Quercetin as a Potential Therapeutic Agent for Malignant Melanoma—A Review of Current Evidence and Future Directions
Abstract
1. Introduction
2. Methods
3. Results
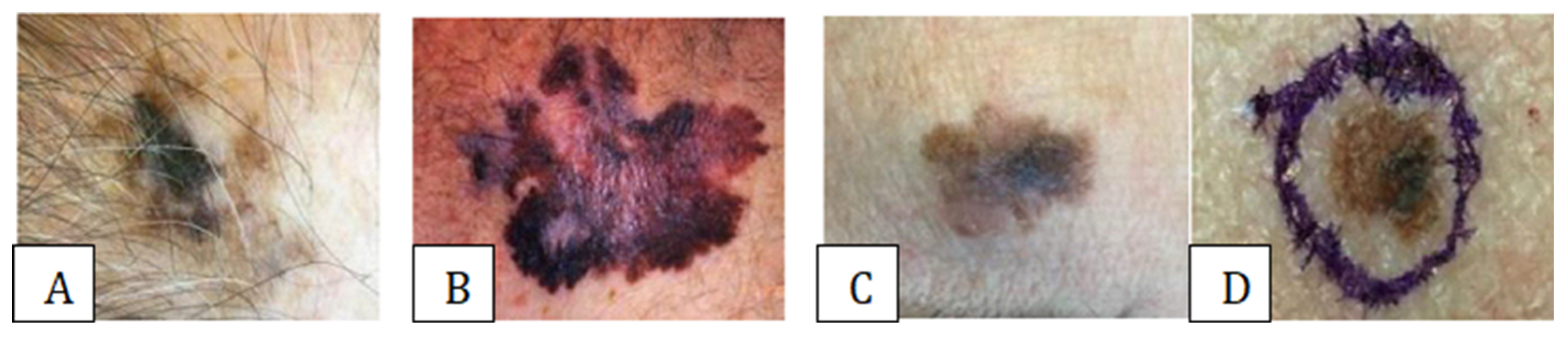
3.1. Assessing and Diagnosing Melanoma Types
3.2. Quercetin
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.-W.; Lee, S.-J.; Kim, W.-Y.; Seo, J.H.; Lee, H.-Y. How Can We Treat Cancer Disease Not Cancer Cells? Cancer Res. Treat. 2017, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Jain, A.; Aziz, M.; Misra, A. FNA Diagnosis of Malignant Melanoma-Recurrent and Metastatic Disease. BMJ Case Rep. 2012, 2012, bcr2012006887. [Google Scholar] [CrossRef]
- Mallardo, D.; Basile, D.; Vitale, M.G. Advances in Melanoma and Skin Cancers. Int. J. Mol. Sci. 2025, 26, 1849. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024. Available online: https://gco.iarc.fr/today (accessed on 23 February 2024).
