‘Lamina External Graft Overlay’: The Use of Segmented Xenogenic Bone Sheets in the Reconstruction of 3D Bone Defects
Abstract
1. Introduction
2. Materials and Methods
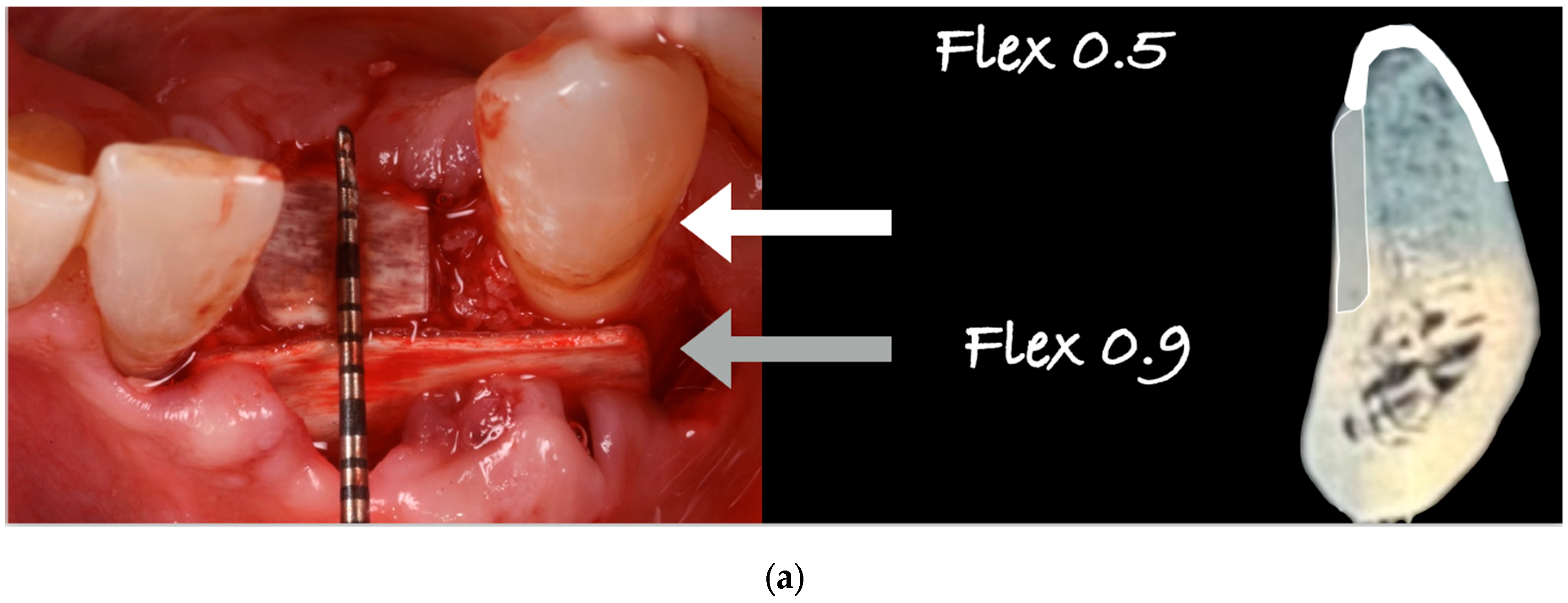
2.1. Biomaterials
2.2. Preparation of FG
2.3. Surgical Technique and ‘Lamina External Graft Overlay’ (L.E.G.O.) Rationale
2.4. Patient Selection
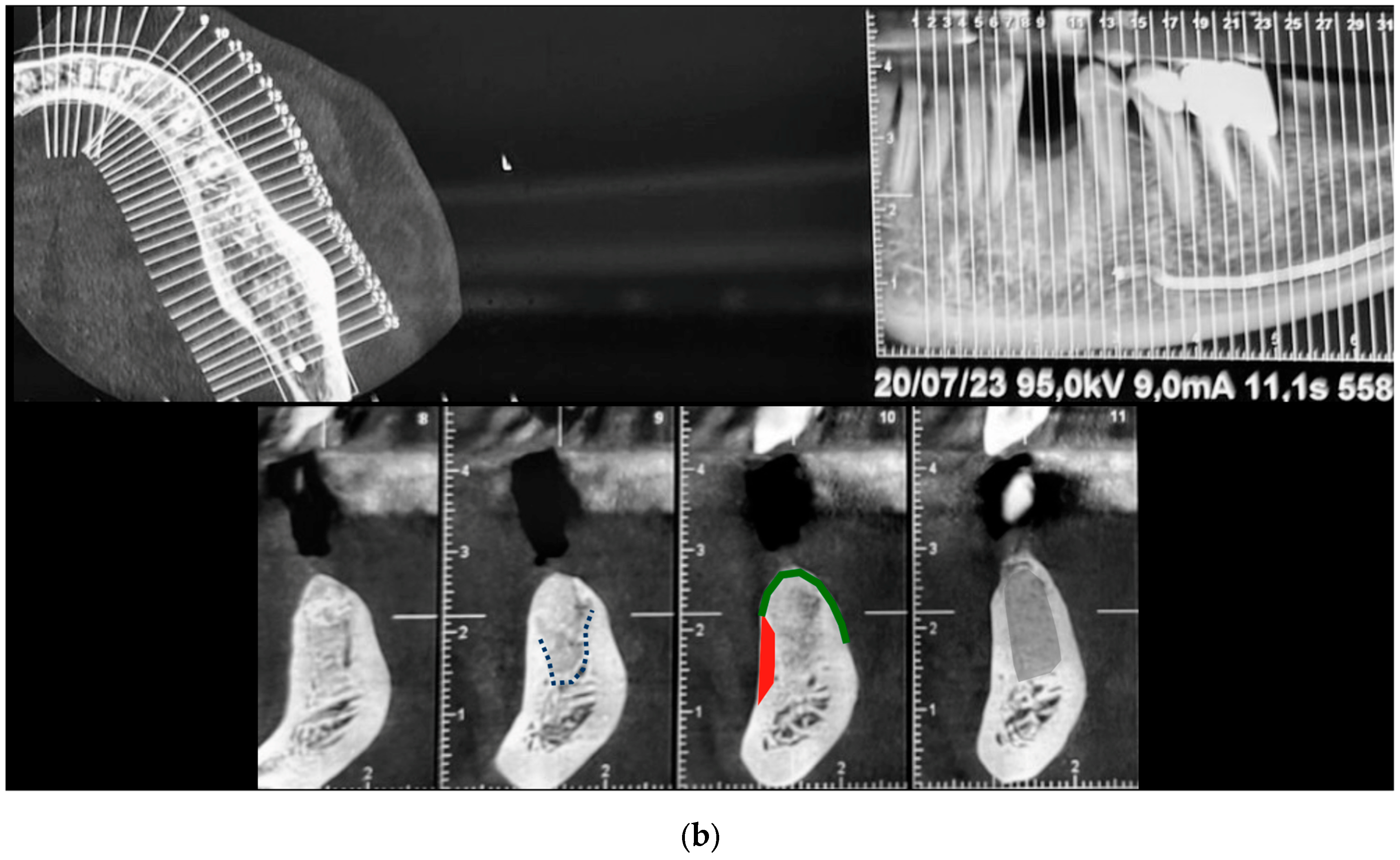
2.5. Histological and Histomorphometric Analysis (Patient N° 3)
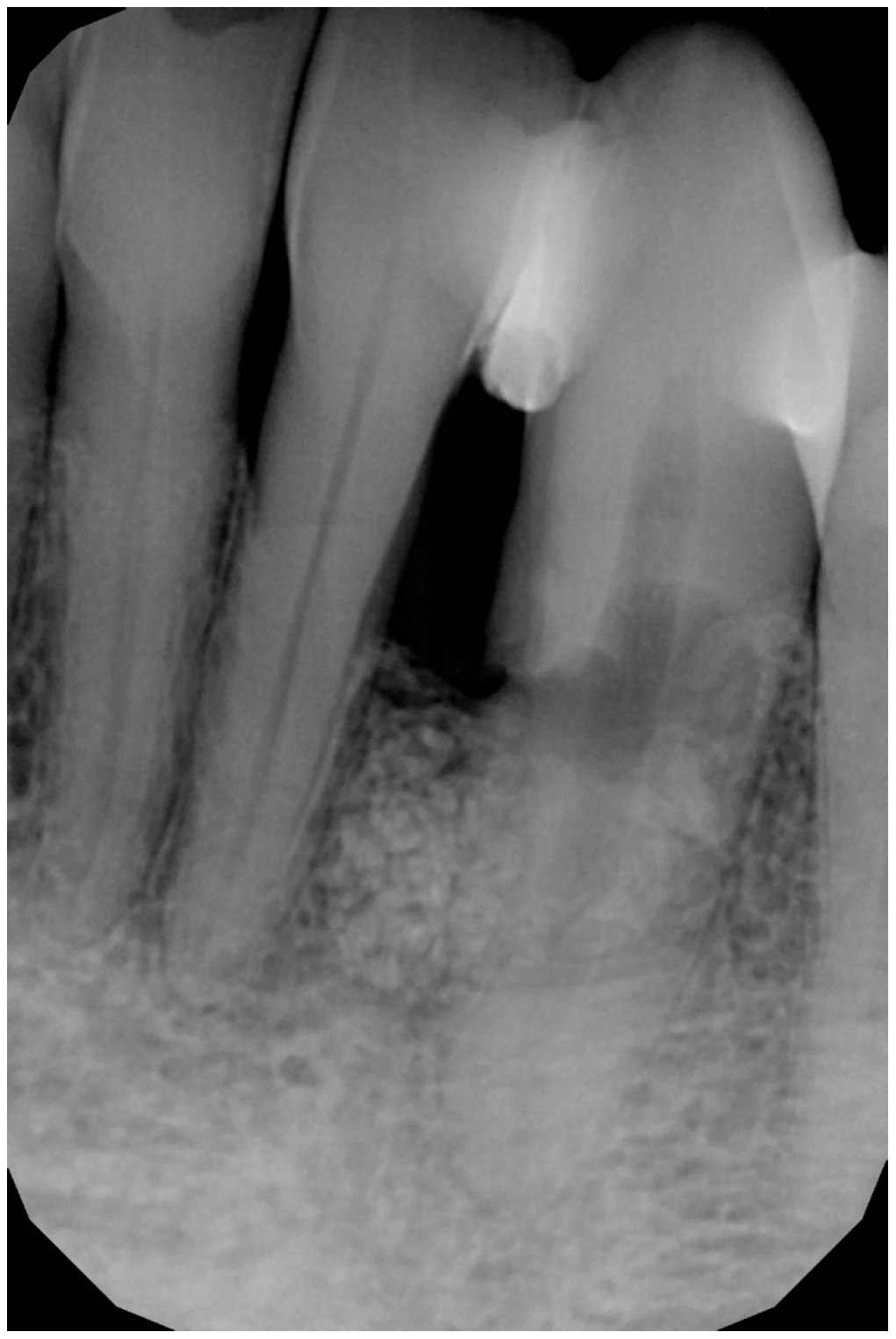
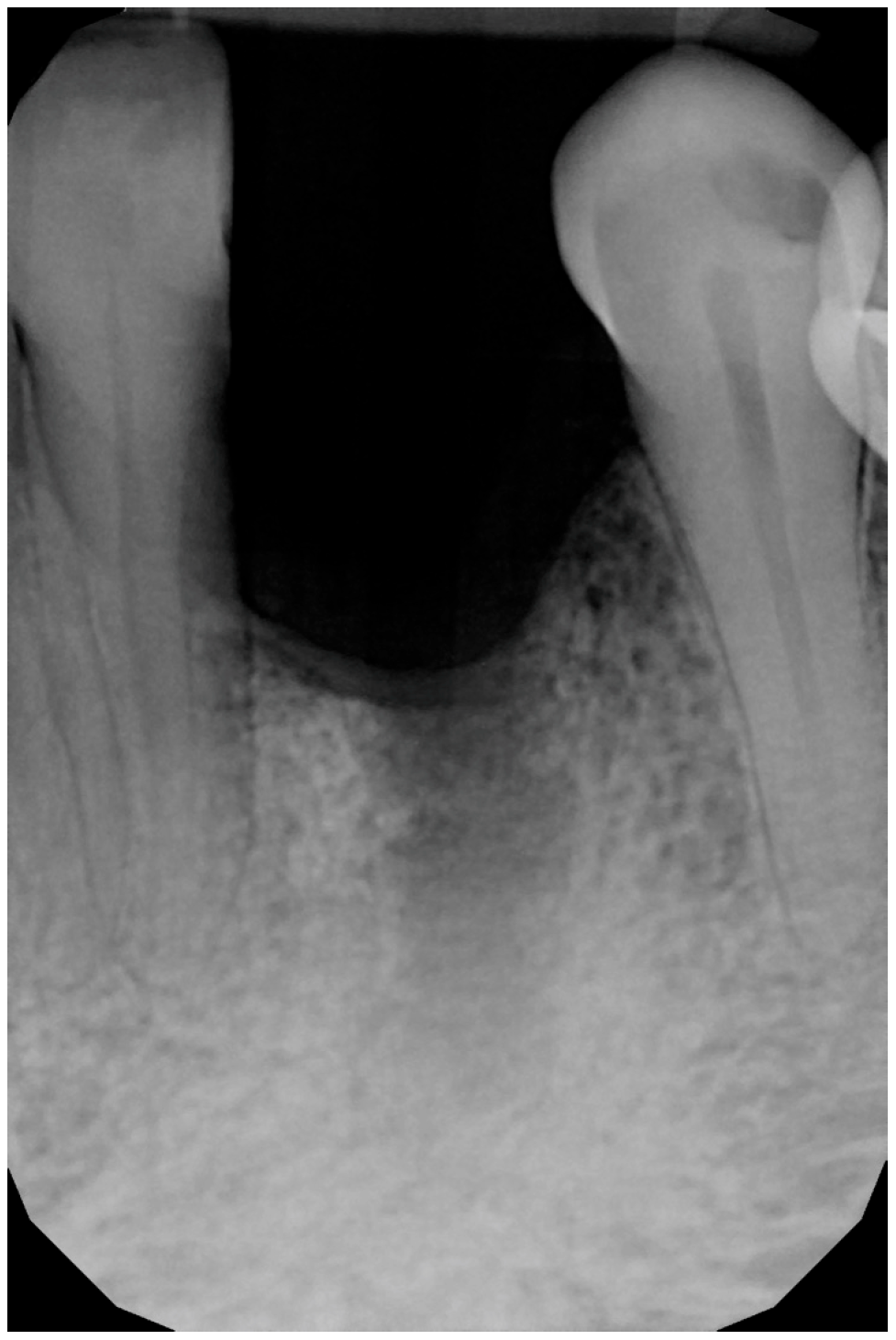
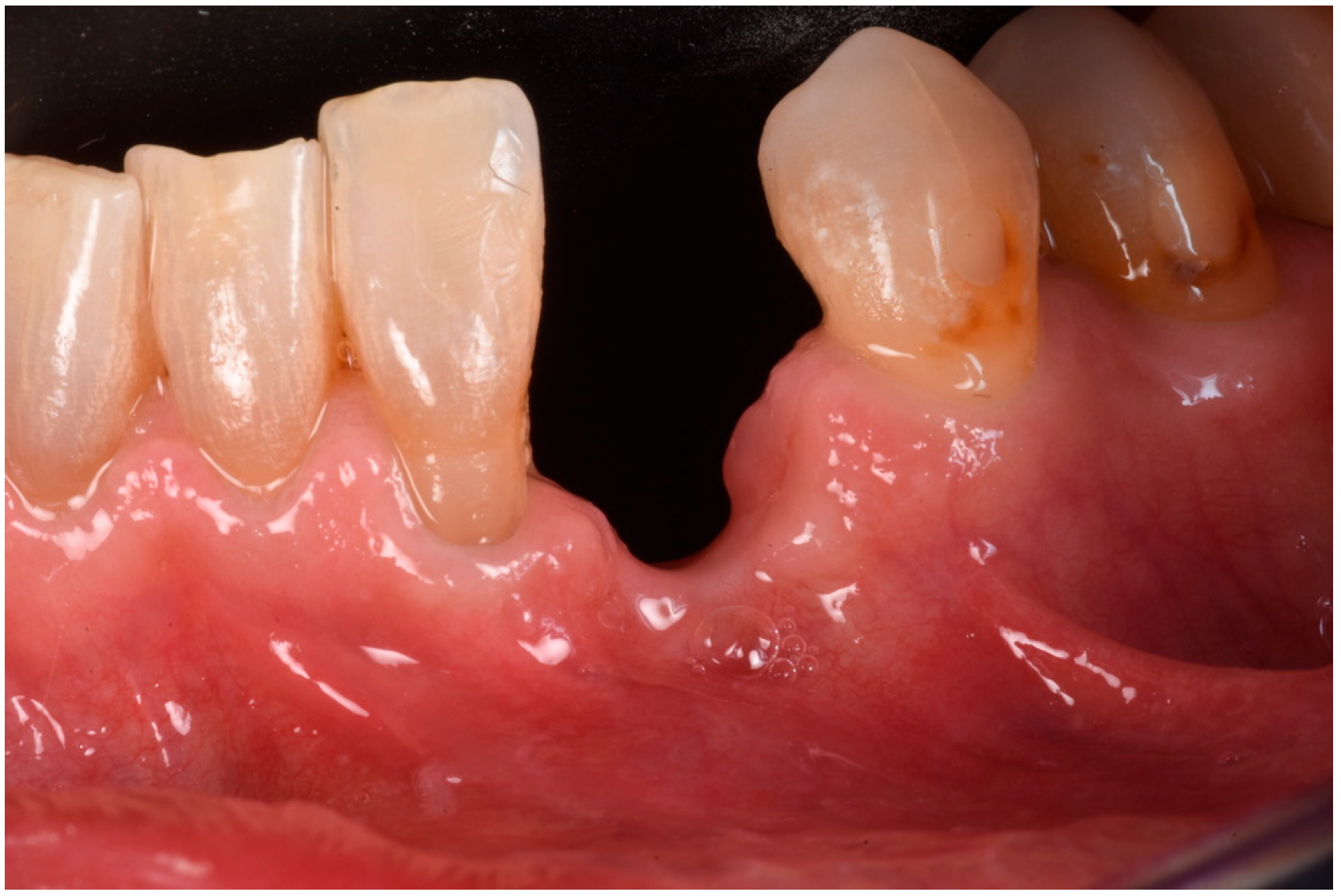
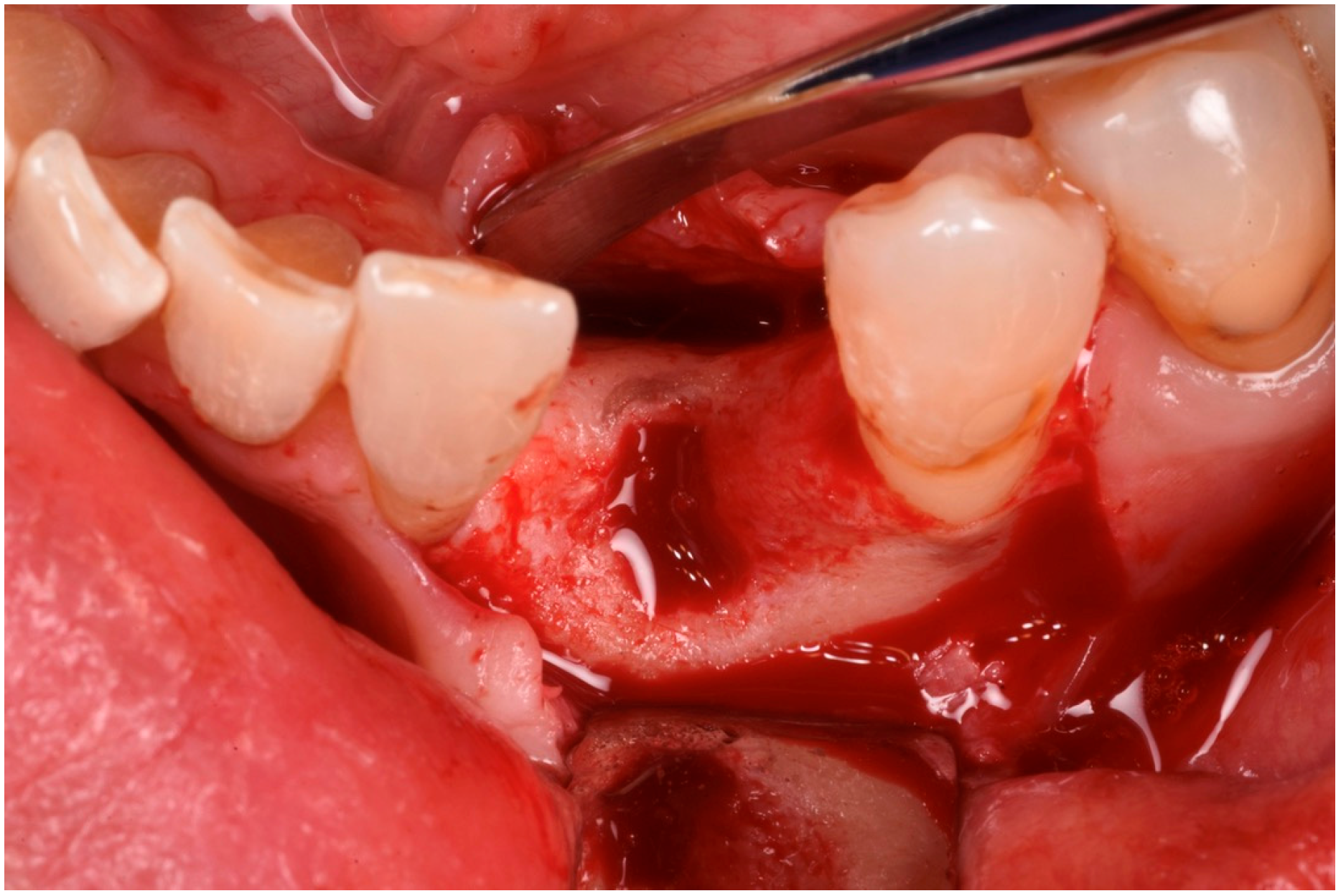
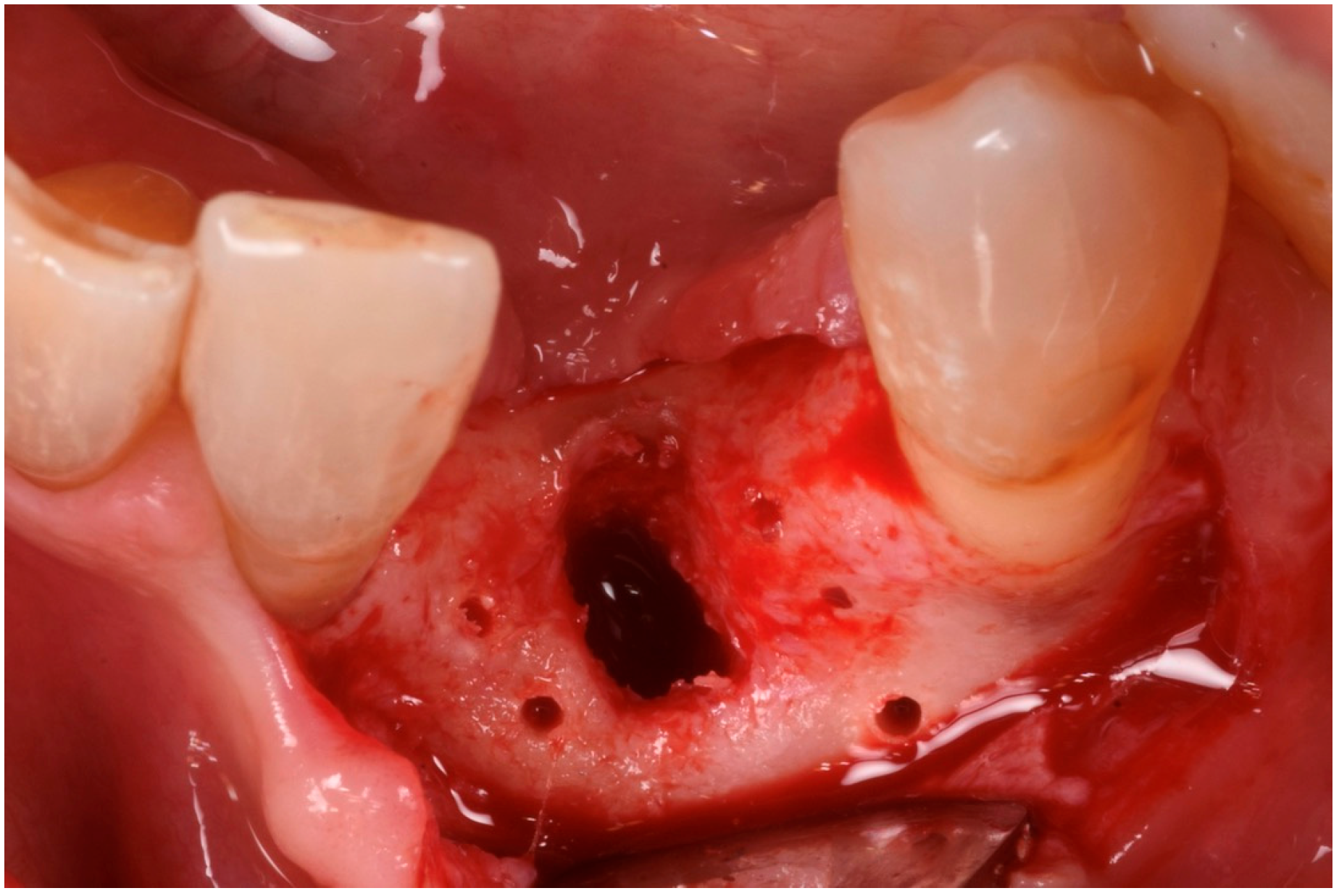
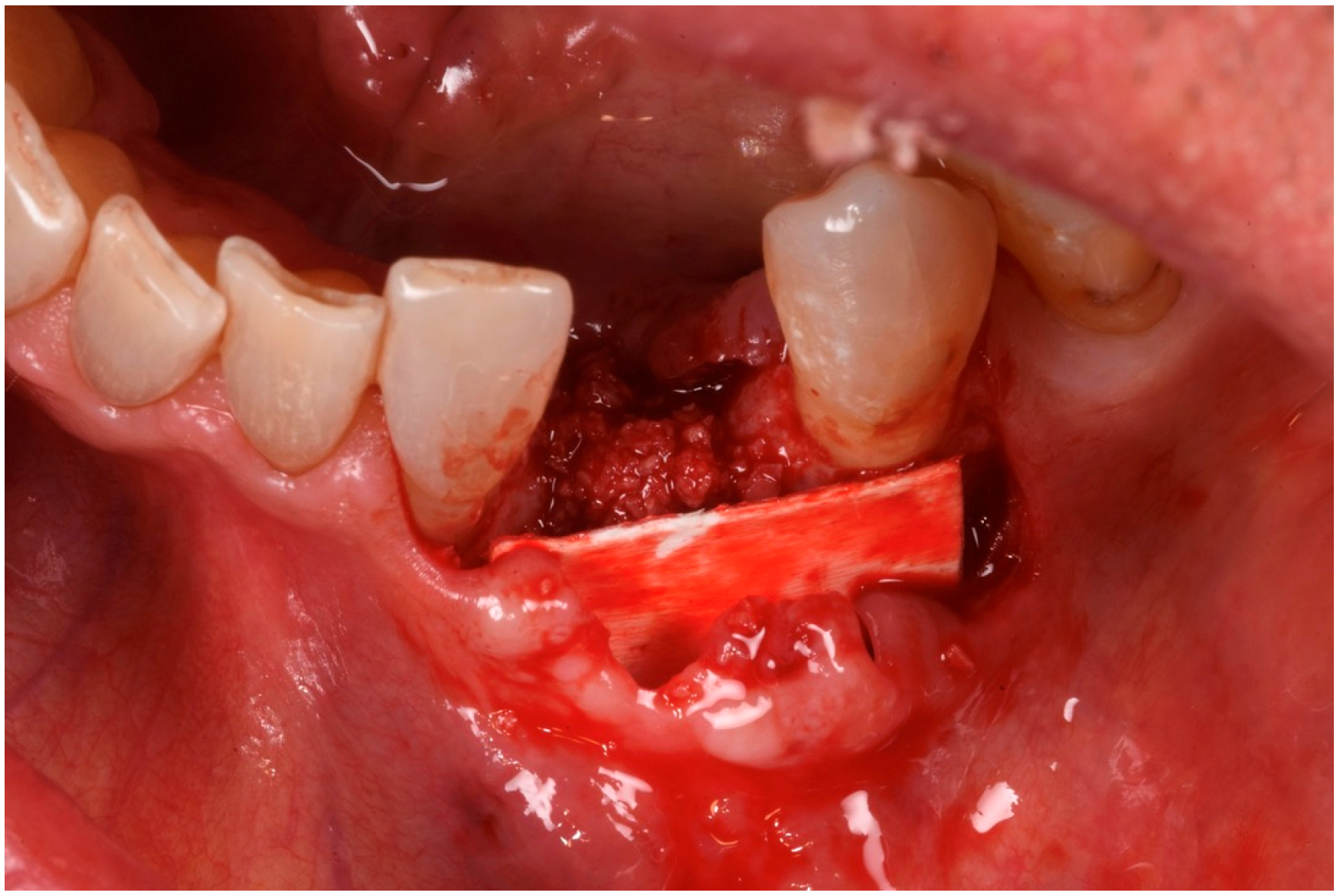
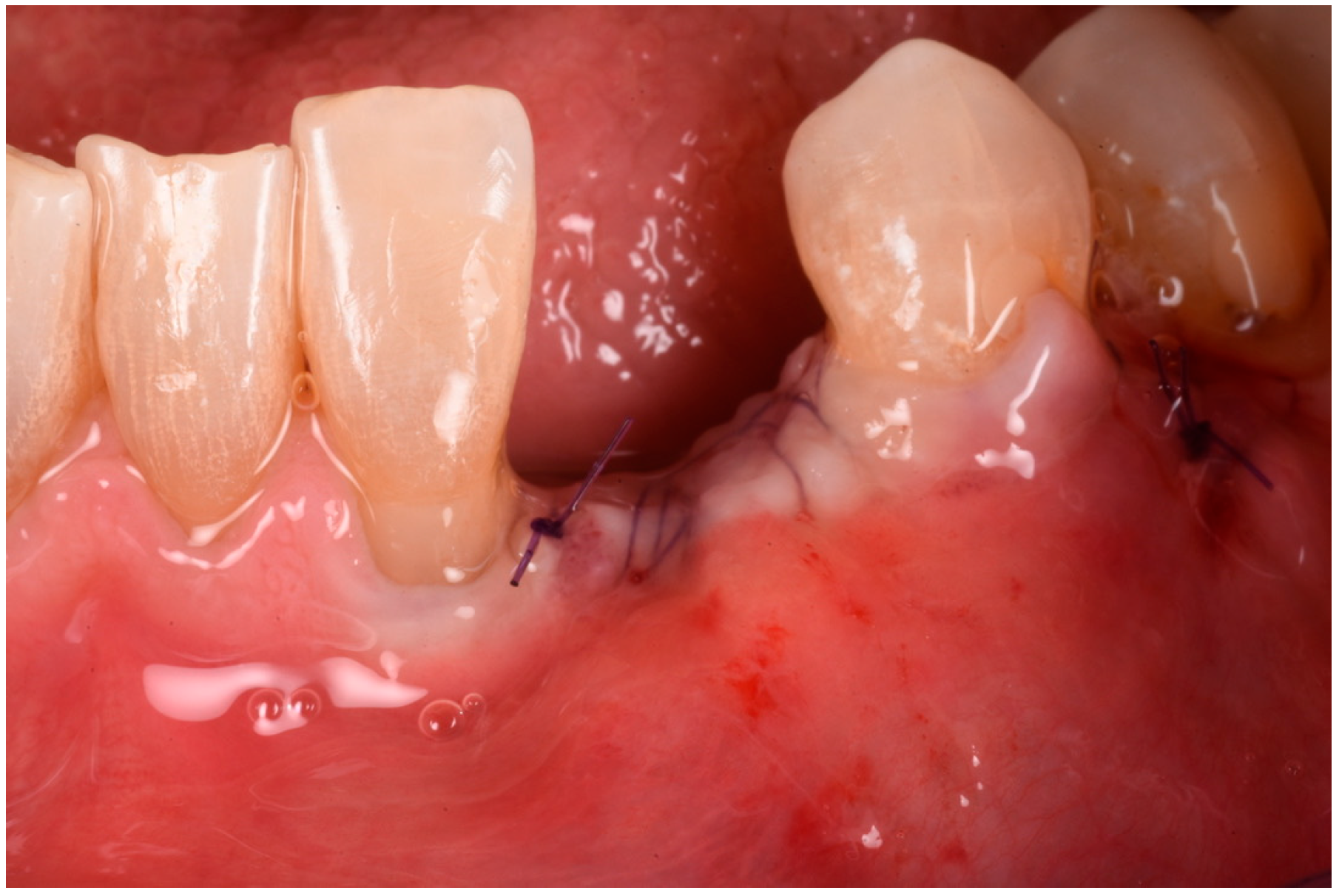
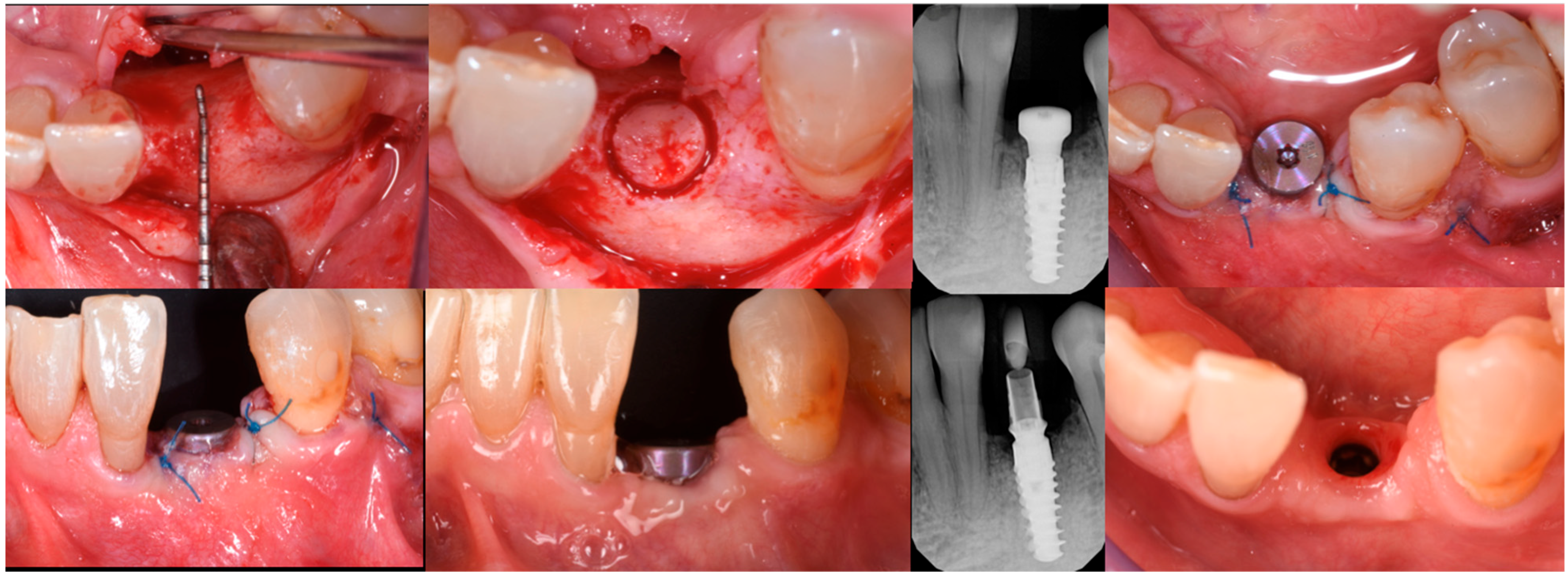
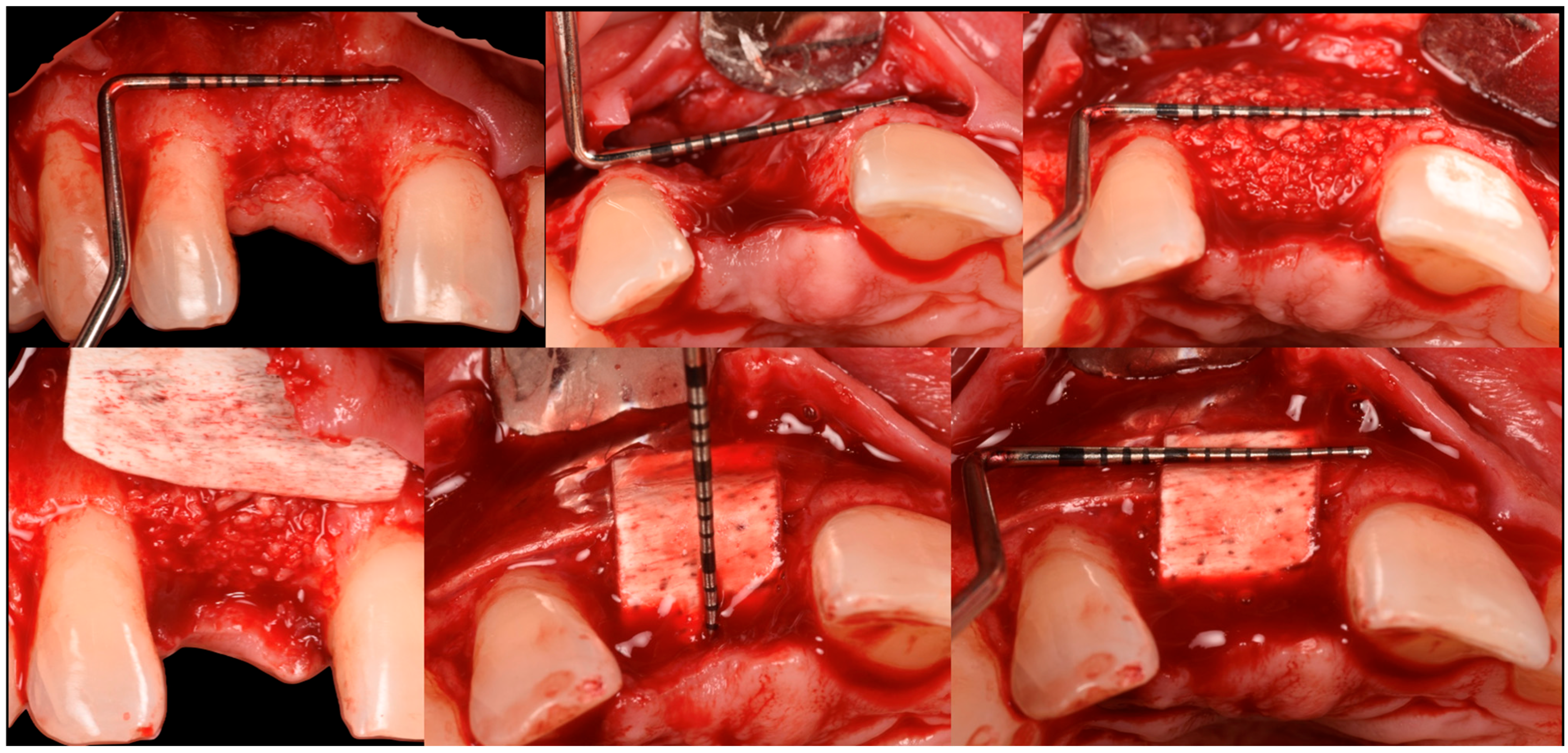
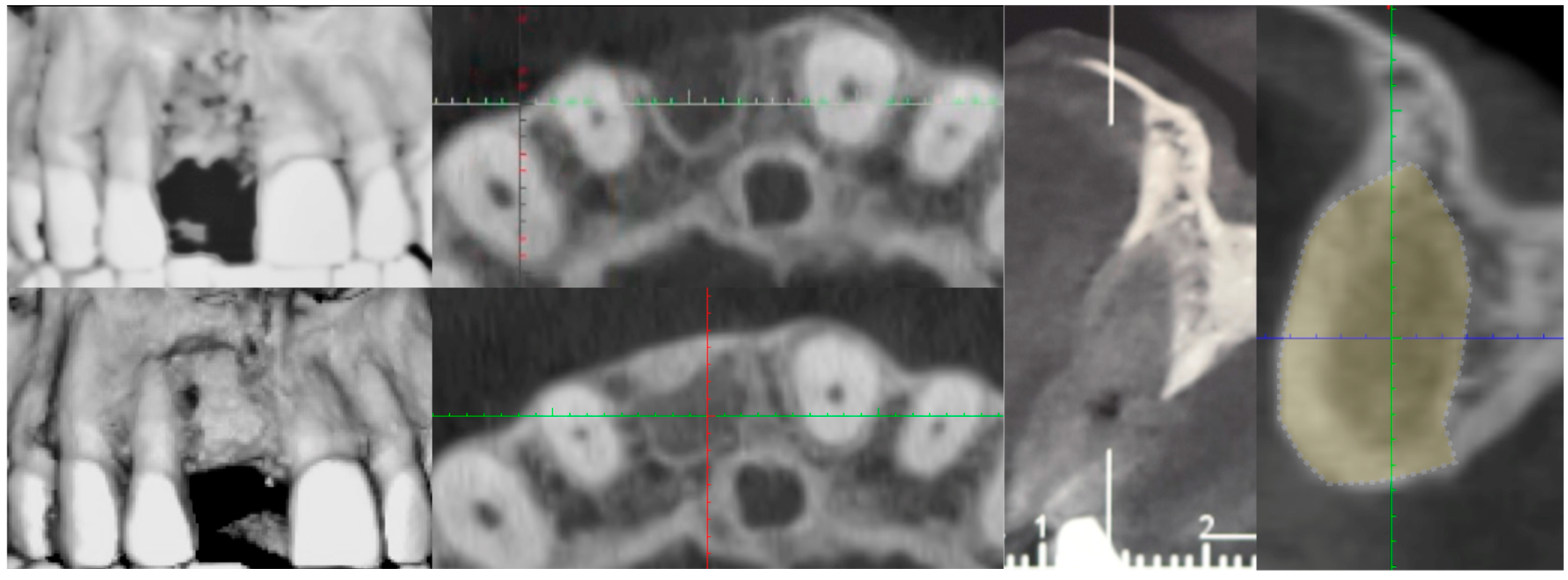
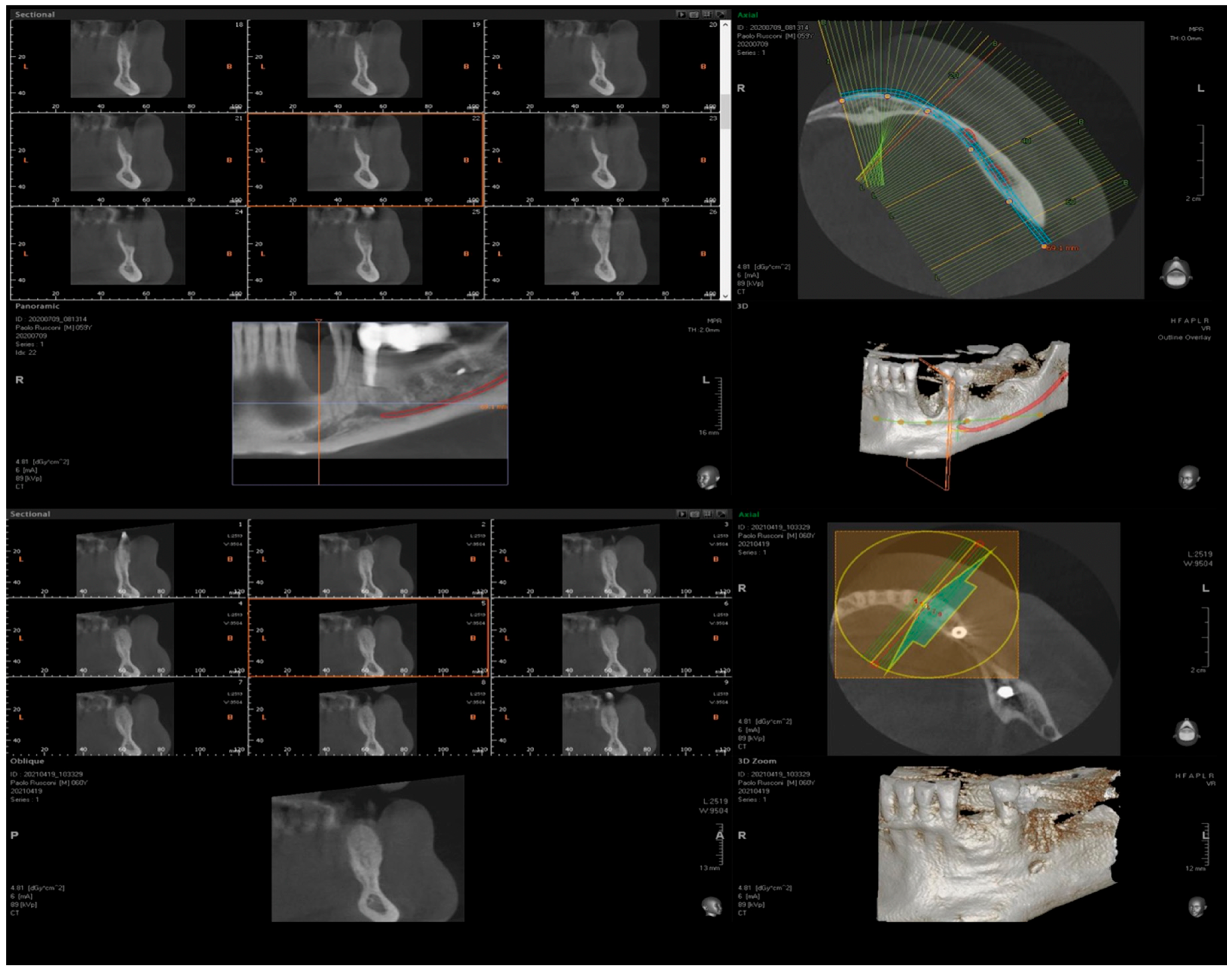
3. Surgical Technique and Case Presentation (Patient N° 3)
3.1. Clinical History
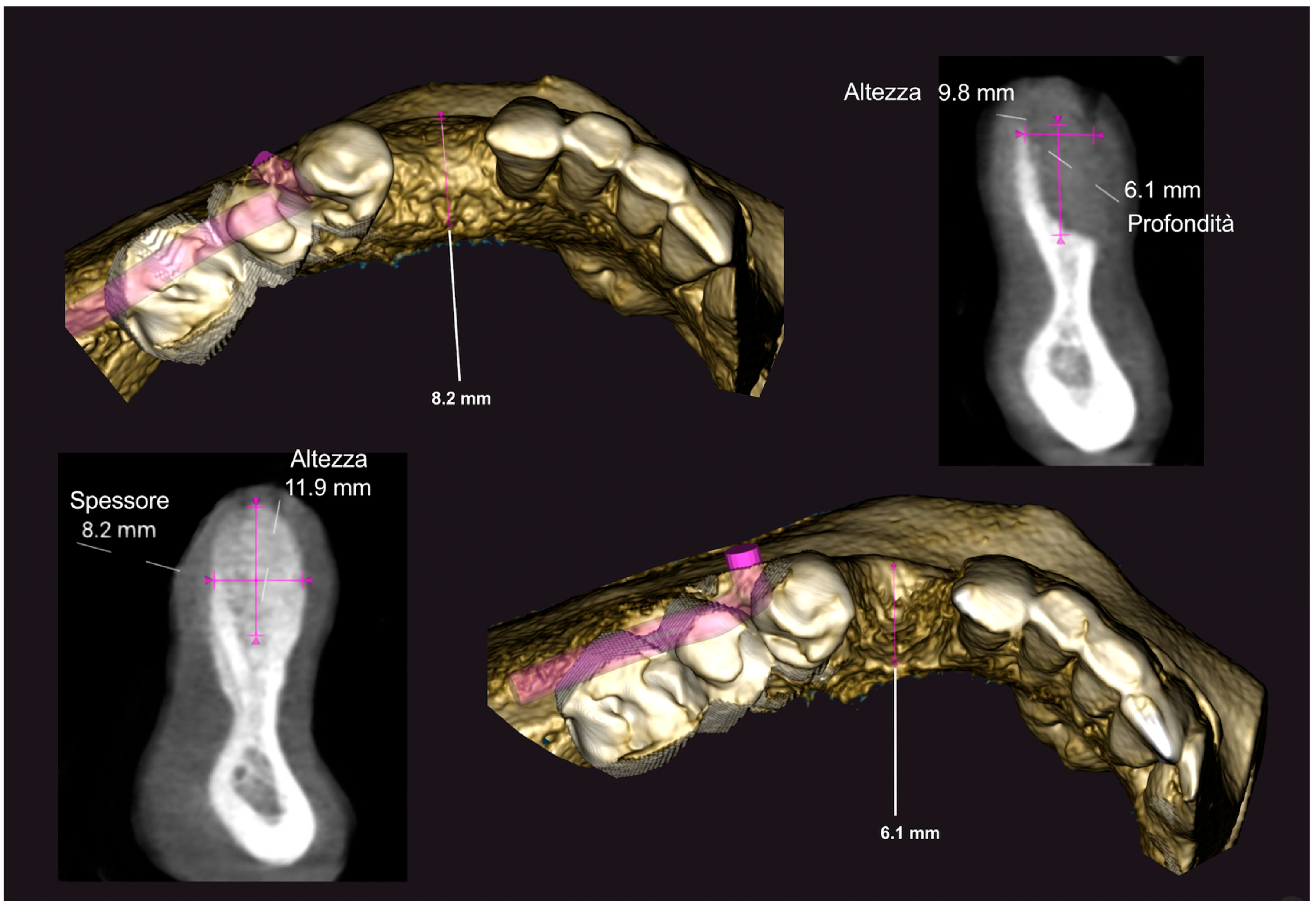
3.2. Hard Tissue Augmentation
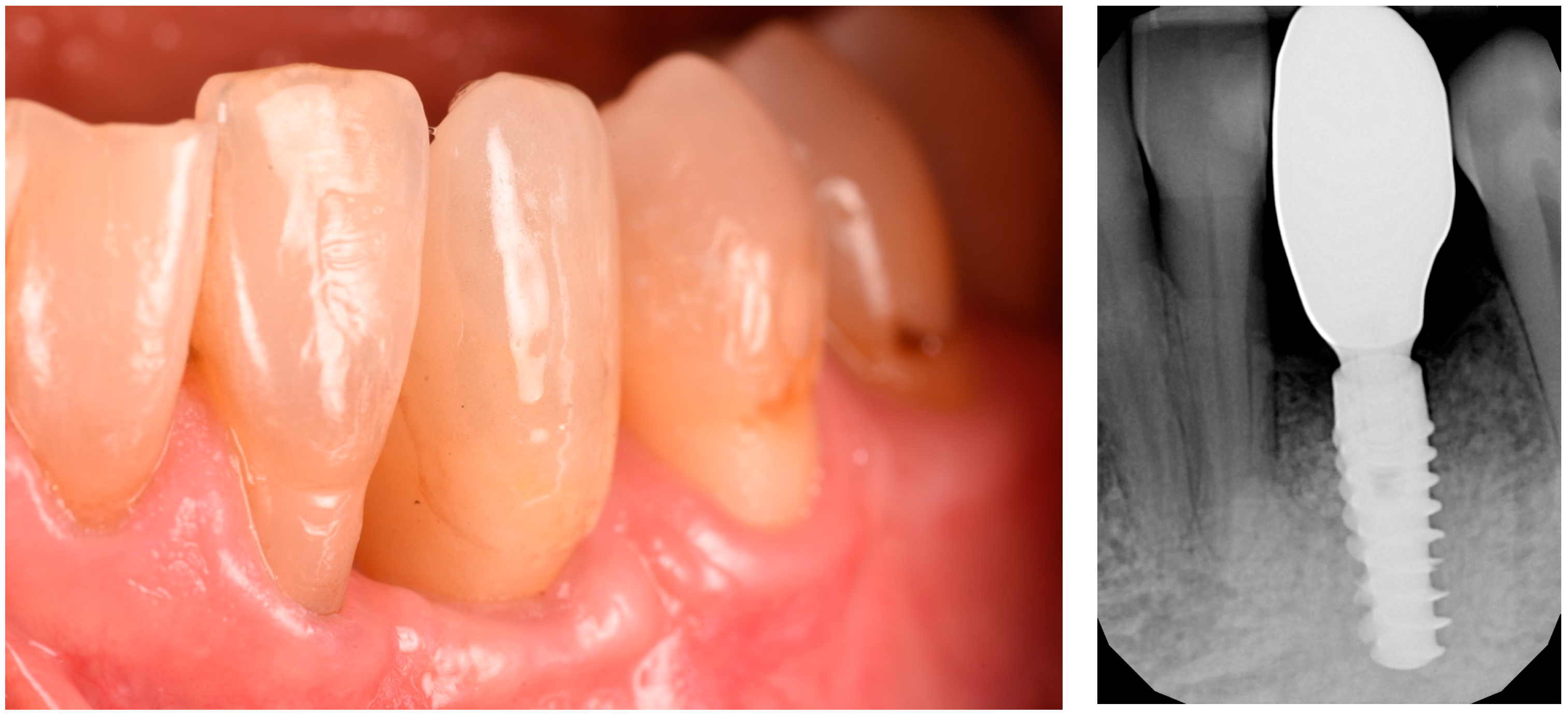
3.3. Implant Insertion and Follow-Up
3.4. Histological Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammerle, C.H.