Abstract
Background and Objectives: Mitral valve transcatheter edge-to-edge repair (TEER) is a widely adopted therapeutic approach for managing significant mitral regurgitation (MR) in high-risk surgical candidates. While procedural safety and efficacy have been demonstrated, the impact of institutional expertise on outcomes remains unclear. We aimed at evaluating whether the institutional monthly volume of TEER influences short- and long-term clinical results. Materials and Methods: This analysis from the multicenter, prospective GIOTTO trial study evaluated the impact of institutional monthly volume on outcomes of TEER to remedy significant mitral regurgitation. Centers were stratified into tertiles based on monthly volumes (≤2.0 cases/month, 2.1–3.5 cases/month, >3.5 cases/month), and key clinical, echocardiographic, and procedural outcomes were analyzed. Statistical analysis was based on standard bivariate tests as well as unadjusted and multivariable adjusted Cox models. Results: A total of 2213 patients were included, stratified into tertiles based on institutional procedural volume: 645 (29.1%) patients in the first tertile, 947 (42.8%) patients in the second tertile, and 621 (28.1%) patients in the third tertile. Several baseline differences were found, with some features disfavoring less busy centers (e.g., functional class and surgical risk, both p < 0.05), and others suggesting a worse risk profile in those treated in busier institutions (e.g., frailty and history of prior mitral valve intervention, both p < 0.05). Procedural success rates were higher in busier centers (p < 0.001), and hospital stay was also shorter there (p < 0.001). Long-term follow-up (median 14 months) suggested worse outcomes in patients treated in less busy centers at unadjusted analysis (e.g., p = 0.018 for death, p = 0.015 for cardiac death, p = 0.014 for death or hospitalization for heart failure, p < 0.001 for cardiac death or hospitalization for heart failure), even if these associations proved no longer significant after multivariable adjustment, except for cardiac death or hospitalization for heart failure, which appeared significantly less common in the busiest centers (p < 0.05). Similar trends were observed when focusing on tertiles of overall center volume and when comparing for each center the first 50 cases with the following ones. Conclusions: High institutional monthly volume of TEER mitral valve repair appears to correlate with an improved procedural success rate and shorter hospitalizations. Similarly favorable results were found for long-term rates of cardiac death or hospitalization for heart failure. These findings inform on the importance of operator experience and center expertise in achieving state-of-the-art results with TEER, while confirming the usefulness of the proctoring approach when naïve centers begin a TEER program.
1. Introduction
Transcatheter edge-to-edge repair (TEER) has emerged as an effective alternative to surgical valve repair in high-risk patients, and to conservative medical therapy only in carefully selected individuals considered unfit for surgery [1,2]. Indeed, given its ability to improve functional outcomes and reduce heart failure hospitalizations, TEER is increasingly performed worldwide [3]. However, the early and long-term effectiveness of TEER is impacted by several factors, ranging from patient to anatomic and procedural ones [4,5,6,7,8,9,10,11].
On top of patient, procedural, and operator features, institutional characteristics have been the focus of attentive recommendations and analysis [12]. Indeed, in most settings, TEER is only provided as long as surgical mitral valve repair/replacement is also available. Yet, this minimum requirement might be too lenient because expertise in patient screening and selection, procedural efficiency, and seamless quality of care are paramount to achieve optimal outcomes with TEER [13]. In particular, a number of cutoffs have been proposed and tested formally in the past, under the key premise that higher-volume centers may provide better results, shortly after the procedure as well as subsequently. However, results have been inconsistent so far, with some studies suggesting that no evident cutoff can be envisioned, and others ending up recommending a minimum yearly volume ranging from 8 to 24 cases [14,15,16].
We hypothesized that the institutional monthly volume of TEER could influence short- and long-term clinical results of this technically demanding procedure, in the sense that institutions exhibiting higher monthly volumes could provide better outcomes in comparison to centers with a lower monthly caseload. We thus aimed at appraising the impact of the institutional monthly volume of TEER on early and long-term outcomes, and in order to test this hypothesis, we chose to leverage the extensive and detailed dataset of the ongoing prospective GIOTTO (GIse registry Of Transcatheter treatment of mitral valve regurgitaTiOn) registry, an Italian multicenter observational study including patients undergoing TEER with a MitraClip (Abbott Vascular, Santa Clara, CA, USA) [5]. Indeed, key strengths of GIOTTO include its contemporary stance and follow-up that goes well beyond discharge.
