Comparison of the Safety and Efficacy of Remimazolam and Propofol for Sedation in Adults Undergoing Colonoscopy: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligible Criteria
2.2. Search Strategy
2.3. Study Selection and Data Extraction
2.4. Outcome Measures
2.5. Assessment of Risk of Bias and Quality of Evidence
2.6. Statistical Analysis
3. Results
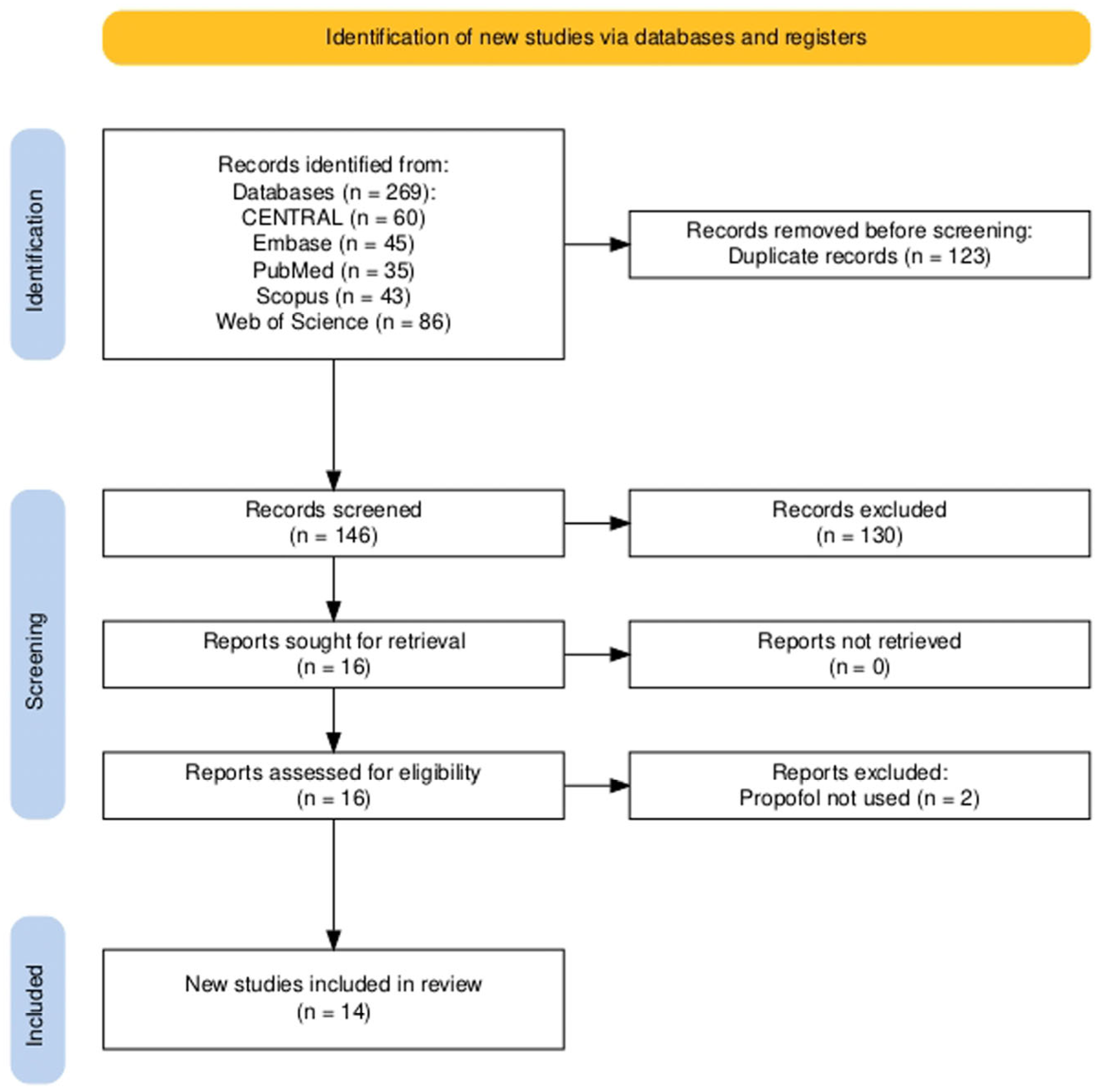
3.1. Study Selection
3.2. Characteristics of Studies and Participants
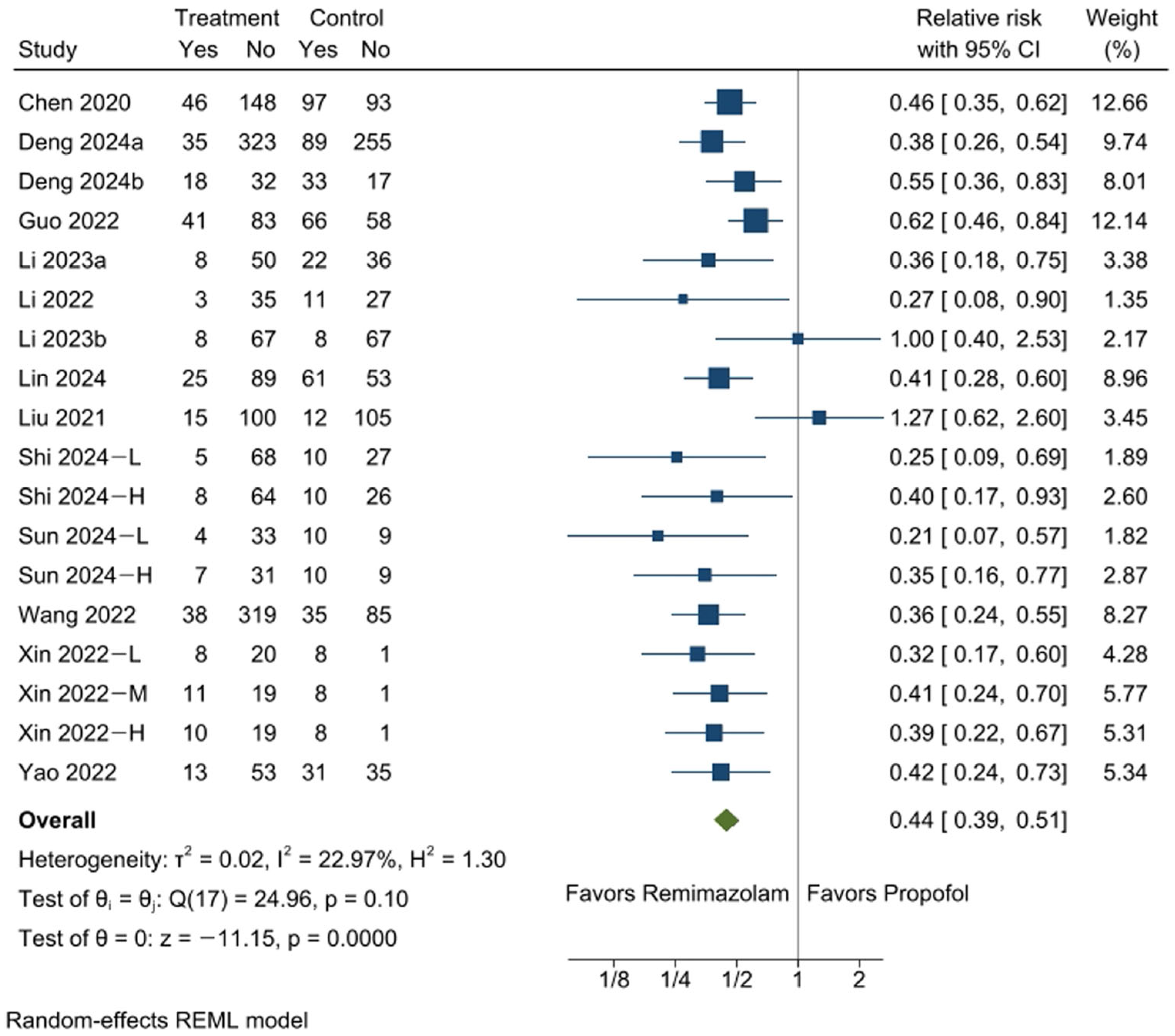
3.3. Incidence of Hypotension
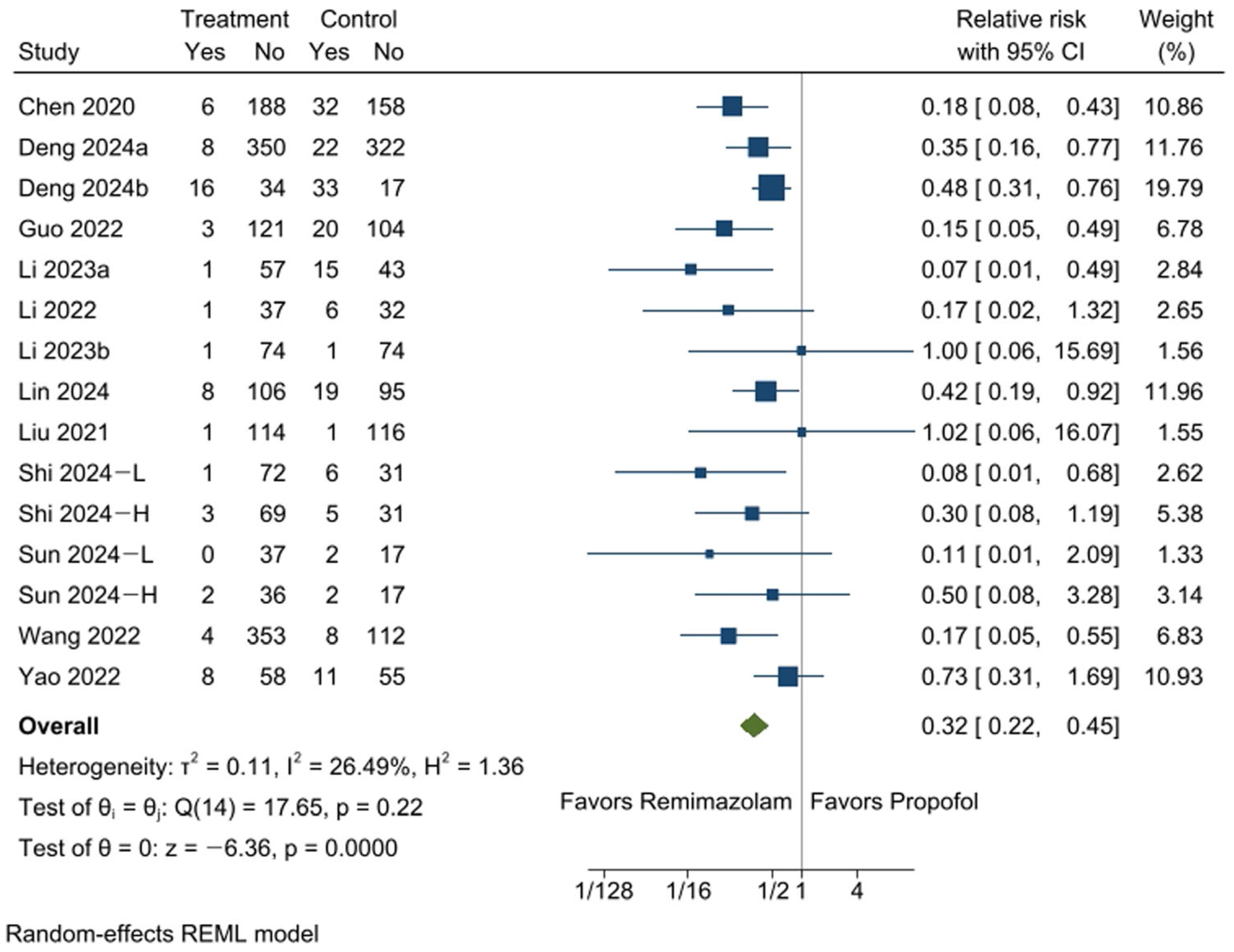
3.4. Incidence of Bradycardia
3.5. Incidence of Respiratory Depression
3.6. Incidence of Injection Pain
3.7. Procedure Success Rates
3.8. Time to Target Sedation Depth
3.9. Emergence Time from Sedation
3.10. Post-Procedural Unit Stay Time
3.11. Risk of Bias
3.12. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCT | Randomized controlled trial |
| RR | Relative risk |
| MD | Mean difference |
| CI | Confidence interval |
| PK | Pharmacokinetics |
| PD | Pharmacodynamics |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RoB 2 | Cochrane Risk of Bias tool 2 |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
References
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; Kubik, M.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Quaye, A.N.; Hisey, W.M.; Mackenzie, T.A.; Robinson, C.M.; Richard, J.M.; Anderson, J.C.; Warters, R.D.; Butterly, L.F. Association Between Colonoscopy Sedation Type and Polyp Detection: A Registry-Based Cohort Study. Anesthesiology 2024, 140, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- de Wit, F.; van Vliet, A.L.; de Wilde, R.B.; Jansen, J.R.; Vuyk, J.; Aarts, L.P.; de Jonge, E.; Veelo, D.P.; Geerts, B.F. The effect of propofol on haemodynamics: Cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br. J. Anaesth. 2016, 116, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yang, K.H.; Lee, C.S.; Lee, H.S.; Moon, S.Y.; Hwang, S.I.; Song, J.H. The effect-site concentration of propofol producing respiratory depression during spinal anesthesia. Korean J. Anesthesiol. 2011, 61, 122–126. [Google Scholar] [CrossRef]