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Hujoel, P.; Becker, B.E. Effect of barrier membranes and autologous bone grafts on ridge width preservation around implants. Clin. Implant Dent. Relat. Res. 2002, 4, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rocchietta, I.; Fontana, F.; Simion, M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: A systematic review. J. Clin. Periodontol. 2008, 35, 203–215. [Google Scholar] [CrossRef]
- Urban, I.A.; Jovanovic, S.A.; Lozada, J.L. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: A retrospective study of 35 patients 12 to 72 months after loading. Int. J. Oral Maxillofac. Implant. 2009, 24, 502–510. [Google Scholar]
- Wagner-Ecker, M.; Voltz, P.; Egermann, M.; Richter, W. The collagen component of biological bone graft substitutes promotes ectopic bone formation by human mesenchymal stem cells. Acta Biomater. 2013, 9, 7298–7307. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Y.Y.; Zhao, J.N.; Wu, S.J.; Xiong, Z.; Lu, R. Effect of type I collagen on the adhesion, proliferation, and osteoblastic gene expression of bone marrow-derived mesenchymal stem cells. Chin. J. Traumatol. 2004, 7, 358–362. [Google Scholar]
- Regazzoni, C.; Winterhalter, K.H.; Rohrer, L. Type I collagen induces expression of bone morphogenetic protein receptor type II. Biochem. Biophys. Res. Commun. 2001, 283, 316–322. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Banyard, D.A.; Bourgeois, J.M.; Widgerow, A.D.; Evans, G.R. Regenerative biomaterials: A review. Plast. Reconstr. Surg. 2015, 135, 1740–1748. