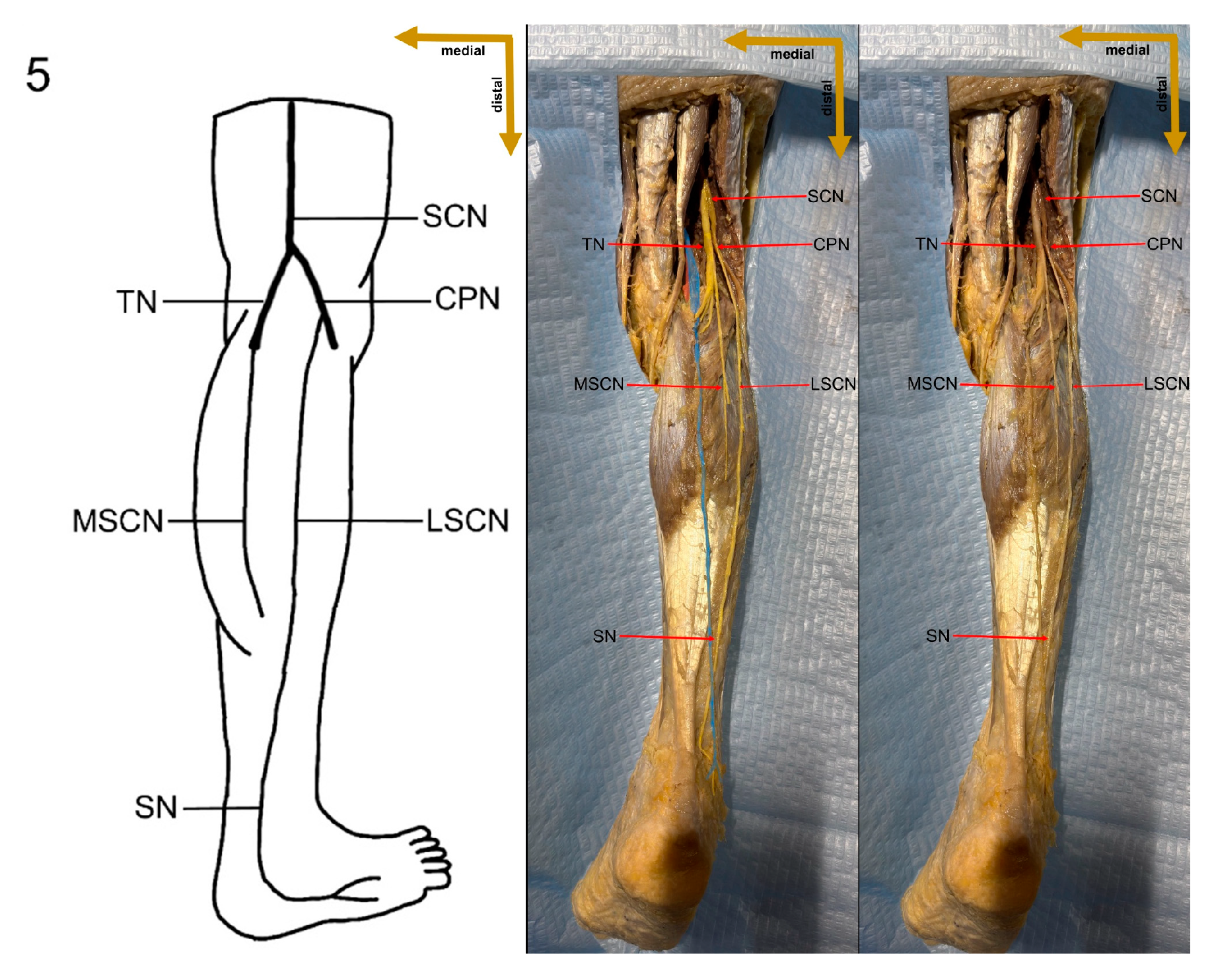
1. Introduction
The sural nerve (SN) is a significant pure sensory cutaneous nerve that innervates the base of the fifth metatarsal, as well as the lateral ankle and foot [
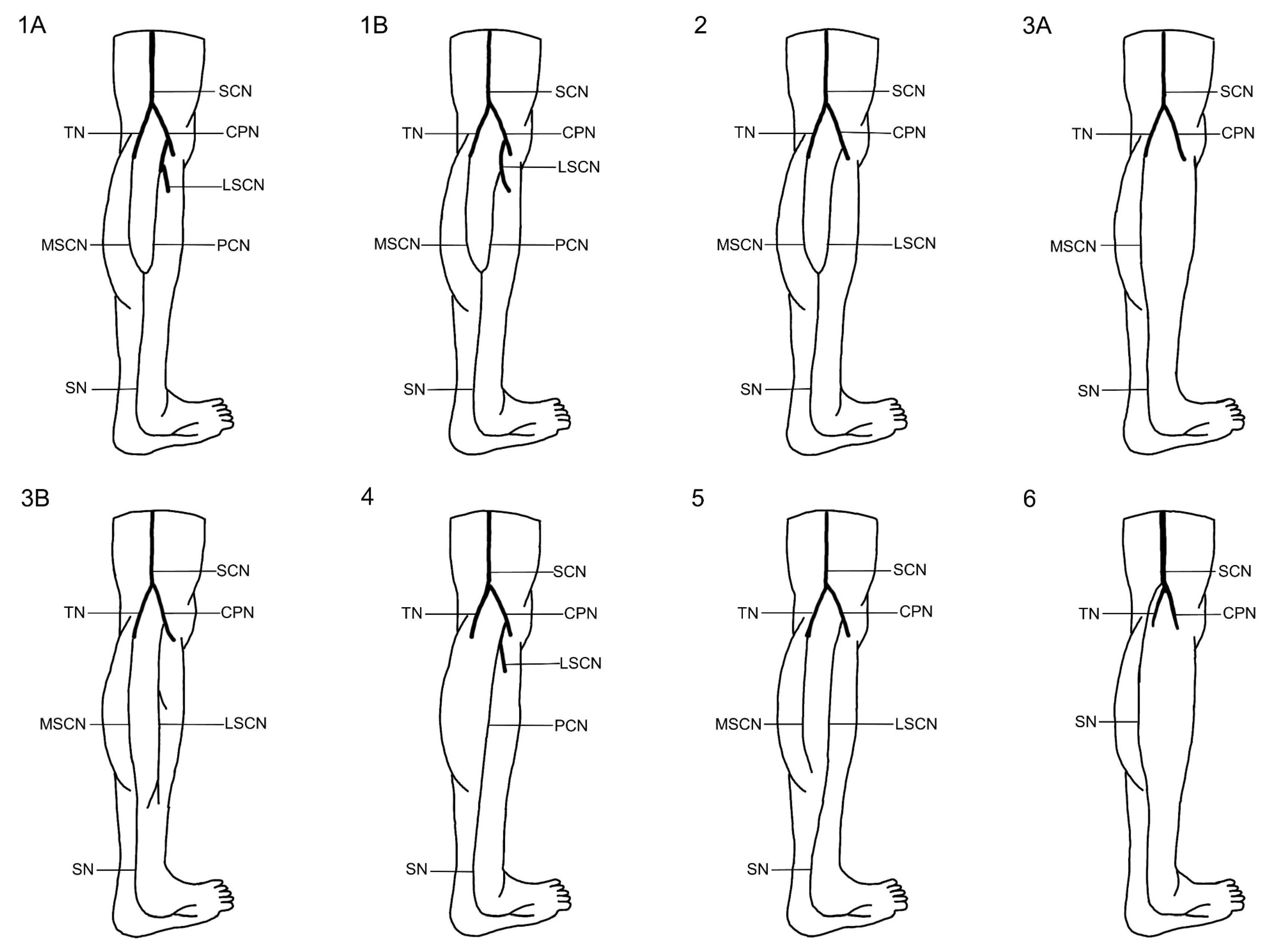
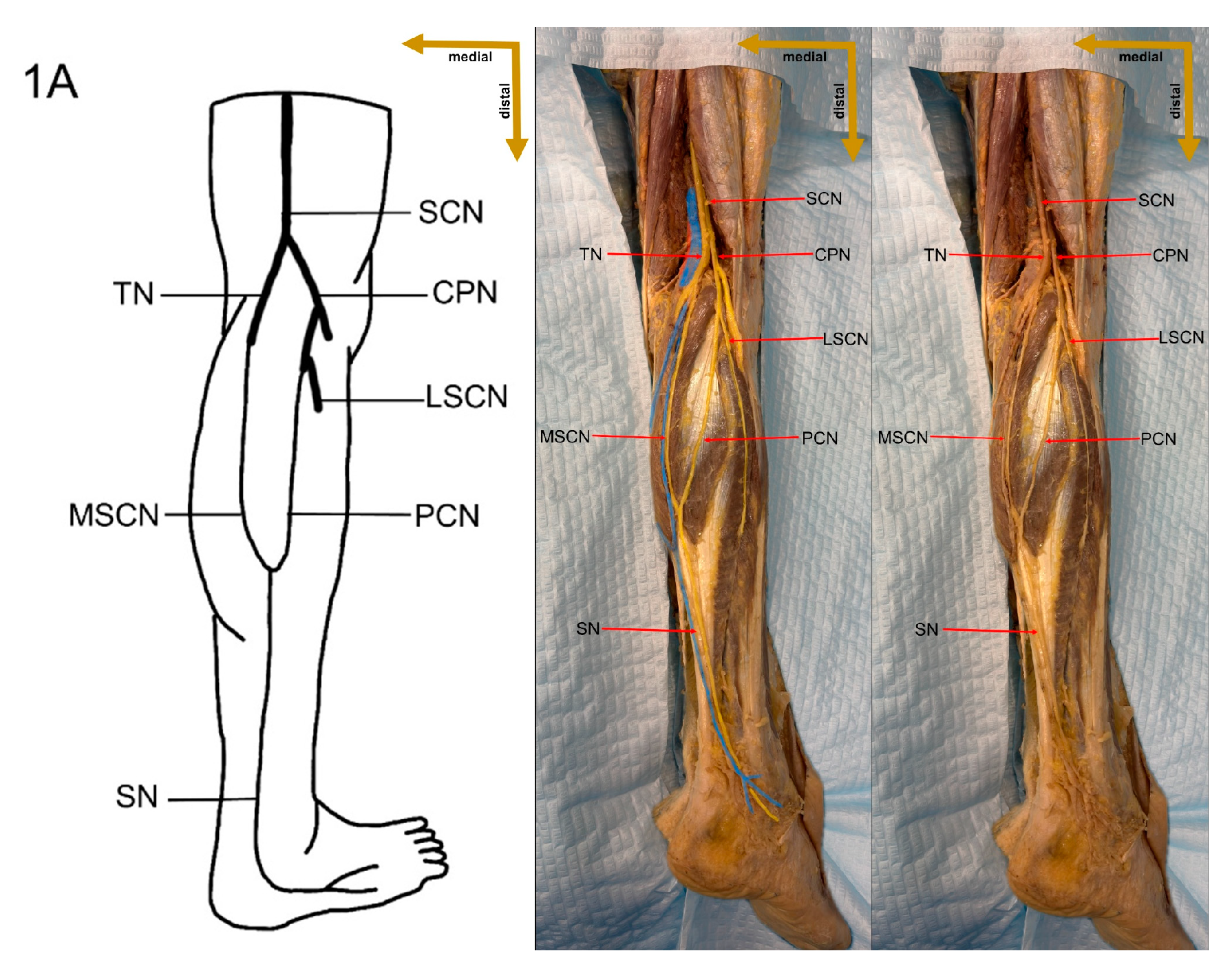
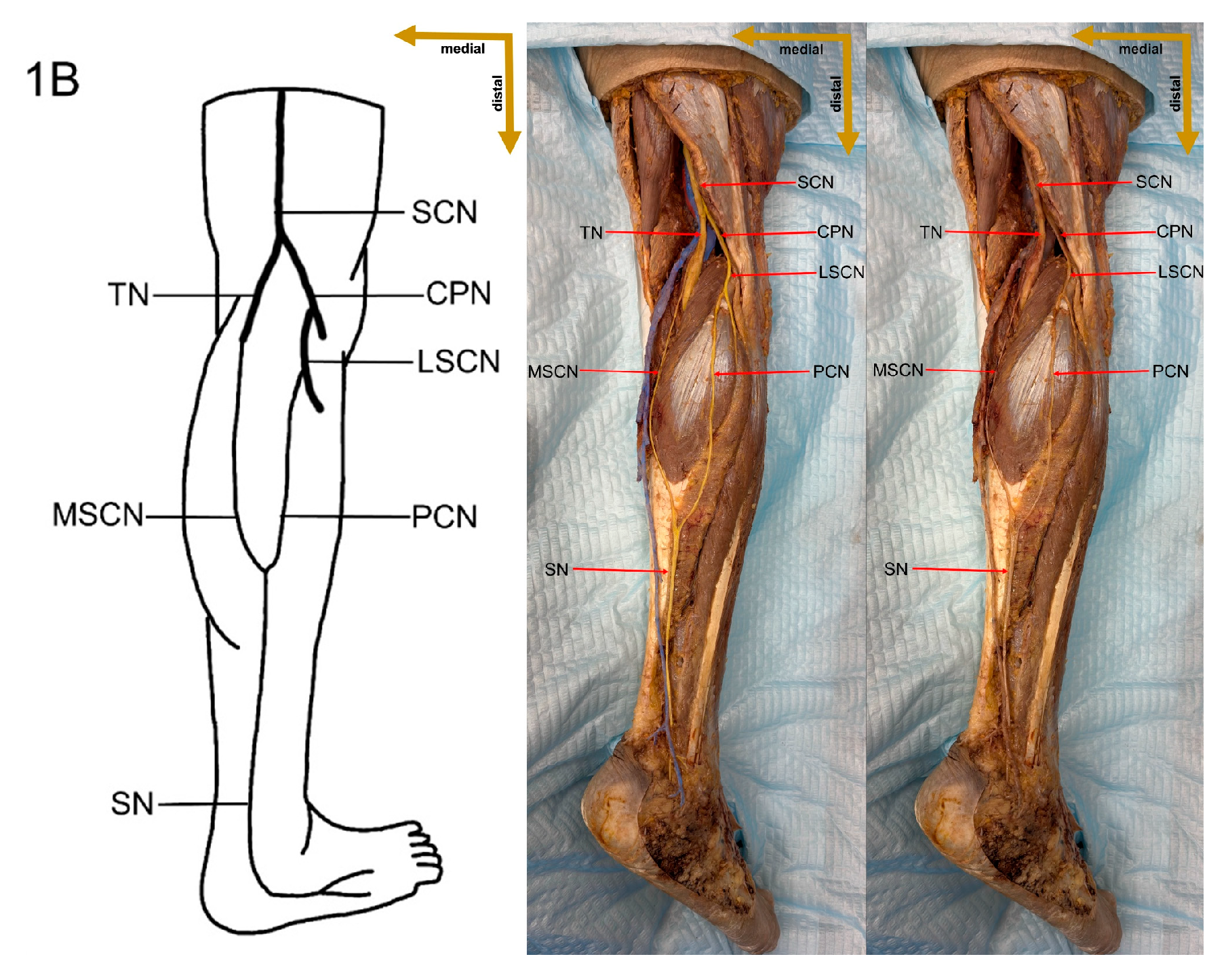
1]. It is formed by contributions from the tibial nerve (TN) and the common peroneal nerve (CPN), with several distinct variations in its formation reported in the literature [
2,
3,
4]. The peroneal communicating branch (PCB) of the common peroneal nerve and the medial sural cutaneous nerve (MSCN), a branch of the tibial nerve, are two major components that frequently unite to form the SN [
5,
6,
7,
8,
9]. However, in some cases, the SN may arise directly from the MSCN, the lateral sural cutaneous nerve (LSCN), or as a fusion of the MSCN and LSCN, branching from the common peroneal nerve [
7]. The sural nerve is typically formed within the anatomical region extending from the popliteal fossa to the level of the ankle joint [
10]. It runs along the lateral border of the Achilles tendon (TC), near the small saphenous vein (SSV), and between the heads of the gastrocnemius muscle [
11,
12]. As it courses posteriorly and inferiorly toward the lateral malleolus, its superficial location becomes particularly relevant for surgical procedures. Due to its predictable anatomical landmarks and accessibility, the sural nerve is often harvested near the lateral malleolus for nerve grafting. Despite its relatively consistent trajectory, the sural nerve exhibits considerable anatomical variability in its origin and formation. Multiple classification systems have identified at least four distinct formation patterns, which influence its course, branching patterns, and potential clinical implications. These variations must be carefully considered in both surgical and diagnostic procedures to minimize complications and optimize patient outcomes [
13,
14,
15].
Sural nerve pathology often presents with nonspecific symptoms, such as hypoesthesia and neuropathic pain, significantly impacting the quality of life [
16,
17]. Although high-frequency ultrasound is commonly used for SN imaging, the nerve’s small size and complex fascicular structure pose challenges. Due to the anatomical variability in the sural nerve (SN) at its origin, locating it distally and tracing it proximally is often a more effective approach for identification [
18]. Magnetic resonance imaging (MRI) provides better visualization of the SN’s anatomical course, making it a valuable tool for surgical planning. However, MRI has limitations in clearly differentiating fascicular structures. In contrast, micro-CT, when combined with contrast agents, allows for high-resolution three-dimensional reconstruction, offering superior detail in fascicular organization. These advanced imaging techniques play a crucial role in early diagnosis, guiding treatment, and preventing iatrogenic injury during surgical procedures [