- Slominski, R.M.; Kim, T.-K.; Janjetovic, Z.; Brożyna, A.A.; Podgorska, E.; Dixon, K.M.; Mason, R.S.; Tuckey, R.C.; Sharma, R.; Crossman, D.K.; et al. Malignant Melanoma: An Overview, New Perspectives, and Vitamin D Signaling. Cancers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Giubellino, A. The Current State of Treatment and Future Directions in Cutaneous Malignant Melanoma. Biomedicines 2022, 10, 822. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Y.; Liu, Q.; Zhang, H.; Jia, L.; Chai, Y.; Jiang, H.; Wu, M.; Li, Y. Global trends in melanoma burden: A comprehensive analysis from the Global Burden of Disease Study, 1990–2021. J. Am. Acad. Dermatol. 2025, 92, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.; Nabavi, S.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Leachman, S.A.; Cassidy, P.B.; Chen, S.C.; Curiel, C.; Geller, A.; Gareau, D.; Pellacani, G.; Grichnik, J.M.; Malvehy, J.; North, J.; et al. Methods of Melanoma Detection. In Melanoma; Springer: Cham, Switzerland, 2016; pp. 51–105. [Google Scholar]
- Cassano, R.; Cuconato, M.; Calviello, G.; Serini, S.; Trombino, S. Recent Advances in Nanotechnology for treating Melanoma. Molecules 2021, 26, 785. [Google Scholar] [CrossRef]
- Cazzato, G. Histopathological Diagnosis of Malignant Melanoma at the Dawn of 2023: Knowledge Gained and New Challenges. Dermatopathology 2023, 10, 91–92. [Google Scholar] [CrossRef]
- Wright, F.C.; Souter, L.H.; Kellett, S.; Easson, A.; Murray, C.; Toye, J.; McCready, D.; Nessim, C.; Ghazarian, D.; Hong, N.J.L.; et al. Primary Excision Margins, Sentinel Lymph Node Biopsy, and Completion Lymph Node Dissection in Cutaneous Melanoma: A Clinical Practice Guideline. Curr. Oncol. 2019, 26, 541–550. [Google Scholar] [CrossRef]
- Lazar, A.M.; Costea, D.O.; Popp, C.G.; Mastalier, B. Skin Malignant Melanoma and Matrix Metalloproteinases: Promising Links to Efficient Therapies. Int. J. Mol. Sci. 2024, 25, 7804. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Harris, Z.; Donovan, M.G.; Branco, G.M.; Limesand, K.H.; Burd, R. Quercetin as an Emerging Anti-Melanoma Agent: A Four-Focus Area Therapeutic Development Strategy. Front. Nutr. 2016, 3, 48. [Google Scholar] [CrossRef]