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Kao, S.T.; Scott, D.D. A review of bone substitutes. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 513–521. [Google Scholar] [CrossRef]
- Rossi, R.; Memè, L.; Strappa, E.M.; Bambini, F. Restoration of Severe Bone and Soft Tissue Atrophy by Means of a Xenogenic Bone Sheet (Flex Cortical Sheet): A Case Report. Appl. Sci. 2023, 13, 692. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Piattelli, A.; Zaniol, T.; Iezzi, G. Implant and Prosthetic Success Following Peri-Implant Guided Bone Regeneration in the Esthetic Zone Using an Equine Cortical Bone Membrane and an Equine Enzyme-Treated Bone Graft: A Retrospective Study with 9-Year Follow-Up. Int. J. Oral Maxillofac. Implant. 2020, 35, 824–832. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Garagiola, U.; Andreasi Bassi, M. Preserving the Bone Profile in Anterior Maxilla Using an Equine Cortical Bone Membrane and an Equine Enzyme-Treated Bone Graft: A Case Report with 5-Year Follow-Up. J. Contemp. Dent. Pract. 2017, 18, 614–621. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Cazzaniga, A.; Andreasi Bassi, M.; Ludovichetti, M.; Ammirabile, G.; Celletti, R. The use of cortical heterologous sheets for sinus lift bone grafting: A modification of Tulasne’s technique with 7-year follow-up. Int. J. Immunopathol. Pharmacol. 2013, 26, 549–556. [Google Scholar] [CrossRef]