2. Methods
This study was based on the dataset accrued in the GIOTTO trial, which is sponsored by the Italian Society of Invasive Cardiology (GISE—Società Italiana di Cardiologia Interventistica, Milan, Italy) and is registered online at ClinicalTrials.gov (NCT03521921) [5]. Notably, ethical approval was obtained from all participating institutions, and all patients provided written informed consent.
For the purpose of this analysis, which we labelled GIOTTO-VAT (volume and time), we mainly focused on comparing tertiles of cases per month, center-wise, with the first tertile up to 2.0 cases per month, the second tertile with more than 2.0 and up to 3.5 cases per month, and the third tertile with more than 3.5 cases per month. Exploratory analyses were conducted according to tertiles of total volume, center-wise, with the first tertile up to 100 cases, the second tertile with more than 100 and up to 200 cases, and the third tertile with more than 200 cases, as well as distinguishing between the first 50 cases per center and the subsequent ones.
Details on baseline variables were collected, including demographic data, comorbidities, functional class, prior cardiac procedures, and medication history. Echocardiographic parameters being assessed included left atrial diameter, left ventricular dimensions, mitral valve gradient, and severity of tricuspid regurgitation. Procedural variables of interest included number and generation of MitraClip devices implanted, fluoroscopy time, device time, and procedural success. Fatal and non-fatal outcomes occurring during the index hospitalization and during follow-up were systematically collected, with specific attention to the following events: death, cardiac death, the composite of death or hospitalization, and the composite of death or hospitalization for heart failure.
Descriptive statistics were computed for all variables, with medians and first and third quartiles provided for continuous variables, and counts and percentages for categorical variables. Bivariate analysis was based on Kruskal–Wallis tests for continuous variables and Fisher exact tests for categorical variables. Censored outcomes were analyzed with Cox proportional hazard models, unadjusted as well as adjusted for potential confounders. Notably, the following potential confounders were forced into the adjusted models: age, gender, smoking history, dyslipidemia, degenerative etiology, baseline functional class, prior mitral valve repair, prior stroke, frailty, peripheral artery disease, surgical risk scores, left atrial diameters, left ventricular diameters, left ventricular volumes, tenting area, severe calcification, prolapse, severity of tricuspid regurgitation, concomitant ECG abnormalities, atrial fibrillation, and significant coronary artery disease. No missing data imputation was performed. Statistical significance was set at a 2-tailed p-value of 0.05, without multiplicity adjustments, and all analyses were conducted using Stata 18 (StataCorp, College Station, TX, USA).
3. Results
A total of 2213 patients were included, with 645 (29.1%) individuals treated in centers performing ≤ 2.0 cases/month, 947 (42.8%) treated in institutions reporting between 2.1 and 3.5 cases/month, and 621 (28.1%) patients treated in hospitals with >3.5 cases/month (Table 1). Several differences according to such stratifications were found in key baseline features, with some suggesting a higher complexity in patients treated in lower-volume centers, such as age, functional class, and surgical risk score (all p < 0.05), and others suggesting a higher risk in those treated in busier centers, such as smoking, dyslipidemia, prior mitral valve intervention, prior stroke, peripheral artery disease, and frailty score (all p < 0.05).

Table 1.
Baseline clinical features according to tertiles of monthly volume, center-wise *.
Other significant differences were found for left ventricular dimensions and function, and mitral valve tenting area (Table 2), disfavoring less busy centers (all p < 0.05), and for left atrial dimensions, mitral valve calcification, mitral valve prolapse, tricuspid regurgitation severity, concomitant ECG abnormalities, and prevalence of atrial fibrillation, disfavoring higher-volume institutions (all p < 0.05).

Table 2.
Baseline imaging and ECG features according to tertiles of monthly volume, center-wise *.