- Kim, S.H.; Fechner, J. Remimazolam—Current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J. Anesthesiol. 2022, 75, 307–315. [Google Scholar] [CrossRef]
- Sahinovic, M.M.; Struys, M.; Absalom, A.R. Clinical Pharmacokinetics and Pharmacodynamics of Propofol. Clin. Pharmacokinet. 2018, 57, 1539–1558. [Google Scholar] [CrossRef]
- Barbosa, E.C.; Espirito Santo, P.A.; Baraldo, S.; Meine, G.C. Remimazolam versus propofol for sedation in gastrointestinal endoscopic procedures: A systematic review and meta-analysis. Br. J. Anaesth. 2024, 132, 1219–1229. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yang, S.T.; Duan, K.M.; Bai, Z.H.; Feng, Y.F.; Guo, Q.L.; Cheng, Z.G.; Wu, H.; Shangguan, W.N.; Wu, X.M.; et al. Efficacy and safety of remimazolam besylate in bronchoscopy for adults: A multicenter, randomized, double-blind, positive-controlled clinical study. Front. Pharmacol. 2022, 13, 1005367. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Lee, D.H.; Park, J.S. Finding the ideal sedative: A non-inferiority study of remimazolam vs propofol in endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 2023, 38, 2160–2166. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, version 6.4; Cochrane: Chichester, UK, 2023. [Google Scholar]
- Li, S.; Zhang, Y.; Wang, H.; Ye, G.; Yao, N.; Zhu, X. Remimazolam tosilate infusion has a lower incidence of hypotension than propofol infusion during painless colonoscopy. Anaesth. Pain Intensive Care 2023, 27, 697–705. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ye, G.; Xiang, J.; Mou, J.; Yao, N.; Zhu, X. Efficacy and safety of remimazolam tosylate combined with sufentanil for elderly patients undergoing colonoscopy sedation. Med. J. Wuhan Univ. 2022, 43, 480–484. [Google Scholar] [CrossRef]