- Foti, V.; Savio, D.; Rossi, R. One-Time Cortical Lamina: A New Technique for Horizontal Ridge Augmentation. A Case Series. Br. J. Healthc. Med. Res. 2021, 8, 22–30. [Google Scholar] [CrossRef]
- Rossi, R.; Ghezzi, C.; Tomecek, M. Cortical lamina: A new device for the treatment of moderate and severe tridimensional bone and soft tissue defects. Int. J. Esthet. Dent. 2020, 15, 454–473. [Google Scholar]
- Rossi, R.; Foce, E. Reconstruction of a horizontal and vertical bone defect using the Cortical Lamina Technique. Arch. Med. Res. 2019, 7, 1–11. [Google Scholar]
- Lopez, M.A.; Andreasi Bassi, M.; Confalone, L.; Carinci, F.; Ormianer, Z.; Lauritano, D. The use of resorbable cortical lamina and micronized collagenated bone in the regeneration of atrophic crestal ridges: A surgical technique. Case series. J. Biol. Regul. Homeost. Agents 2016, 30, 81–85. [Google Scholar]
- Schuh, P.L.; Bolz, W.; Weiss, M.; Niedermaier, R.; Riemann, M.; Stelzle, F.; Wachtel, H. The multi layer technique: An innovative technique of immediate implant placement with simultaneous hard and soft tissue augmentation in the esthetic zone. Implantologie 2015, 23, 443–451. [Google Scholar]