- Peng, D.; Chen, L.; Sun, Y.; Sun, L.; Yin, Q.; Deng, S.; Niu, L.; Lou, F.; Wang, Z.; Xu, Z.; et al. Melanoma Suppression by Quercein Is Correlated with RIG-I and Type I Interferon Signaling. Biomed. Pharmacother. 2020, 125, 109984. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.-H.; Tse, A.K.-W.; Kwan, H.-Y.; Yu, H.; Cheng, C.-Y.; Su, T.; Fong, W.-F.; Yu, Z.-L. Quercetin Exerts Anti-Melanoma Activities and Inhibits STAT3 Signaling. Biochem. Pharmacol. 2014, 87, 424–434. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Khuanekkaphan, M.; Netsomboon, K.; Fristiohady, A.; Asasutjarit, R. Development of Quercetin Solid Dispersion-Loaded Dissolving Microneedles and In Vitro Investigation of Their Anti-Melanoma Activities. Pharmaceutics 2024, 16, 1276. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Ju, W.S.; Kim, K.; Kim, J.; Yu, J.O.; Ryu, J.-S.; Kim, J.-S.; Lee, H.-A.; Koo, D.-B.; Choo, Y.-K. Quercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 5146. [Google Scholar] [CrossRef]
- Ha, A.T.; Rahmawati, L.; You, L.; Hossain, M.A.; Kim, J.-H.; Cho, J.Y. Anti-Inflammatory, Antioxidant, Moisturizing, and Antimelanogenesis Effects of Quercetin 3-O-β-D-Glucuronide in Human Keratinocytes and Melanoma Cells via Activation of NF-ΚB and AP-1 Pathways. Int. J. Mol. Sci. 2021, 23, 433. [Google Scholar] [CrossRef]
- Baghel, S.; Shrivastava, N.; Baghel, P.A.; Rajput, S. A Review of Quercetin: Antioxidant and Anticancer Properties. World J. Pharm. Pharm. Sci. 2012, 1, 146–160. [Google Scholar]
- Natarelli, N.; Aleman, S.J.; Mark, I.M.; Tran, J.T.; Kwak, S.; Botto, E.; Aflatooni, S.; Diaz, M.J.; Lipner, S.R. A Review of Current and Pipeline Drugs for Treatment of Melanoma. Pharmaceuticals 2024, 17, 214. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.Y.; Volate, S.R.; Wargovich, M.J. The Role of Phytochemicals in Inhibition of Cancer and Inflammation: New Directions and Perspectives. J. Food Compos. Anal. 2006, 19, 405–419. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Lee, Y.-H.; Sharma, A.R.; Park, J.-B.; Jagga, S.; Sharma, G.; Lee, S.-S.; Nam, J.-S. Quercetin Induces Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells through Modulation of Foxo3a Activity. Korean J. Physiol. Pharmacol. 2017, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and Apoptosis-Inducing Effects of Quercetin in Vitro and in Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Ren, M.-X.; Deng, X.-H.; Ai, F.; Yuan, G.-Y.; Song, H.-Y. Effect of Quercetin on the Proliferation of the Human Ovarian Cancer Cell Line SKOV-3 in Vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef]