19]. One of the surgical procedures frequently associated with SN injury involves minimally invasive Achilles tendon repair. The incidence of sural nerve paresthesia following surgical intervention for Achilles tendon repair varies significantly, ranging from 1.7% to 23% in percutaneous procedures. However, when all surgical techniques are considered, the overall occurrence of paresthesia can be as high as 60%. Postoperative sural nerve dysfunction may result from either traction or sharp injury, often occurring during the dissection of the Achilles tendon’s connective tissue (peritendineum), at the site of the stab incision, or during the creation of the canal through the lateral calcaneus. Notably, both open and percutaneous repair techniques carry a risk of sural nerve damage. However, percutaneous suturing is the primary cause of nerve entrapment in these cases, highlighting the need for careful surgical planning and technique selection to minimize the risk of complications [
20].
The sural nerve is widely recognized by surgeons as a preferred site for harvesting autologous nerve grafts [
21]. It is particularly advantageous for nerve grafting due to its considerable length, expandable nature, and optimal caliber for revascularization in interfascicular graft replacement [
22]. These characteristics make the sural nerve an excellent choice for repairing nerve defects resulting from traumatic injuries. In clinical practice, the sural nerve is typically identified in relation to the small saphenous vein, which serves as a reliable anatomical landmark. However, due to the inherent variability in its formation, surgeons may need to assess both legs to determine the most suitable graft specimen. This ensures optimal graft selection and improves surgical outcomes. Sural nerve grafts are especially valuable in reconstructive procedures, playing a crucial role in restoring muscle tone in cases of facial nerve palsy. Their effectiveness in nerve repair highlights the importance of understanding sural nerve anatomy and its variations to enhance surgical precision and patient recovery [
23].
Sural nerve biopsy is a valuable diagnostic tool for assessing peripheral neuropathies, particularly in identifying underlying inflammatory mechanisms [
24]. While often unnecessary when neuropathy can be diagnosed through clinical and laboratory tests, it becomes essential in cases of suspected vasculitis or unexplained peripheral neuropathy [
25]. The sural nerve is an ideal candidate for biopsy due to its superficial location, predictable sensory distribution, and purely sensory function, which minimize the risk of motor deficits and ulceration [