- Li, W.; Zhao, J.; Hao, R.; Wang, S.; Chen, M.; Liu, H.; Qi, L.; Hao, Z. The Efficacy and Safety of Remimazolam Besylate Combined with Esketamine for Outpatient Colonoscopy: A Prospective, Randomized, Controlled Clinical Trial. Drug Des. Devel Ther. 2023, 17, 2875–2887. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Xu, X.; Huang, Y.; Xue, S.; Wu, A.; Jin, X.; Wang, Q.; Lyu, J.; Wang, S.; et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: A multicentered, randomized, positive-controlled, phase III clinical trial. Am. J. Transl. Res. 2020, 12, 4594–4603. [Google Scholar]
- Deng, B.R.; Zhang, Y.; Xie, Z.F.; Wang, D.D.; Zeng, T.; Zhang, D.B.; Huang, L.; Wang, Q.Y.; Shen, T.; Wu, Q.L. Comparative Analysis of Hemodynamic Effects of Remimazolam and Propofol Combined with Esketamine in Colonoscopic Procedures in the Elderly. Drug Des. Dev. Ther. 2024, 18, 5269–5280. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, R.; Jiang, W.; Zhai, W.; Chen, X.; Xia, L.; Xiong, C.; Zhou, Y.; Zhang, X.; Peng, Y. Efficacy and safety of remimazolam besylate in patients with obesity undergoing painless colonoscopy: A prospective, double-blind, randomized controlled trial. Signa Vitae 2024, 20, 76–85. [Google Scholar] [CrossRef]
- Guo, L.; Liu, T.; Zhang, Y.; Qi, D. Effect of remimazolam versus propofol sedation on the quality of recovery after colonoscopy: A randomised, controlled, noninferiority trial. Eur. J. Anaesthesiol. 2022, 39, 953–955. [Google Scholar] [CrossRef]
- Lin, S.; Wei, Y.; Zhuo, Y.; Que, S.; Jin, X.; Yao, Y.; Qian, B. Comparing Cognitive Recovery of Remimazolam versus Propofol in Elderly Patients Undergoing Colonoscopy: A Randomized Controlled Trial. Clin. Interv. Aging 2024, 19, 2133–2143. [Google Scholar] [CrossRef]