In terms of procedural details, significant differences were found in rates of implantation of multiple MitraClips, type of MitraClip used, device time, and fluoroscopy time (all p < 0.05; Table 3). Notably, device success rates were similar across tertiles, but procedural success was marginally albeit significantly higher in busier centers (p < 0.001), with concomitantly lower rates of severe residual mitral regurgitation (p = 0.001). Patients in high-volume centers experienced fewer in-hospital bleeding events (p = 0.006) but more vascular complications (p = 0.008), without significant differences in in-hospital mortality (p = 0.123). Length of hospital stay was significantly shorter in higher-volume centers (p < 0.001).

Table 3.
Procedural and in-hospital outcomes according to tertiles of monthly volume, center-wise *.
During a median follow-up of 14 months, details on a total of 539 (24.4%) deaths, 286 (12.9%) cardiac deaths, 685 (31.0%) deaths or hospitalizations, and 359 (16.2%) cardiac deaths or hospitalizations for heart failure were accrued (Table 4). Moderate or severe mitral regurgitation was reported overall in 308 (14.3%). Survival analysis was performed using first unadjusted models (Table 5), and these suggested worse outcomes in less busy centers for death, cardiac death, the composite of death or hospitalization, and the composite of cardiac death or hospitalization for heart failure (all p < 0.05). However, after taking into account potential confounders, all these differences were no longer significant, except for cardiac death or hospitalization for heart failure, which appeared significantly less common in the busiest centers (all p < 0.05; Figure 1).

Table 4.
Cumulative outcomes at follow-up according to tertiles of monthly volume, center-wise *.

Table 5.
Unadjusted and adjusted survival analysis according to tertiles of monthly volume, center-wise *.
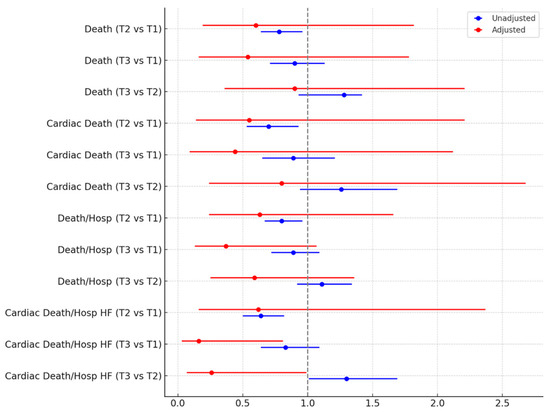
Figure 1.
Hazard ratios (95% confidence intervals) at unadjusted and adjusted Cox proportional hazard analysis comparing different tertiles (T) of monthly volume, center-wise. HF = heart failure; Hosp = hospitalization.
Similar trends were observed when leveraging tertiles of overall center volume and when comparing for each center the first 50 cases with the following ones (Tables S1–S4), with higher procedural success rates in busier/more experienced centers, and similarly trends favoring them for long-term clinical outcomes.
4. Discussion
A high institutional monthly volume of TEER mitral valve repair appears to correlate with improved procedural success rate and shorter hospitalizations. Similarly favorable results were found for long-term rates of cardiac death or hospitalization for heart failure.
The evidence base appraising the impact of institutional volume and expertise on TEER outcomes is complex and heterogeneous, but some studies have indeed suggested that some thresholds are important to achieve satisfactory procedural, in-hospital, and mid-term outcomes (Table S5) [10,12,14,15,16,17]. The findings from the present GIOTTO-VAT study support the concept that institutional volume in TEER may significantly impact on clinical outcomes. Notably, centers with higher monthly case volumes demonstrated greater procedural success. Furthermore, similarly favorable results were evident for long-term outcomes at unadjusted and adjusted analysis. These results align with existing evidence in the field of structural heart interventions, where operator and institutional experience have been shown to positively impact clinical outcomes [10,11].
Interestingly, while higher-volume centers showed better procedural metrics, long-term clinical outcomes, including mortality, were comparable across tertiles. This suggests that while experience improves technical execution and immediate safety, patient selection and underlying clinical conditions remain key determinants of long-term prognosis. Moreover, the impact of experienced proctors affiliated with high-volume centers providing careful guidance to lower-volume institutions cannot be discounted, in person or remotely. Indeed, the impact of proctors should be carefully appraised in future studies, together with detailed analyses on post-procedural care and management protocols.