26]. The procedure is performed with the patient in a supine position under general anesthesia. A small incision is made approximately 10 cm below the popliteal fossa, and a 2–3 cm segment of the nerve is excised. The proximal portion of the nerve is then implanted into the gastrocnemius muscle to prevent the formation of painful neuromas [
27]. A histopathological analysis of the excised nerve segment focuses on the examination of axons, myelin, and diagnostic lesions, such as amyloid deposits, sarcoid tubercles, and vasculitis. Inflammatory neuropathies are often characterized by pathological changes in endothelial cells and pericytes, which play a crucial role in the diagnostic process. Sural nerve biopsy is particularly important in cases of vascular neuropathy, where vasculitis leads to damage to the vasa nervorum, resulting in ischemia and symptoms such as pain, weakness, and sensory deficits [
28,
29,
30,
31]. Vasculitic neuropathy is typically marked by fibrinoid necrosis, asymmetric axonal loss, and perivascular microfasciculation [
32].
In chronic inflammatory demyelinating polyneuropathy (CIDP), biopsy is generally reserved for atypical cases or when differentiation from vasculitis or amyloid deposition is necessary. CIDP typically presents as either symmetric or multifocal neuropathy, characterized by progressive weakness and sensory deficits. Histopathological findings in CIDP include axonal loss, inflammatory infiltrates, onion bulb formations, and segmental demyelination [
32]. In summary, the sural nerve exhibits considerable anatomical variation, not only in its formation but also in its trajectory and branching patterns. These variations have significant clinical implications, particularly in surgical procedures, diagnostic imaging, and nerve grafting. Research has demonstrated that the frequency and types of sural nerve formation variations differ across populations from various geographical regions and ethnic backgrounds, likely due to genetic and developmental factors [
33]. Understanding these anatomical differences is crucial for tailoring surgical approaches, minimizing the risk of iatrogenic injuries, and improving diagnostic accuracy. In nerve grafting procedures, recognizing regional anatomical variability ensures optimal donor site selection and reduces the likelihood of complications. Additionally, population-specific anatomical studies play a vital role in refining nerve conduction studies and enhancing the interpretation of biopsy results in neuropathy cases. Therefore, comprehensive anatomical research across diverse populations is essential for advancing clinical outcomes, improving patient safety, and optimizing both surgical and diagnostic strategies [
33].