- Schuh, P.L.; Wachtel, H.; Beuer, F.; Goker, F.; Del Fabbro, M.; Francetti, L.; Testori, T. Multi-Layer Technique (MLT) with Porcine Collagenated Cortical Bone Lamina for Bone Regeneration Procedures and Immediate Post-Extraction Implantation in the Esthetic Area: A Retrospective Case Series with a Mean Follow-Up of 5 Years. Materials 2021, 14, 5180. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Foce, E.; Scolavino, S. The Cortical Lamina Technique: A new option for alveolare ridge augmentation, procedure, protocol and case report. J. Leban. Dent. Assoc. 2017, 51, 35–41. [Google Scholar]
- Rossi, R.; Barone, A.; Choukroun, J.; D’Amato, S.; Fairbairn, P.J.; Foce, E.; Fontana, F.; Foti, V.; Gluckman, H.; Grassi, A.; et al. Building Better Bone. A Comprehensive Guide to GBR Techniques; Rossi, R., Ed.; Quintessence Publishing: New Malden, UK, 2024. [Google Scholar]
- Perrotti, V.; Nicholls, B.M.; Piattelli, A. Human osteoclast formation and activity on an equine spongy bone substitute. Clin. Oral Implant. Res. 2009, 20, 17–23. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Zaniol, T.; Cinci, L.; Pieri, L. Chemical, Clinical and Histomorphometric Comparison Between Equine Bone Manufactured Through Enzymatic Antigen-Elimination and Bovine Bone Made Non-Antigenic Using a High-Temperature Process in Post-Extractive Socket Grafting. A Comparative Retrospective Clinical Study. Dent. J. 2019, 7, 70. [Google Scholar] [CrossRef]