- Liu, X.; Ding, B.; Shi, F.; Zhang, Y.; Liu, L.; Sha, Y.; Zhao, T. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: A prospective, randomized, single-blind, non-inferiority trial. Drug Des. Dev. Ther. 2021, 15, 4675–4685. [Google Scholar] [CrossRef]
- Shi, H.B.; Zhang, J.Y.; Hu, Z.Q.; Hou, Q.H.; Hu, Q.H.; Dai, Z.G.; Zhou, W.J.; Qi, D.W.; Li, Y.L.; Wang, Q.; et al. The efficacy and safety of remimazolam in painless colonoscopy: A prospective, randomized clinical trial. Front. Med. 2024, 11, 1434767. [Google Scholar] [CrossRef]
- Sun, Q.R.; Cheng, J.T.; Lei, W.P.; Lu, X.L.; Huang, Y.Q.; Sun, J.L. The effects of remimazolam combined with sufentanil on respiration, circulation and sedation level in patients undergoing colonoscopy. BMC Anesthesiol. 2024, 24, 252. [Google Scholar] [CrossRef]
- Wang, X.M.; Hu, X.L.; Bai, N.Y.; Li, L.; Zhang, M.; Cheng, Z.G.; Guo, Q.L. Safety and efficacy of remimazolam besylate in patients undergoing colonoscopy: A multicentre, single-blind, randomized, controlled, phase III trial. Front. Pharmacol. 2022, 13, 900723. [Google Scholar] [CrossRef]
- Xin, Y.Y.; Chu, T.T.; Wang, J.X.; Xu, A.J. Sedative effect of remimazolam combined with alfentanil in colonoscopic polypectomy: A prospective, randomized, controlled clinical trial. BMC Anesthesiol. 2022, 22, 262. [Google Scholar] [CrossRef]
- Yao, Y.S.; Guan, J.S.; Liu, L.W.; Fu, B.B.; Chen, L.; Zheng, X.C. Discharge readiness after remimazolam versus propofol for colonoscopy A randomised, double-blind trial. Eur. J. Anaesthesiol. 2022, 39, 911–917. [Google Scholar] [CrossRef]

| Author (Year) | No. of Group | Sample Size | Age | Induction | Maintenance | Opioid | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | A1 | A2 | Remimazolam | Propofol | Remimazolam | Propofol | |||
| Chen, 2020 [16] | 2 | 194 | 190 | 45 | 44 | 5 mg | 1.5 mg/kg | 2.5 mg | 0.5 mg/kg | Fentanyl 1 µg/kg |