4. Discussion
The sural nerve shows significant anatomical variation across populations, as demonstrated by our study and previous research. In our sample of 18 limbs from Lithuania, Type 1 formation was observed in 5 limbs (27.8%), which is considerably lower precentage than that reported by Huelke (284 of 352 limbs, 80.7%) [
5] and is lower precentage than findings from Serbia (117 of 200 limbs, 58.5%) [
6] and India (36 of 50 limbs, 72%) [
8], but similar precentage to Shankar et al. (30 of 102 limbs, 29.4%) [
7]. Type 2 was identified in 3 limbs (16.6%), a rate lower precentage than that observed in Thailand (102 of 152 limbs, 67.1%) [
10] and Korea (20 of 26 limbs, 76.9%) [
34], yet comparable to reports from Serbia (18 of 200 limbs, 9%) [
6] and the USA (18 of 208 limbs, 8.7%) [
9]. Notably, Type 3 was found in 8 limbs (44.4%), which exceeds the precentage frequencies reported in Serbia (52 of 200 limbs, 26%) [
6], India (27 of 102 limbs, 26.5%) [
7], and the USA (72 of 208 limbs, 34.6%) [
9]. Additionally, Type 4 was present in 1 limb (5.6%), a higher precentage rate than that documented by Huelke (1 of 352 limbs, 0.3%) [
5] and Steele et al. (1 of 208 limbs, 0.5%) [
9] but lower precentage than the 22.5% observed by Shankar et al. (23 of 102 limbs) [
7]. Type 5 formation was also seen in 1 limb (5.6%), which is higher precentage than the rates from Serbia (3 of 200 limbs, 1.5%) [
6] and the USA (1 of 208 limbs, 0.5%) [
9], while Type 6 was absent, consistent with several studies, although Shankar et al. reported it in 14 of 102 limbs (13.7%) [
7]. Types 7 and 8 were not observed, aligning with the findings of Huelke [
5] and Kavyashree et al. [
8], whereas Steele et al. [
9] documented these types in 30 of 208 limbs (14.4%) and Pyun and Kwon documented them in 2 of 26 limbs (7.7%) [
34]. These inter-study differences may reflect genetic SN formation variations between populations, as genetic predispositions can influence peripheral nerve morphology (
Table 7) [
7,
10].
Analysis of the site of sural nerve formation across the leg reveals notable inter-study differences that may reflect population-based anatomical variability. In our study, SN formation in the upper quarter of the leg was observed in 1 of 18 limbs (5.6%), which is slightly higher precentage than that reported by Ugrenovic et al. [
6] (3 of 200 limbs, 1.6%) and Kavyashree et al. [
8] (1 of 50 limbs, 2.8%), yet much lower precentage than that documented by Huelke (86 of 352 limbs, 24.3%) [
5]. In the second quarter of the leg, our findings showed SN formation in 7 of 18 limbs (38.8%), a frequency higher precentage than that noted by Huelke [
5] (59 of 352 limbs, 16.9%) and Ugrenovic et al. [
6] (56 of 200 limbs, 28.0%), and considerably higher precentage than Kavyashree et al. (3 of 50 limbs, 5.6%) [
8]. In the third quarter, which encompasses the popliteal fossa and proximal calf region, our study reported SN formation in 9 of 18 limbs (50.0%); this aligns with the findings of Ugrenovic et al. [
6] (130 of 200 limbs, 64.8%) and P’an MT [
36] (147 of 286 limbs, 51.5%), while exceeding Huelke’s [
5] report (129 of 352 limbs, 36.6%) and Kavyashree et al.’s observation (17 of 50 limbs, 33.3%) [
8]. Finally, in the fourth quarter of the leg, corresponding to the distal calf and ankle region, our study found SN formation in 1 of 18 limbs (5.6%), a value matching that of Ugrenovic et al. [
6] (11 of 200 limbs, 5.6%), but substantially lower precentage than those reported by Kavyashree et al. [
8] (29 of 50 limbs, 58.3%) and Huelke (78 of 352 limbs, 22.2%) (
Table 8) [
5].
Table 9 compares the symmetry of sural nerve formation between legs across different populations. In the USA, Huelke (1957) reported symmetry in 291 of 352 cases (82.7%) and asymmetry in 61 of 352 cases (17.3%) [
5]. In Serbia, Urgenovic et al. (2005) found symmetry in 124 of 200 cases (62%) and asymmetry in 76 of 200 cases (38%) [
6]. In India, Shankar et al. (2010) observed symmetry in 62 of 102 cases (60.8%) and asymmetry in 40 of 102 cases (39.2%) [
7], while Kavyashree et al. (2013) documented symmetry in 30 of 50 cases (60%) and asymmetry in 20 of 50 cases (40%) [
8]. In Thailand, Mahakkanukrauh and Chomsung (2002) reported symmetry in 30 of 152 cases (19.7%) and asymmetry in 122 of 152 cases (80.3%) [
10]. In China, P’an MT (1939) noted symmetry in 240 of 286 cases (83.9%) and asymmetry in 46 of 286 cases (16.1%) [
36]. In our present study in Lithuania, symmetry was observed in 10 of 18 cases (55.6%) and asymmetry in 8 of 18 cases (44.4%). These findings indicate that our Lithuanian sample shows a relatively high prevalence of asymmetry compared to most previous studies—except for the Thailand study [
10].
Table 10 shows a comprehensive comparison of sural nerve length across different geographical regions based on various cadaveric studies. Steele et al. reported the longest SN length in the USA (32.97 ± 14.12 cm), suggesting notable anatomical variation among populations [
9]. In contrast, Sekiya and Kumaki in Japan recorded the shortest SN length (12.4 ± 6.06 cm) [
37]. Intermediate values were observed in studies from India and Thailand, with Kavyashree et al. documenting a mean SN length of 19.02 ± 7.66 cm in India, and Mahakkanukrauh and Chomsung reporting 14.41 ± 5.79 cm in Thailand [
8,
10]. In our present study in Lithuania, the mean SN length was 21.99 ± 6.27 cm, a value that is closer to the Indian dataset but still longer than those reported in Japan and Thailand (
Table 10).
The mean sural nerve diameter and standard deviation across different populations based on various cadaveric studies is shown in
Table 11.
The highest mean SN diameter was observed in a study conducted in Thailand by Mahakkanukrauh and Chomsung (2002) (3.61 ± 0.07 mm), indicating relatively low variability in SN thickness [
10]. Steele et al. (2021) documented a mean SN diameter of 2.74 ± 0.93 mm in a USA-based study, demonstrating a wider range of individual variation (
Table 11) [
9].