- Khoury, F.; Hanser, T. Three-Dimensional Vertical Alveolar Ridge Augmentation in the Posterior Maxilla: A 10-Year Clinical Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 471–480. [Google Scholar] [CrossRef]
- De Stavola, L.; Tunkel, J. Results of vertical bone augmentation with autogenous bone block grafts and the tunnel technique: A clinical prospective study of 10 consecutively treated patients. Int. J. Periodontics Restor. Dent. 2013, 33, 651–659. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Gastaldi, G.; Vinci, R.; Cinci, L.; Pieri, L.; Gherlone, E. Histomorphometric comparison of enzyme-deantigenic equine bone and anorganic bovine bone in sinus augmentation: A randomized clinical trial with 3-year follow-up. Int. J. Oral Maxillofac. Implant. 2015, 30, 1161–1167. [Google Scholar] [CrossRef]
- Canullo, L.; Wiel Marin, G.; Tallarico, M.; Canciani, E.; Musto, F.; Dellavia, C. Histological and Histomorphometrical Evaluation of Postextractive Sites Grafted with Mg-Enriched Nano-Hydroxyapatite: A Randomized Controlled Trial Comparing 4 Versus 12 Months of Healing. Clin. Implant Dent. Relat. Res. 2016, 18, 973–983. [Google Scholar] [CrossRef]