| Deng, 2024a [17] | 2 | 358 | 344 | 75 | 75 | 0.15 mg/kg | 1.5 mg/kg | 0.05 mg/kg | 0.5 mg/kg | Esketamine 0.3 mg/kg |
| Deng, 2024b [18] | 2 | 50 | 50 | 46 | 47 | 0.15 mg/kg | 2 mg/kg | 2.5 mg | 0.5 mg/kg | Sufentanil 0.1 µg/kg |
| Guo, 2022 [19] | 2 | 124 | 124 | 47 | 48 | 5 mg | 1.5 mg/kg | 2.5 mg | 0.5 mg/kg | Fentanyl 1 µg/kg |
| Li, 2023a [13] | 2 | 58 | 58 | 49 | 48 | 0.05–0.15 mg/kg | 0.5–1.5 mg/kg | 0.3–0.5 mg/kg/h | 3–5 mg/kg/h | Sufentanil 0.1 µg/kg |
| Li, 2022 [14] | 2 | 38 | 38 | 68 | 68 | 0.05–0.15 mg/kg | 0.5–1.5 mg/kg | 0.3–0.5 mg/kg/h | 3–5 mg/kg/h | Sufentanil 0.1 µg/kg |
| Li, 2023b [15] | 2 | 75 | 75 | 50 | 50 | 0.15–0.25 mg/kg | 1–2 mg/kg | 1–2 mg/kg/h | 2–4 mg/kg/h | Esketamine 0.15 mg/kg |
| Lin, 2024 [20] | 2 | 114 | 114 | 71 | 71 | 0.2 mg/kg | 1 mg/kg | 0.1 mg/kg | 0.5 mg/kg | Sufentanil 0.05 µg/kg |
| Liu, 2021 [21] | 2 | 115 | 117 | 69 | 69 | 0.15 mg/kg | 0.1 mL/kg | 0.075 mg/kg | 0.05 mL/kg | Fentanyl 0.5 µg/kg |
| Shi, 2024 [22] | 3 | 73 | 73 | 58 | 60 | 0.15 mg/kg | 2 mg/kg | 0.04 mg/kg | 0.4 mg/kg | Nalbuphine 0.2 mg/kg |
| 72 | 57 | 2 mg/kg | 2 mg/kg | 0.04 mg/kg | 0.4 mg/kg | Nalbuphine 0.2 mg/kg | ||||
| Sun, 2024 [23] | 3 | 37 | 38 | 53 | 53 | 0.2 mg/kg | 2 mg/kg | 2.5 mg | 30 mg | Sufentanil 5 µg |
| 38 | 48 | 0.25 mg/kg | 2 mg/kg | 2.5 mg | 30 mg | Sufentanil 5 µg | ||||
| Wang, 2022 [24] | 2 | 357 | 120 | 44 | 47 | 7 mg/kg | 1.5 mg/kg | 2.5 mg | 0.5 mg/kg | Fentanyl 50 µg |
| Xin, 2022 [25] | 4 | 28 | 27 | 54 | 56 | 0.1 mg/kg | 2 mg/kg | 2.5 mg | 1/3 to 1/2 of initial dose | Alfentanil 10 µg/kg |
| 30 | 50 | 0.15 mg/kg | 2 mg/kg | 2.5 mg | 1/3 to 1/2 of initial dose | Alfentanil 10 µg/kg | ||||
| 29 | 52 | 0.2 mg/kg | 2 mg/kg | 2.5 mg | 1/3 to 1/2 of initial dose | Alfentanil 10 µg/kg | ||||
| Yao, 2022 [26] | 3 | 66 | 66 | 49 | 48 | 0.2 mg/kg | 1 mg/kg | 6 mg | 30 mg | Sufentanil 5 µg |