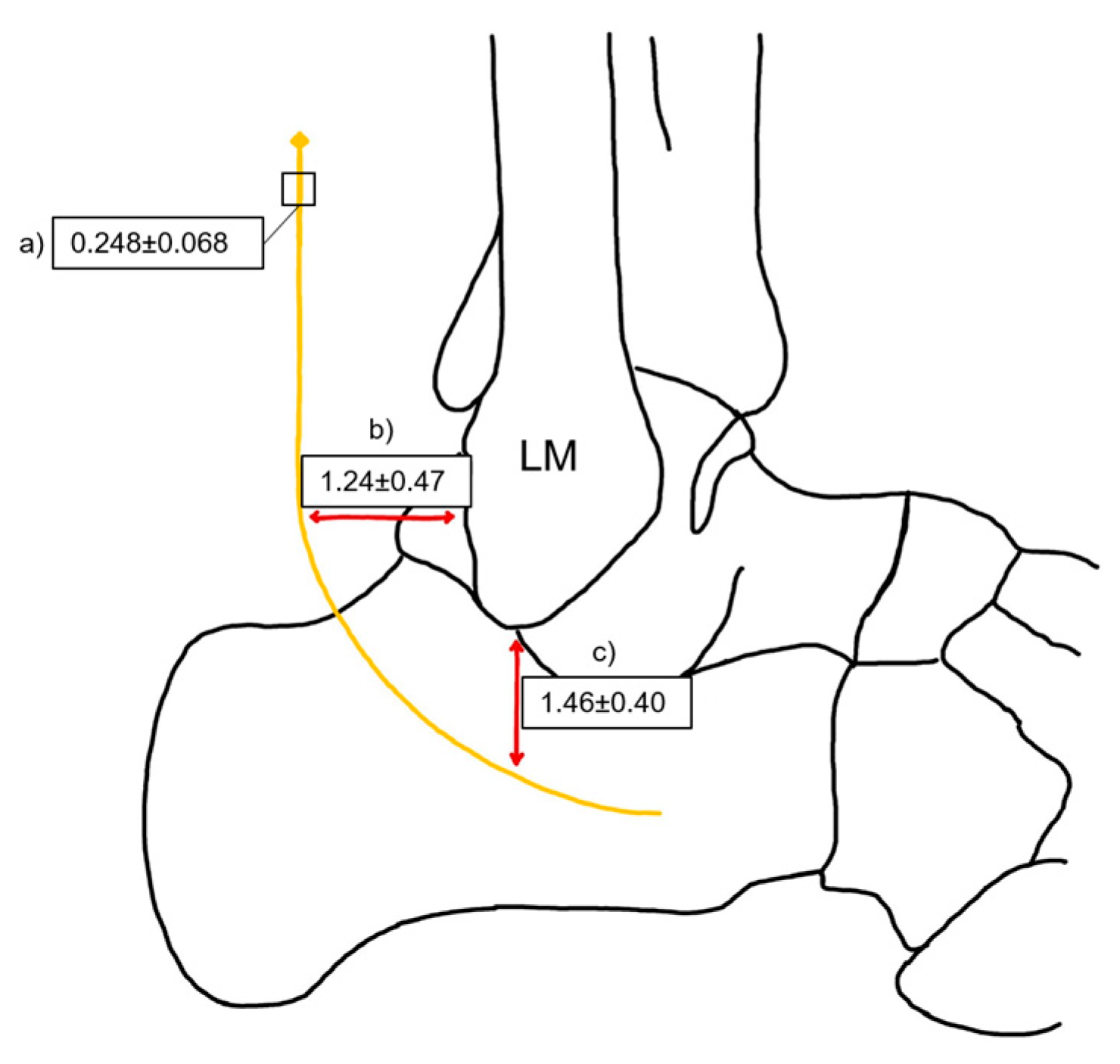
The present study, conducted in Lithuania, reported an intermediate mean SN diameter of 2.48 ± 0.68 mm. This finding suggests that the SN morphology in the Lithuanian population is closer to that observed in the USA, but smaller than that documented in the Thailand population (
Table 11) [
9,
10].
The distance between the sural nerve and key anatomical landmarks of the lateral malleolus across different populations is shown in
Table 12. Notably, the distance from the SN to the posterior border of the LM varies slightly, with the highest mean value recorded in the USA (1.7 ± 0.7 cm) and the lowest in Turkey and Lithuania (1.3 cm) [
9,
35]. Similarly, the distance from the SN to the distal tip of the LM shows regional differences, with England reporting the highest mean (2.3 ± 0.2 cm), while Lithuania and Turkey present the shortest values (1.5 ± 0.4 cm and 1.3 ± 0.7 cm, respectively) (
Table 12) [
38].
Understanding these variations in the sural nerve formation is crucial for several reasons. First, the SN plays a vital role in the diagnosis and management of diabetic peripheral neuropathy (DPN). It is a common complication of diabetes, often involving the SN and associated with sensory deficits, neuropathic pain, and an increased risk of non-traumatic lower limb amputations [
39,
40]. While nerve conduction studies remain the gold standard for DPN diagnosis, imaging modalities such as high-frequency ultrasound and SN biopsy offer valuable insights into the morphological changes that occur in neuropathic conditions [
41,
42]. The early and accurate identification of these changes is critical for timely intervention and improved patient outcomes. Given the clinical significance of the SN, it is essential to perform tissue biopsies when necessary and to account for potential variations in its length, diameter, and localization across different populations. These anatomical differences may influence biopsy accuracy, nerve conduction studies, and surgical interventions, emphasizing the need for population-specific reference data. A comprehensive understanding of these parameters enhances diagnostic precision and ensures more effective treatment strategies, particularly in diabetic patients who are at high risk for neuropathic complications [
43,
44].
Furthermore, isolated SN neuropathy (mononeuropathy) and SN entrapment are clinical entities that underscore the importance of detailed anatomical knowledge. The SN’s superficial location makes it particularly vulnerable to trauma, compression from space-occupying lesions, and iatrogenic injury during surgical procedures [
45,
46]. In cases of mononeuropathy, patients may present with pain, numbness, and paresthesia, which can mimic other neurological conditions [
47]. Sural nerve entrapment similarly manifests with pain, burning, tenderness, and abnormal sensations in the posterolateral region of the distal leg and the lateral aspect of the foot extending to the fifth digit [
48]. Precise anatomical mapping, therefore, facilitates correct diagnosis and targeted treatment.
The sural nerve is widely utilized in nerve grafting due to its favorable anatomical properties, making it a preferred donor nerve in reconstructive surgeries. Its straight course, minimal branching, and optimal caliber contribute to successful nerve regeneration [
49]. Sural nerve grafting is particularly valuable for repairing long nerve defects and is often chosen over other autologous grafts for its specific advantages [
50]. Nerve grafts are typically required for segmental nerve loss exceeding 1–2 cm, as a careful mobilization of nerve stumps can often reduce smaller gaps. Synthetic conduits, however, generally provide reliable results only for defects smaller than 5 mm [
51]. The success of nerve grafting or direct nerve repair depends on the presence of viable proximal and distal nerve stumps. When a proximal stump is unavailable, such as in skull-base injuries, a nerve transfer to the distal stump may provide a more effective reconstructive approach [
52,
53]. The considerable length of the sural nerve makes it particularly valuable for facial nerve reinnervation, allowing cross-face grafting from a healthy facial nerve to the paralyzed side [
54,
55]. Additionally, sural nerve grafts are frequently used in nerve elongation procedures, particularly in cases of brachial plexus injuries [
56].