A notable finding was the reduced hospitalization duration in high-volume centers compared to lower-volume ones. This may reflect more efficient perioperative management, shorter procedural times, and faster post-operative recovery [18]. Shorter hospital stays can also reduce healthcare costs, which is a significant consideration in the growing adoption of TEER for mitral regurgitation management, thus reinforcing the call for established expertise in all phases of management of patients with significant mitral regurgitation [19].
Our results prove informatively complementary to those reported by Grayburn and colleagues, who analyzed the American College of Cardiology/Society of Thoracic Surgeons Transcatheter Valve Therapy Registry and the Society of Thoracic Surgeons Adult Cardiac Surgery Database comparing institutions with high volumes for mitral valve repair, including surgical interventions [20]. In this study of 41,834 patients, the TEER success rate was similar in low- vs. high-volume centers, but 1-year survival and freedom from heart failure readmission were better in higher-volume hospitals.
This study has several key limitations that should be carefully considered by readers. First, despite some clear hints that higher volumes over time are associated with better outcomes in patients undergoing TEER for significant mitral regurgitation, results were not altogether consistent [21]. In addition, even adjusted analyses cannot be considered devoid of risk of residual confounding, such as ethnic features, which were not collected in our study. This is a key drawback, as minorities often have a more difficult time in accessing high-volume centers [22]. Furthermore, the risk of duplicity and type I error inflation due to a plethora of statistical tests remains substantial, and thus external replication of the present findings is paramount [23].
5. Conclusions
The present GIOTTO-VAT study suggests that hospitals with a higher volume of cases over time may yield improved procedural success rates, with ensuing shorter hospitalizations. Long-term rates of cardiac death or hospitalization for heart failure also favored centers with higher case/month figures. These findings inform on the importance of operator experience and center expertise in achieving state-of-the-art results with TEER, while confirming the usefulness of the proctoring approach when naïve centers begin a TEER program.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina61050904/s1, Table S1: Key outcomes according to tertiles of total volume, center-wise; Table S2: Key outcomes according to the first 50 cases versus the subsequent ones, center-wise; Table S3: Unadjusted and adjusted analysis according to tertiles of total volume, center-wise *; Table S4: Unadjusted and adjusted analysis according to the first 50 cases versus the subsequent ones, center-wise (hazard ratios < 1 indicate better outcomes after the first 50 cases) *; Table S5: Cut-offs for institutional volume of transcatheter edge-to-edge repair (TEER) proposed or analyzed in the scholarly literature.
Author Contributions
Conceptualization, N.C., C.T. and A.G.; methodology, N.C., P.F., F.F., A.M. (Alberto Morello), G.B.-Z., P.D., A.P.R., F.B., A.L.B., A.M. (Annalisa Mongiardo), S.G., F.D.F., M.A. (Marianna Adamo), M.M., F.M., G.T., F.G., F.R., E.V., M.F., L.F., F.C., A.S., M.P. and A.G.; validation, N.C., P.F., M.A. (Michele Albanese), F.R. and A.G.; formal analysis, A.M. (Alberto Morello), G.B.-Z., P.D., S.G., M.A. (Marianna Adamo), G.T., F.C. and A.S.; investigation, F.F., M.C., M.A. (Michele Albanese), A.P.R., F.B., A.L.B., A.M. (Annalisa Mongiardo), S.G., F.D.F., M.M., F.M., F.G., E.V., L.F., C.T. and A.G.; resources, N.C., A.M. (Annalisa Mongiardo), M.M. and M.F.; data curation, N.C., M.C., G.B.-Z., M.A. (Marianna Adamo), G.T. and M.P.; writing—original draft, G.B.-Z. and F.R.; writing—review and editing, N.C., P.F., F.F., M.C., M.A. (Michele Albanese), A.M. (Alberto Morello), P.D., A.P.R., F.B., A.L.B., A.M. (Annalisa Mongiardo), S.G., F.D.F., M.A. (Marianna Adamo), M.M., F.M., G.T., F.G., E.V., M.F., L.F., F.C., A.S., M.P., C.T. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
The GIOTTO (GIse registry Of Transcatheter treatment of mitral valve regurgitaTiOn) registry is sponsored by the Italian Society of Invasive Cardiology, with an unrestricted grant by Abbott Vascular, Santa Clara, CA, USA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of IRCCS Policlinico San Donato, Milan, Italy (protocol code: GISE/01/2014/GIOTTO; date of approval: 3 March 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available because of inclusion of patient details. Requests to access the datasets should be directed to the corresponding author.