- Roshanazadeh, M.; Babaahmadi Rezaei, H.; Rashidi, M. Quercetin Enhances the Suppressive Effects of Doxorubicin on the Migration of MDA-MB-231 Breast Cancer Cell Line. Int. J. Cancer Manag. 2021, 14, e119049. [Google Scholar] [CrossRef]
- Rezaie, F.; Mokhtari, M.J.; Kalani, M. Quercetin Arrests in G2 Phase, Upregulates INXS LncRNA and Downregulates UCA1 LncRNA in MCF-7 Cells. Int. J. Mol. Cell Med. 2021, 10, 208–216. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, X.; Mu, J.; Zou, Y.; Xu, L.; Chen, J.; Zhu, X.; Li, B.; Zeng, Z.; Wu, X.; et al. Quercetin Induces MGMT+ Glioblastoma Cells Apoptosis via Dual Inhibition of Wnt3a/β-Catenin and Akt/NF-ΚB Signaling Pathways. Phytomedicine 2023, 118, 154933. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Glycerin as Used in Cosmetics. Int. J. Toxicol. 2019, 38, 6S–22S. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Same, S.; Adibkia, K.; Zarza, K.; Shokri, J.; Taghaee, M.; Kouhsoltani, M. A Comparative Histological Study on the Skin Occlusion Performance of a Cream Made of Solid Lipid Nanoparticles and Vaseline. Res. Pharm. Sci. 2015, 10, 378–387. [Google Scholar]
- Feril, L.; Ogawa, K.; Watanabe, A.; Ogawa, R.; Cui, Z.-G.; Kondo, T.; Tachibana, K. Anticancer Potential of EDTA: A Preliminary in Vitro Study. Mathews J. Cancer Sci. 2017, 2, 1–3. [Google Scholar]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of Quercetin from Quercetin Aglycone and Rutin in Healthy Volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef]
- Costa, R.; Costa Lima, S.A.; Gameiro, P.; Reis, S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent Advances on the Improvement of Quercetin Bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Quercetin/β-Cyclodextrin Inclusion Complex Embedded Nanofibres: Slow Release and High Solubility. Food Chem. 2016, 197, 864–871. [Google Scholar] [CrossRef]
- Azzi, J.; Jraij, A.; Auezova, L.; Fourmentin, S.; Greige-Gerges, H. Novel Findings for Quercetin Encapsulation and Preservation with Cyclodextrins, Liposomes, and Drug-in-Cyclodextrin-in-Liposomes. Food Hydrocoll. 2018, 81, 328–340. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Zhu, Y.; Chen, Y.; Zou, L.; Liu, W.; Cheng, C.; Fu, D.; Liu, C. Enhancement of the Solubility, Stability and Bioaccessibility of Quercetin Using Protein-Based Excipient Emulsions. Food Res. Int. 2018, 114, 30–37. [Google Scholar] [CrossRef]