- Carmagnola, D.; Pispero, A.; Pellegrini, G.; Sutera, S.; Henin, D.; Lodi, G.; Achilli, A.; Dellavia, C. Maxillary sinus lift augmentation: A randomized clinical trial with histological data comparing deproteinized bovine bone grafting vs. graftless procedure with a 5–12-year follow-up. Clin. Implant Dent. Relat. Res. 2024, 26, 972–985. [Google Scholar] [CrossRef]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265–273. [Google Scholar]
- Marian, D.; Toro, G.; D’Amico, G.; Trotta, M.C.; D’Amico, M.; Petre, A.; Lile, I.; Hermenean, A.; Fratila, A. Challenges and Innovations in Alveolar Bone Regeneration: A Narrative Review on Materials, Techniques, Clinical Outcomes, and Future Directions. Medicina 2024, 61, 20. [Google Scholar] [CrossRef]
- Raabe, C.; Cafferata, E.A.; Couso-Queiruga, E.; Chappuis, V.; Ramanauskaite, A.; Schwarz, F. Impact of Two Flap Advancement Techniques and Periosteal Suturing on Graft Displacement During Guided Bone Regeneration. Clin. Implant. Dent. Relat. Res. 2025, 27, e13434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alarcón-Apablaza, J.; Godoy-Sánchez, K.; Jarpa-Parra, M.; Garrido-Miranda, K.; Fuentes, R. Tissue Sources Influence the Morphological and Morphometric Characteristics of Collagen Membranes for Guided Bone Regeneration. Polymers 2024, 16, 3499. [Google Scholar] [CrossRef]






| Horizontal Bone Width (mm) | Vertical Bone (mm) | ||||
|---|---|---|---|---|---|
