After Transcatheter Aortic Valve Implantation. Medicina. 2025; 61(4):686. https://doi.org/10.3390/medicina61040686
Chicago/Turabian StyleBaudo, Massimo, Serge Sicouri, Francesco Cabrucci, Yoshiyuki Yamashita, Dimitrios E. Magouliotis, Sarah M. Carnila, Sandra V. Abramson, Katie M. Hawthorne, Harish Jarrett, Roberto Rodriguez, and et al. 2025. "Evolution of Untreated Moderate Mitral Regurgitation After Transcatheter Aortic Valve Implantation" Medicina 61, no. 4: 686. https://doi.org/10.3390/medicina61040686
APA StyleBaudo, M., Sicouri, S., Cabrucci, F., Yamashita, Y., Magouliotis, D. E., Carnila, S. M., Abramson, S. V., Hawthorne, K. M., Jarrett, H., Rodriguez, R., Goldman, S. M., Coady, P. M., Gnall, E. M., Gray, W. A., Gelsomino, S., & Ramlawi, B. (2025). Evolution of Untreated Moderate Mitral Regurgitation After Transcatheter Aortic Valve Implantation. Medicina, 61(4), 686. https://doi.org/10.3390/medicina61040686