- Censi, R.; Martena, V.; Hoti, E.; Malaj, L.; Di Martino, P. Permeation and Skin Retention of Quercetin from Microemulsions Containing Transcutol® P. Drug Dev. Ind. Pharm. 2012, 38, 1128–1133. [Google Scholar] [CrossRef]
- Han, S.B.; Kwon, S.S.; Jeong, Y.M.; Yu, E.R.; Park, S.N. Physical Characterization and in Vitro Skin Permeation of Solid Lipid Nanoparticles for Transdermal Delivery of Quercetin. Int. J. Cosmet. Sci. 2014, 36, 588–597. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Silva, L.B.; Kawakami, C.M.; Araújo, M.M.; Del Lama, M.P.F.M.; Naal, R.M.Z.G.; Maria-Engler, S.S.; Gaspar, L.R.; Marcato, P.D. Topical Formulation of Quercetin Encapsulated in Natural Lipid Nanocarriers: Evaluation of Biological Properties and Phototoxic Effect. J. Drug Deliv. Sci. Technol. 2019, 53, 101148. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, S.; Rath, S.K. A Versatile Flavonoid Quercetin: Study of Its Toxicity and Differential Gene Expression in the Liver of Mice. Phytomed. Plus 2022, 2, 100148. [Google Scholar] [CrossRef]
- Dunnick, J. Toxicity and Carcinogenicity Studies of Quercetin, a Natural Component of Foods. Fundam. Appl. Toxicol. 1992, 19, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The Photodegradation of Quercetin: Relation to Oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef]
- Katiyar, S.K. Green Tea Prevents Non-Melanoma Skin Cancer by Enhancing DNA Repair. Arch. Biochem. Biophys. 2011, 508, 152–158. [Google Scholar] [CrossRef]
- Nihal, M.; Roelke, C.T.; Wood, G.S. Anti-Melanoma Effects of Vorinostat in Combination with Polyphenolic Antioxidant (−)-Epigallocatechin-3-Gallate (EGCG). Pharm. Res. 2010, 27, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Kuttan, G.; Pratheeshkumar, P.; Manu, K.A.; Kuttan, R. Inhibition of Tumor Progression by Naturally Occurring Terpenoids. Pharm. Biol. 2011, 49, 995–1007. [Google Scholar] [CrossRef]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and Quercetin Synergistically Inhibit Cancer Cell Proliferation in Multiple Cancer Cells and Modulate Wnt/β-Catenin Signaling and Apoptotic Pathways in A375 Cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, Y. Curcumin Inhibits the Growth and Metastasis of Melanoma via MiR-222-3p/SOX10/Notch Axis. Dis. Markers 2022, 2022, 3129781. [Google Scholar] [CrossRef]
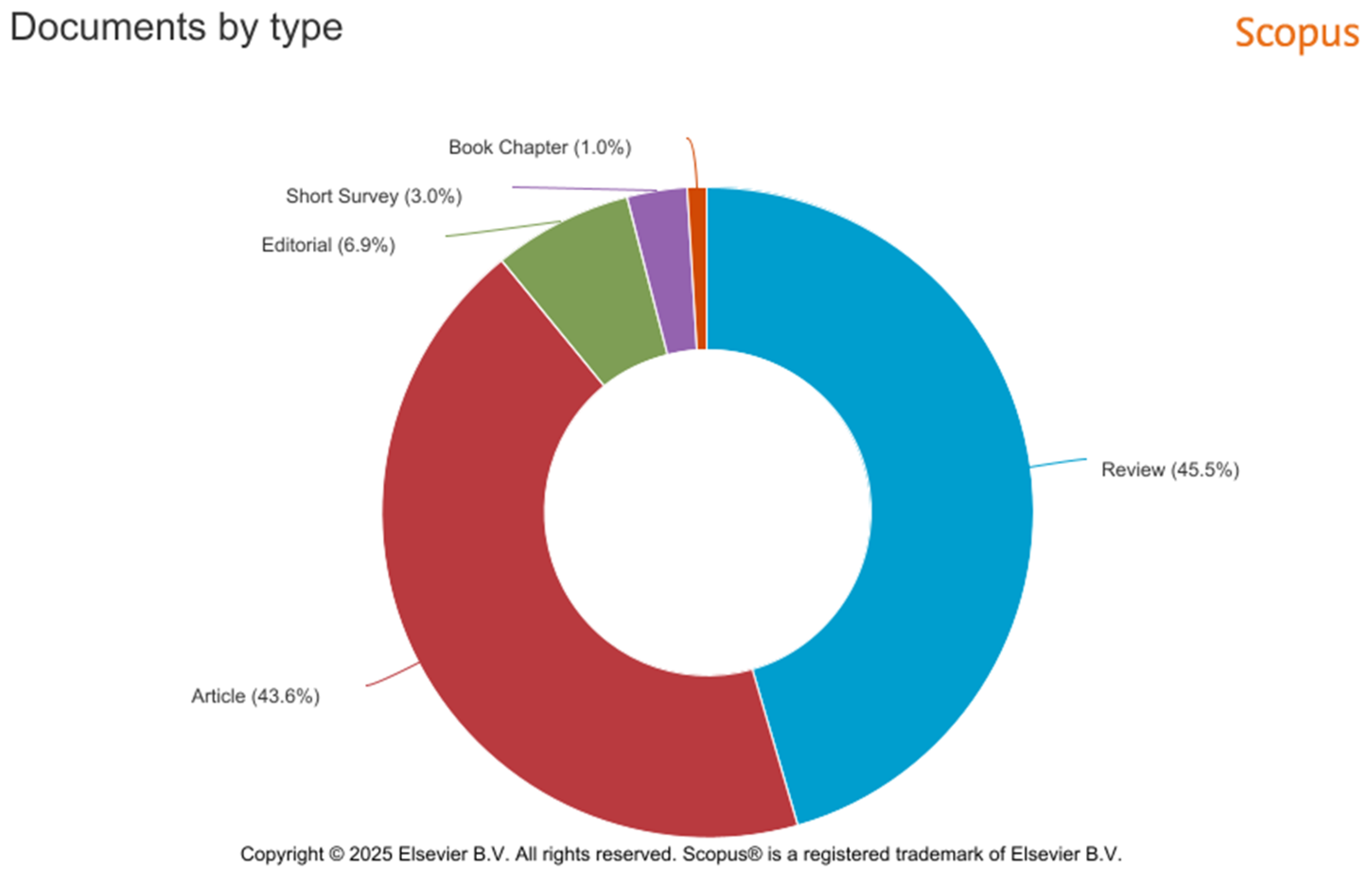
| SCOPUS | PUBMED | |
|---|---|---|
| Year | Documents | Documents |
| 2025 | 6 | 1 |
| 2024 | 20 | 14 |
| 2023 | 5 | 7 |
| 2022 | 22 | 6 |