Conflicts of Interest
Giuseppe Biondi-Zoccai has consulted for Aleph, Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Menarini, Microport, Opsens Medical, Terumo, and Translumina, outside the present work. All other authors report no conflicts of interest.
Central Illustration
Impact of institutional monthly volume ratio of transcatheter edge-to-edge repair (TEER) on patient outcomes (forest plot based on hazard ratios, with accompanying 95% confidence intervals).
References
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Egorova, N.; Moskowitz, G.; Giustino, G.; Ailawadi, G.; Acker, M.A.; Gillinov, M.; Moskowitz, A.; Gelijns, A. Trends in MitraClip, mitral valve repair, and mitral valve replacement from 2000 to 2016. J. Thorac. Cardiovasc. Surg. 2021, 162, 551–562.e4. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, W.; Zhang, D.; Ye, G.; Ding, C. Mortality and Clinical Predictors After Percutaneous Mitral Valve Repair for Secondary Mitral Regurgitation: A Systematic Review and Meta-Regression Analysis. Front. Cardiovasc. Med. 2022, 9, 918712. [Google Scholar] [CrossRef]
- Giordano, A.; Biondi-Zoccai, G.; Finizio, F.; Ferraro, P.; Denti, P.; Rubbio, A.P.; Petronio, A.S.; Bartorelli, A.L.; Mongiardo, A.; De Felice, F.; et al. Characteristics and outcomes of MitraClip in octogenarians: Evidence from 1853 patients in the GIOTTO registry. Int. J. Cardiol. 2021, 342, 65–71. [Google Scholar] [CrossRef]
- van-Roessel, A.M.; Asmarats, L.; Li, C.H.P.; Millán, X.; Fernández-Peregrina, E.; Menduiña, I.; Sanchez-Ceña, J.; Arzamendi, D. Mitral transcatheter edge-to-edge repair: Patient selection, current devices, and clinical outcomes. Expert. Rev. Med. Devices 2024, 21, 187–196. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Bruno, A.; Lombardo, M.; Muti, P. Echocardiographic Assessment of Mitral Valve Prolapse Prevalence before and after the Year 1999: A Systematic Review. J. Clin. Med. 2024, 13, 6160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stolz, L.; Stocker, T.J.; Lurz, P.; Hausleiter, J. Growing Evidence for Edge-to-Edge Repair in Secondary Mitral Regurgitation: What to Learn From COAPT, MITRA-FR, and RESHAPE-HF2. JACC Cardiovasc. Interv. 2025, 18, 927–932. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Soulaidopoulos, S.; Pyrpyris, N.; Sagris, Μ.; Aznaouridis, K.; Beneki, E.; Theofilis, P.; Tsioufis, P.; Tatakis, F.; Fragkoulis, C.; et al. Transcatheter Edge-to-Edge Repair for Severe Mitral Regurgitation in Patients with Cardiogenic Shock: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2025, 14, e034932. [Google Scholar] [CrossRef]
- Chhatriwalla, A.K.; Vemulapalli, S.; Holmes, D.R., Jr.; Dai, D.; Li, Z.; Ailawadi, G.; Glower, D.; Kar, S.; Mack, M.J.; Rymer, J.; et al. Institutional Experience With Transcatheter Mitral Valve Repair and Clinical Outcomes: Insights from the TVT Registry. JACC Cardiovasc. Interv. 2019, 12, 1342–1352. [Google Scholar] [CrossRef]
- Chhatriwalla, A.K.; Vemulapalli, S.; Szerlip, M.; Kodali, S.; Hahn, R.T.; Saxon, J.T.; Mack, M.J.; Ailawadi, G.; Rymer, J.; Manandhar, P.; et al. Operator Experience and Outcomes of Transcatheter Mitral Valve Repair in the United States. J. Am. Coll. Cardiol. 2019, 74, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; O’Gara, P.T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.; Satpathy, R.; et al. 2019 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document: Operator and Institutional Recommendations and Requirements for Transcatheter Mitral Valve Intervention: A Joint Report of the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and The Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2020, 76, 96–117. [Google Scholar] [PubMed]