The effectiveness of sural nerve grafting is influenced by several anatomical factors, including nerve length, diameter, and branching pattern. Our study highlights significant variability in SN morphology across different populations, with notable differences in both length and diameter. These parameters are crucial in reconstructive surgery, as the harvested nerve must be appropriately matched to the defect to ensure optimal functional recovery. In our study, the mean sural nerve length (21.99 ± 6.27 cm) falls within an intermediate range compared to previous reports, suggesting that graft suitability may differ among populations. Likewise, the diameter of the SN, which averaged 2.48 ± 0.68 mm in our sample, plays a key role in graft integration and revascularization. A thicker nerve may provide a more robust scaffold for axonal regeneration, while a thinner nerve may be more prone to atrophy or incomplete reinnervation [
57]. These findings underscore the importance of considering population-specific anatomical variations when selecting donor nerves for grafting procedures.
Beyond limb and facial nerve repair, sural nerve grafts have also been used in corneal neurotization, particularly in patients with neurotrophic keratitis—a condition characterized by corneal anesthesia, a loss of the blink reflex, and reduced tear production, leading to ulceration, scarring, and eventual corneal opacification. In such cases, sural nerve grafts, along with the great auricular nerve, have been used to restore corneal sensation by connecting a functional sensory nerve, such as the supratrochlear or supraorbital nerve, to the affected cornea. This enables axonal regeneration and sensory restoration [
58]. Given the variability in SN morphology observed in our study, the success of such procedures may be influenced by regional differences in nerve structure. For instance, variations in SN diameter could affect the density of regenerating axons and the overall sensory recovery in corneal neurotization [
59]. These diverse applications highlight the clinical significance of the sural nerve and emphasize the necessity of understanding its anatomical variations to improve surgical planning and patient outcomes.
The Limits of the Study
One of the primary limitations of this study is the small sample size. A total of nine cadavers (18 limbs) were examined, which restricts the generalizability of the findings to the broader Lithuanian population and other ethnic groups. Comparative studies in the literature typically include a minimum of 25 cadavers, providing a higher level of evidence and greater statistical power. Although notable differences in sural nerve formation were observed within the Lithuanian population, the limited sample size reduces the reliability of these findings and precludes definitive conclusions. Future studies with larger and more diverse samples are necessary to validate these results and enhance their applicability.