| 2021 | 11 | 9 |
| 2020 | 8 | 8 |
| 2019 | 8 | 5 |
| 2018 | 5 | 5 |
| 2017 | 5 | 1 |
| 2016 | 4 | 3 |
| 2015 | 7 | 6 |
| Cancer Type | Study | Mechanism | Effect and Potential Application | |
|---|---|---|---|---|
| Authors and Title | Year | |||
| Melanoma cells, SK-MEL-28 and G-361 lines, alongside non-tumorigenic HaCaT epidermal cells | Sang Young Seo et al. Quercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells [20] | 2024 | Inhibition of the FAK/paxillin/Akt signaling pathway. | Antiproliferative, antimigratory, and cell cycle arrest effects. Reduces the expression levels of ganglioside GD3. |
| Melanoma cells, human skin fibroblast cells (HFF-1 cells; SCRC-1041), and human malignant melanoma cells (A375 cells; CRL-1619) | Monsicha Khuanekkaphan et al. Development of Quercetin Solid Dispersion-Loaded Dissolving Microneedles and In Vitro Investigation of Their Anti-Melanoma Activities [19] | 2024 | It suppressed Bcl-2 gene expression. | Induction of cell apoptosis. Because of its increased solubility, the optimized Q-SD-DMN can be used in further in vivo studies as a synergistic method of melanoma treatment. |
| Keratinocytes (HaCaT) and melanoma (B16F10) cells | Anh Thu Ha et al. Anti-Inflammatory, Antioxidant, Moisturizing, and Antimelanogenesis Effects of Quercetin 3-O-β-D-Glucuronide in Human Keratinocytes and Melanoma Cells via Activation of NF-κB and AP-1 Pathways [21] | 2021 | Activation of NF-κB and AP-1 pathways. | Anti-inflammatory, antioxidant, moisturizing, and antimelanogenesis properties in human keratinocytes and melanoma cells. Bearing to glucuronic acid, quercetin can be used to protect skin cells. |
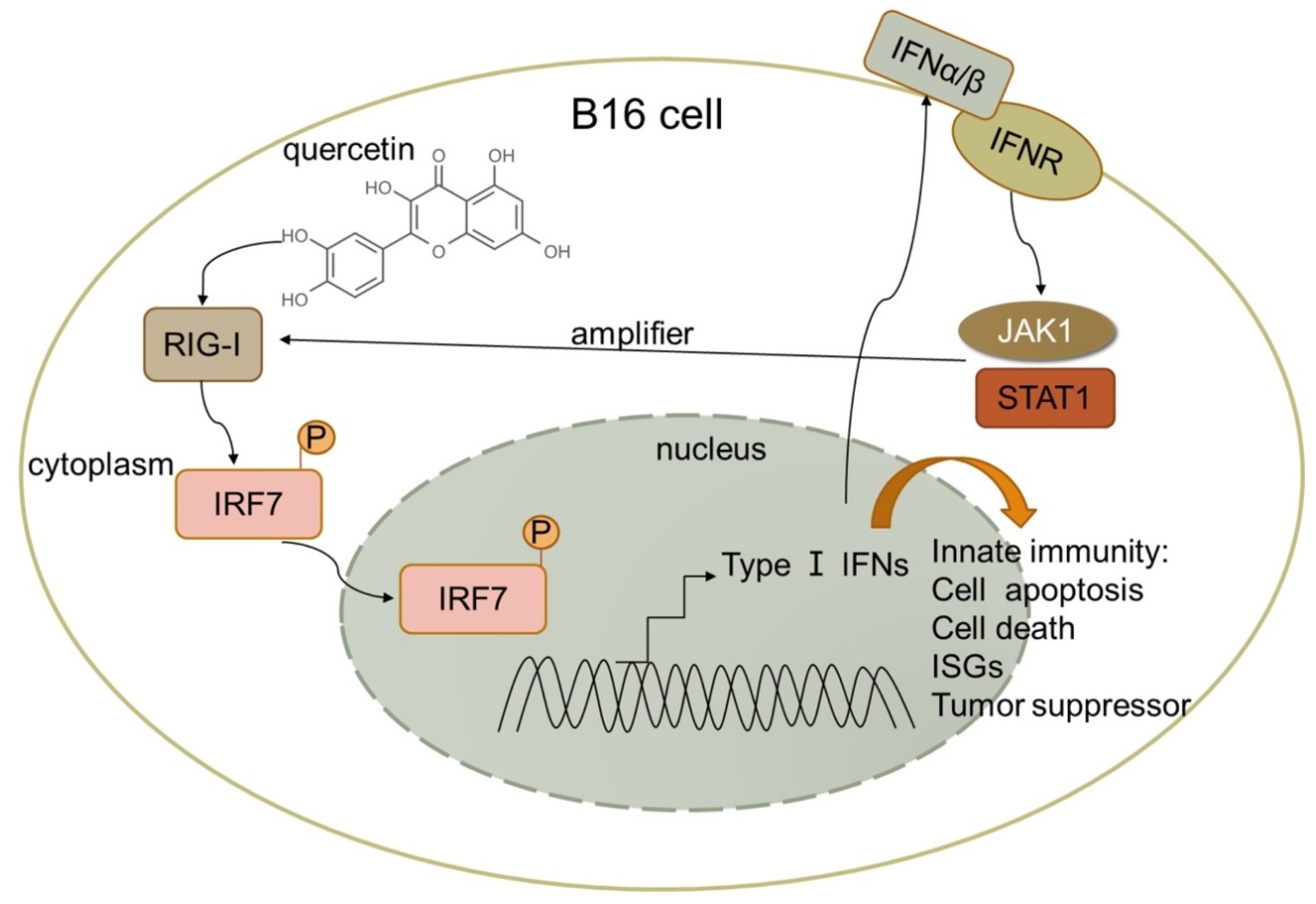
| Malignant melanoma cells, B16 and A375 | Danhong Peng et al. Melanoma suppression by quercetin is correlated with RIG-I and type I interferon signaling [16] | 2020 | Quercetin upregulated IFN-α and IFN-β expression by activating RIG-I promoter in B16 cells. RIG-I likely amplifies antitumor effects by activating signal transduction and activator of transcription 1 (STAT1) in the IFN-JAK-STAT pathway in an autocrine and paracrine manner. | Quercetin inhibited mouse melanoma growth in vivo, suppressed proliferation, and promoted apoptosis. |