| Baseline | At 8 Months | Augmentation Obtained | Defect Depth | Augmentation Obtained at 8 Months | |
| Patient #1 | 1.2 | 8.3 | 7.1 | −9.8 | 11.9 |
| Patient #2 | 2 | 7.8 | 5.8 | −11.2 | 12.5 |
| Patient #3 | 1.5 | 8 | 6.5 | −9.3 | 9 |
| Patient #4 | 0 | 7.2 | 7.2 | −6.8 | 8 |
| Patient #5 | 2.5 | 6.8 | 4.3 | −6 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, R.; Bambini, F.; Dellavia, C.; Henin, D.; Memè, L. ‘Lamina External Graft Overlay’: The Use of Segmented Xenogenic Bone Sheets in the Reconstruction of 3D Bone Defects. Medicina 2025, 61, 683. https://doi.org/10.3390/medicina61040683
Rossi R, Bambini F, Dellavia C, Henin D, Memè L. ‘Lamina External Graft Overlay’: The Use of Segmented Xenogenic Bone Sheets in the Reconstruction of 3D Bone Defects. Medicina. 2025; 61(4):683. https://doi.org/10.3390/medicina61040683
Chicago/Turabian StyleRossi, Roberto, Fabrizio Bambini, Claudia Dellavia, Dolaji Henin, and Lucia Memè. 2025. "‘Lamina External Graft Overlay’: The Use of Segmented Xenogenic Bone Sheets in the Reconstruction of 3D Bone Defects" Medicina 61, no. 4: 683. https://doi.org/10.3390/medicina61040683
APA StyleRossi, R., Bambini, F., Dellavia, C., Henin, D., & Memè, L. (2025). ‘Lamina External Graft Overlay’: The Use of Segmented Xenogenic Bone Sheets in the Reconstruction of 3D Bone Defects. Medicina, 61(4), 683. https://doi.org/10.3390/medicina61040683