Another limitation of this study is the gender imbalance in the sample, which included eight female cadavers and only one male cadaver. This uneven distribution prevents a meaningful analysis of sex differences in sural nerve morphology. While a direct comparison was possible, interpreting the results in this way would be inappropriate. Instead, the study presents morphological data that future researchers can use for comparative analysis. To better understand potential gender-related differences in sural nerve formation, future studies should include a more balanced sample.
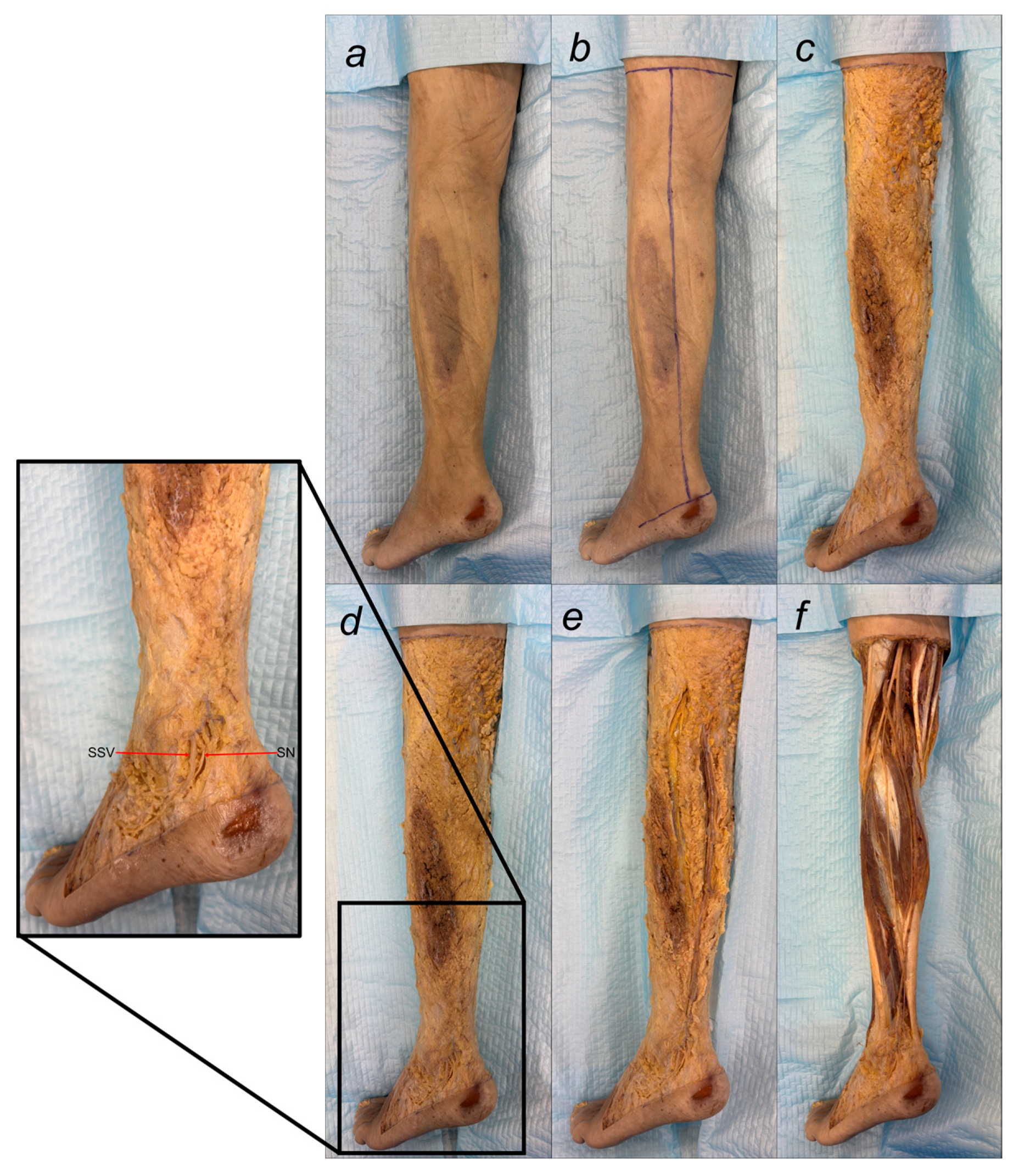
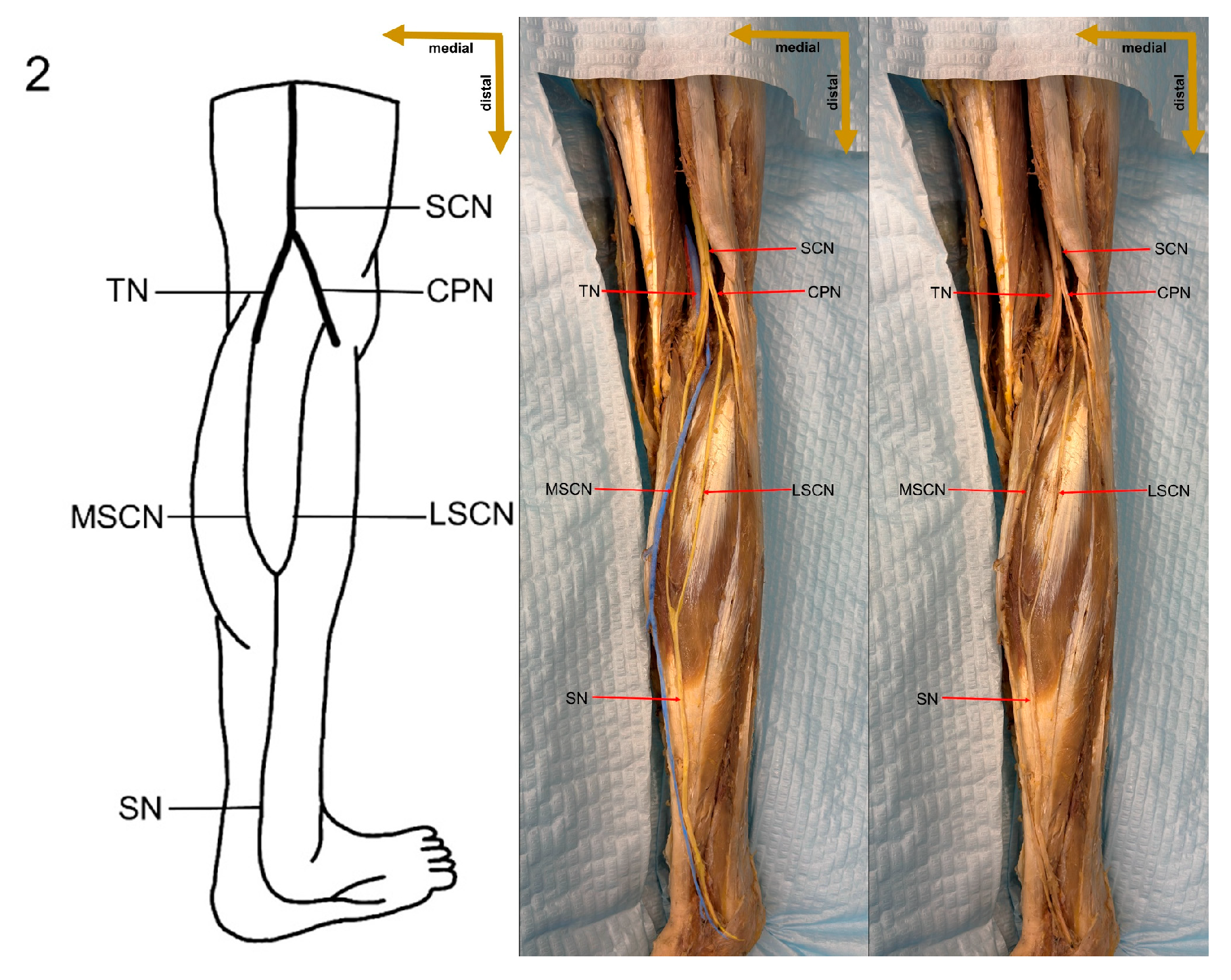
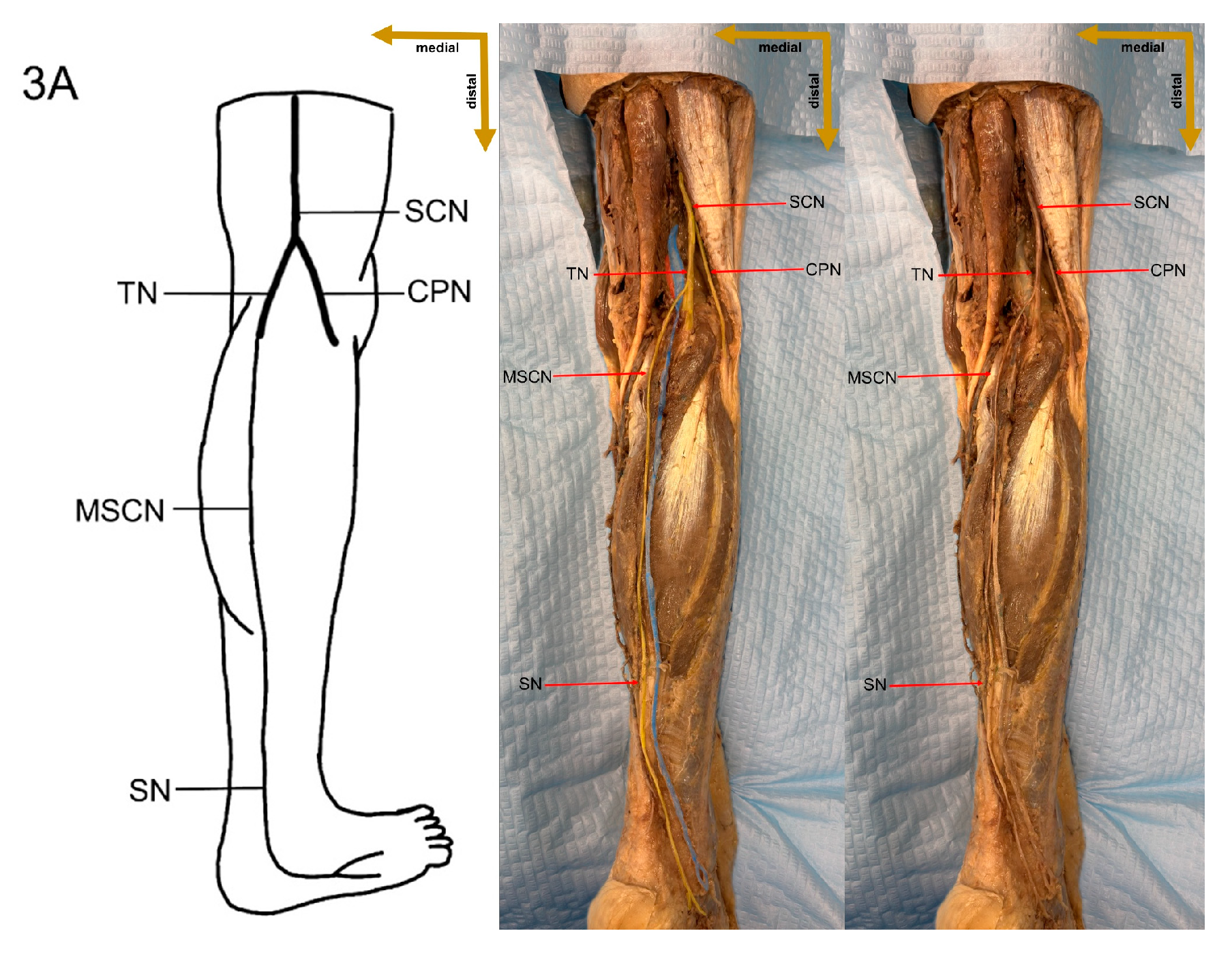
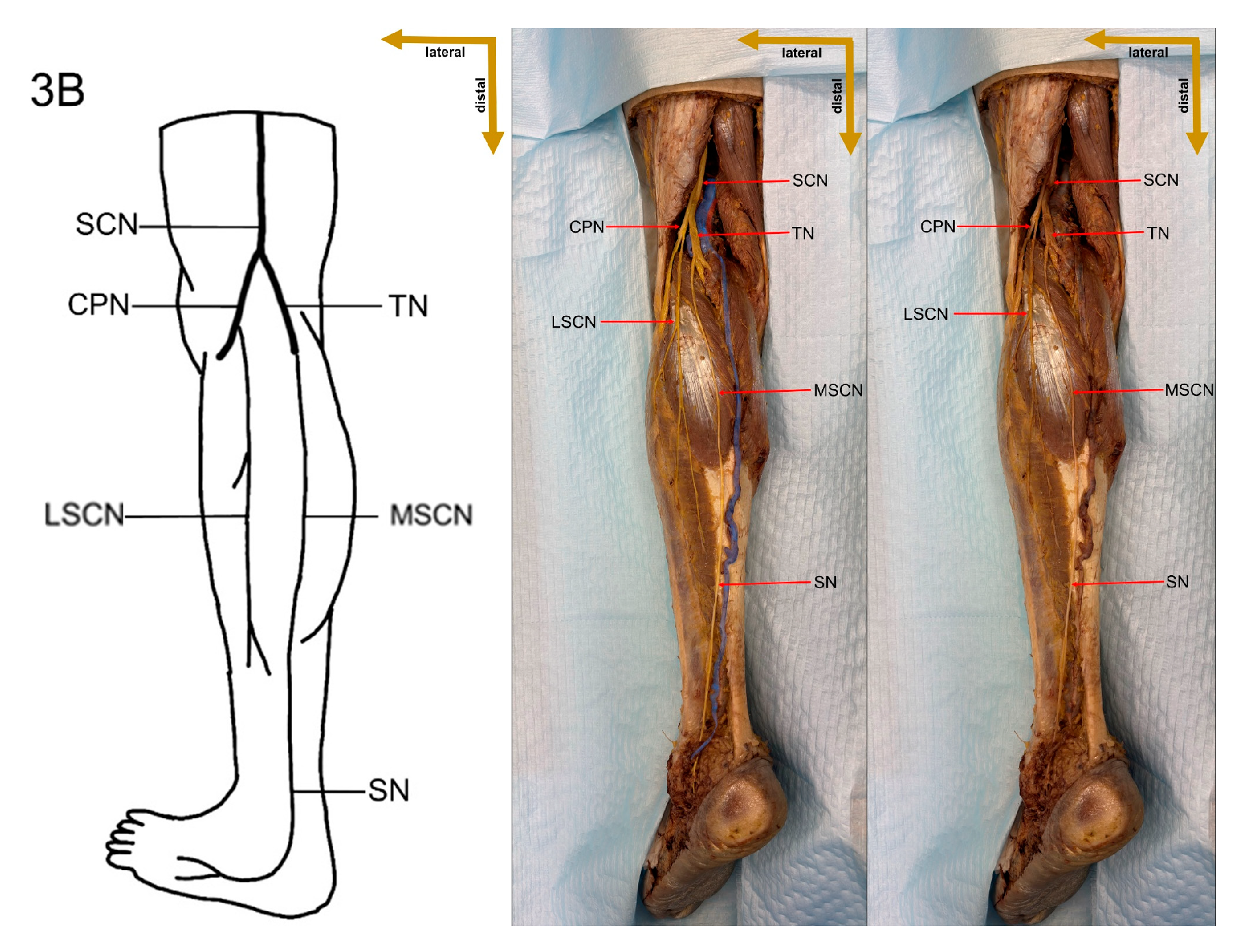
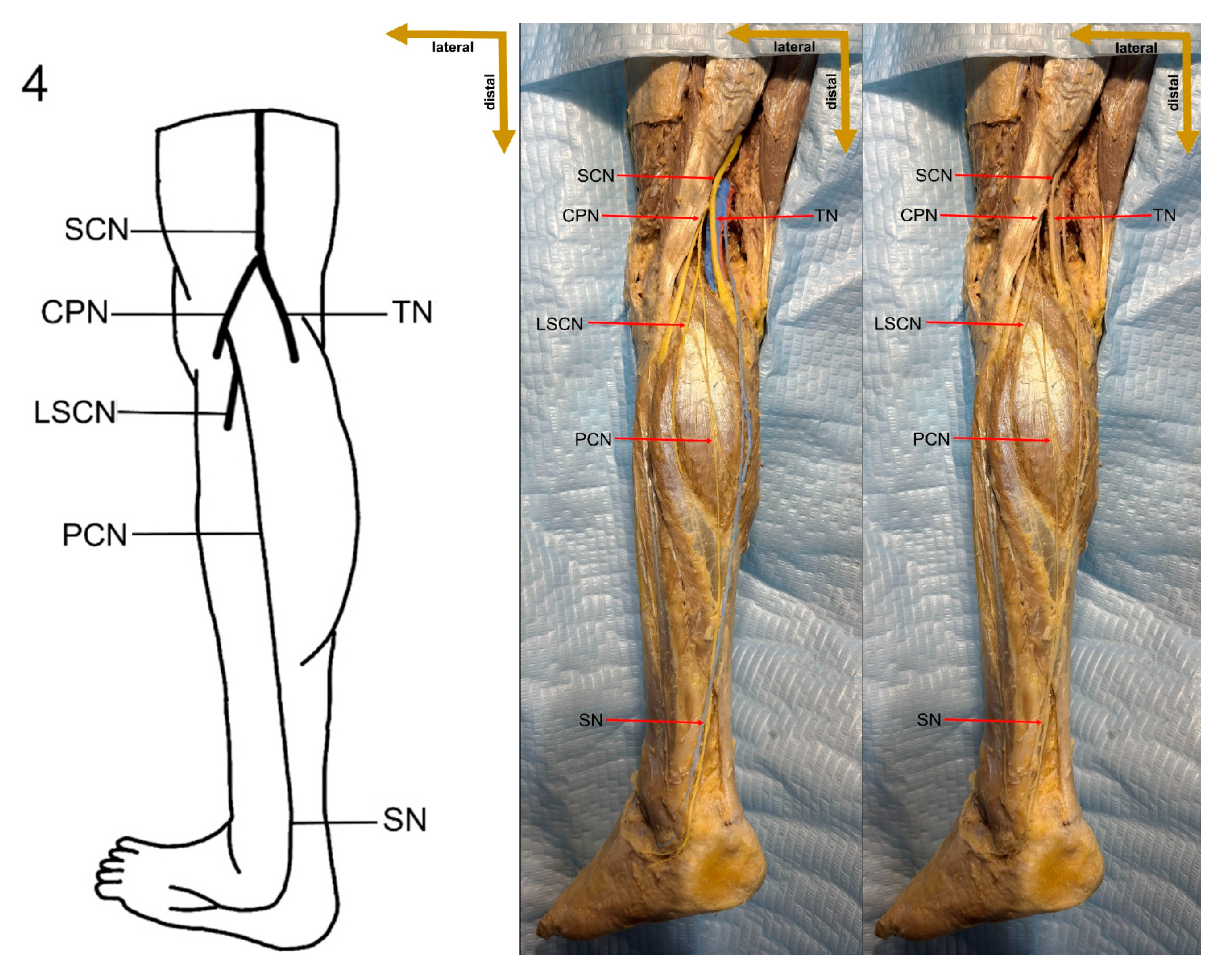
Despite these limitations, this study provides valuable preliminary data on sural nerve morphology in the Lithuanian population. This study is among the first to clearly define its methodology, providing both a detailed explanation and visual representation through images (
Figure 1). These images illustrate each step of the process, enhancing clarity and reproducibility. In addition to the theoretical description of sural nerve variations, the study presents these variations directly on cadaveric specimens. This visual approach allows future researchers to better understand the structures and how to identify them, improving accuracy in anatomical studies.