| MDA-MB-231 breast cancer cell line | Mohammadreza Roshanazadeh et al. Quercetin Enhances the Suppressive Effects of Doxorubicin on the Migration of MDA-MB-231 Breast Cancer Cell Line [28] | 2021 | Quercetin can affect the migration of MDA-MB-231 cells by reducing metastasis-related gene expression and significantly enhancing the inhibitory effects of doxorubicin on this expression. | Quercetin inhibits the viability and migration of MDA-MB-231 cancer cells and synergistically enhances the effects of dox on these cells’ survival and migration. |
| MCF-7 cells | Fatemeh Rezaie et al. Quercetin Arrests in G2 phase, Upregulates INXS LncRNA and Downregulates UCA1 LncRNA in MCF-7 Cells [29] | 2022 | Quercetin might increase cell death by upregulating INXS and downregulating UCA1 lncRNAs in MCF-7 cells. | Quercetin-induced cell cycle arrest at the G2 phase in MCF-7 cells. |
| Glioblastoma cells, T98G | Wanyu Wang et al. Quercetin induces MGMT+ glioblastoma cell apoptosis via dual inhibition of Wnt3a/β-Catenin and Akt/NF-κB signaling pathways. [30] | 2023 | Quercetin-induced apoptosis through decreasing MGMT expression. MGMT downregulation was achieved through dual inhibition of Wnt3a/β-Catenin and Akt/NF-κB signaling pathways. | Quercetin-induced S-phase arrest, DNA damage, and cell apoptosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoinoiu, T.; Dumitrascu, V.; Pit, D.; Schipor, D.-A.; Jabri-Tabrizi, M.; Hoinoiu, B.; Petreuș, D.E.; Seiman, C. Quercetin as a Potential Therapeutic Agent for Malignant Melanoma—A Review of Current Evidence and Future Directions. Medicina 2025, 61, 656. https://doi.org/10.3390/medicina61040656
Hoinoiu T, Dumitrascu V, Pit D, Schipor D-A, Jabri-Tabrizi M, Hoinoiu B, Petreuș DE, Seiman C. Quercetin as a Potential Therapeutic Agent for Malignant Melanoma—A Review of Current Evidence and Future Directions. Medicina. 2025; 61(4):656. https://doi.org/10.3390/medicina61040656
Chicago/Turabian StyleHoinoiu, Teodora, Victor Dumitrascu, Daniel Pit, David-Alexandru Schipor, Madalina Jabri-Tabrizi, Bogdan Hoinoiu, David Emanuel Petreuș, and Corina Seiman. 2025. "Quercetin as a Potential Therapeutic Agent for Malignant Melanoma—A Review of Current Evidence and Future Directions" Medicina 61, no. 4: 656. https://doi.org/10.3390/medicina61040656
APA StyleHoinoiu, T., Dumitrascu, V., Pit, D., Schipor, D.-A., Jabri-Tabrizi, M., Hoinoiu, B., Petreuș, D. E., & Seiman, C. (2025). Quercetin as a Potential Therapeutic Agent for Malignant Melanoma—A Review of Current Evidence and Future Directions. Medicina, 61(4), 656. https://doi.org/10.3390/medicina61040656