- Basso, C.; Musumeci, G.; Saia, F.; Tarantini, F. Percutaneous Approaches to Mitral Valve Disease; Minerva Medica: Torino, Italy, 2019. [Google Scholar]
- Kolte, D.; Butala, N.M.; Kennedy, K.F.; Wasfy, J.H.; Jena, A.B.; Sakhuja, R.; Langer, N.; Melnitchouk, S.; Sundt, T.M., III; Passeri, J.J.; et al. Association Between Hospital Cardiovascular Procedural Volumes and Transcatheter Mitral Valve Repair Outcomes. Cardiovasc. Revasc. Med. 2022, 36, 27–33. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Schmidtmann, I.; Münzel, T.; Baldus, S.; von Bardeleben, R.S. Centre procedural volume and adverse in-hospital outcomes in patients undergoing percutaneous transvenous edge-to-edge mitral valve repair using MitraClip® in Germany. Eur. J. Heart Fail. 2021, 23, 1380–1389. [Google Scholar] [CrossRef]
- Shoji, S.; Kuno, T.; Malik, A.; Briasoulis, A.; Inohara, T.; Kampaktsis, P.N.; Kohsaka, S.; Latib, A. Association between institutional volume of transcatheter mitral valve repair and readmission rates: A report from the Nationwide Readmission Database. Int. J. Cardiol. 2023, 383, 70–74. [Google Scholar] [CrossRef]
- Bansal, K.; Pawar, S.; Gupta, T.; Gilani, F.; Khera, S.; Kolte, D. Association Between Hospital Volume and 30-Day Readmissions After Transcatheter Mitral Valve Edge-to-Edge Repair. Am. J. Cardiol. 2023, 203, 149–156. [Google Scholar] [CrossRef]
- Nita, N.; Schneider, L.; Dahme, T.; Markovic, S.; Keßler, M.; Rottbauer, W.; Tadic, M. Trends in Transcatheter Edge-to-Edge Mitral Valve Repair Over a Decade: Data From the MiTra ULM Registry. Front. Cardiovasc. Med. 2022, 9, 850356. [Google Scholar] [CrossRef]
- Stone, G.W. Volume-Outcome Relationships for Transcatheter Mitral Valve Repair: More Is Better. JACC Cardiovasc. Interv. 2019, 12, 1353–1355. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Mack, M.J.; Manandhar, P.; Kosinski, A.S.; Sannino, A.; Smith, R.L., II; Szerlip, M.; Vemulapalli, S. Comparison of Transcatheter Edge-to-Edge Mitral Valve Repair for Primary Mitral Regurgitation Outcomes to Hospital Volumes of Surgical Mitral Valve Repair. Circ. Cardiovasc. Interv. 2024, 17, e013581. [Google Scholar] [CrossRef]
- Halm, E.A.; Lee, C.; Chassin, M.R. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann. Intern. Med. 2002, 137, 511–520. [Google Scholar] [CrossRef]
- Steitieh, D.; Zaidi, A.; Xu, S.; Cheung, J.W.; Feldman, D.N.; Reisman, M.; Mallya, S.; Paul, T.K.; Singh, H.S.; Bergman, G.; et al. Racial Disparities in Access to High-Volume Mitral Valve Transcatheter Edge-to-Edge Repair Centers. J. Soc. Cardiovasc. Angiogr. Inter. 2022, 1, 100398. [Google Scholar] [CrossRef] [PubMed]
- Safieddine, M.; Chapelle, C.; Ollier, E.; Ferdynus, C.; Bertoletti, L.; Mismetti, P.; Cucherat, M.; Laporte, S. Compared to randomized studies, observational studies may overestimate the effectiveness of DOACs: A metaepidemiological approach. J. Clin. Epidemiol. 2021, 130, 49–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).