| No. of Studies | No. of Participants | RR (95% CI) | p Value | I2 | |
|---|---|---|---|---|---|
| Injection pain | 12 (13 comparisons) | 2960 | 0.14 [0.09, 0.24] | 0.0000 | 64% |
| Procedure success rates | 9 (13 comparisons) | 2115 | 0.99 [0.97, 1.00] | 0.0958 | 32% |
| No. of studies | No. of participants | MD (95% CI) | p value | I2 | |
| Time to target sedation depth | 11 (12 comparisons) | 1801 | 15.97 [8.30, 23.64] | 0.0000 | 99% |
| Emergence time from sedation | 14 (18 comparisons) | 3290 | −0.91 [−1.69, −0.31] | 0.0230 | 97% |
| Post-procedural unit stay time | 13 (17 comparisons) | 3158 | −2.20 [−3.23, −1.17] | 0.0981 | 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, B.-W.; Na, H.-S.; Park, S.-H.; Bang, S.; Shin, H.-J. Comparison of the Safety and Efficacy of Remimazolam and Propofol for Sedation in Adults Undergoing Colonoscopy: A Meta-Analysis of Randomized Controlled Trials. Medicina 2025, 61, 646. https://doi.org/10.3390/medicina61040646
Koo B-W, Na H-S, Park S-H, Bang S, Shin H-J. Comparison of the Safety and Efficacy of Remimazolam and Propofol for Sedation in Adults Undergoing Colonoscopy: A Meta-Analysis of Randomized Controlled Trials. Medicina. 2025; 61(4):646. https://doi.org/10.3390/medicina61040646
Chicago/Turabian StyleKoo, Bon-Wook, Hyo-Seok Na, Sang-Hi Park, Seunguk Bang, and Hyun-Jung Shin. 2025. "Comparison of the Safety and Efficacy of Remimazolam and Propofol for Sedation in Adults Undergoing Colonoscopy: A Meta-Analysis of Randomized Controlled Trials" Medicina 61, no. 4: 646. https://doi.org/10.3390/medicina61040646
APA StyleKoo, B.-W., Na, H.-S., Park, S.-H., Bang, S., & Shin, H.-J. (2025). Comparison of the Safety and Efficacy of Remimazolam and Propofol for Sedation in Adults Undergoing Colonoscopy: A Meta-Analysis of Randomized Controlled Trials. Medicina, 61(4), 646. https://doi.org/10.3390/medicina61040646







