Knee-Sparing Resection and Reconstruction Surgery for Bone Sarcoma Using 3D-Surgical Approach: Average of 5-Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
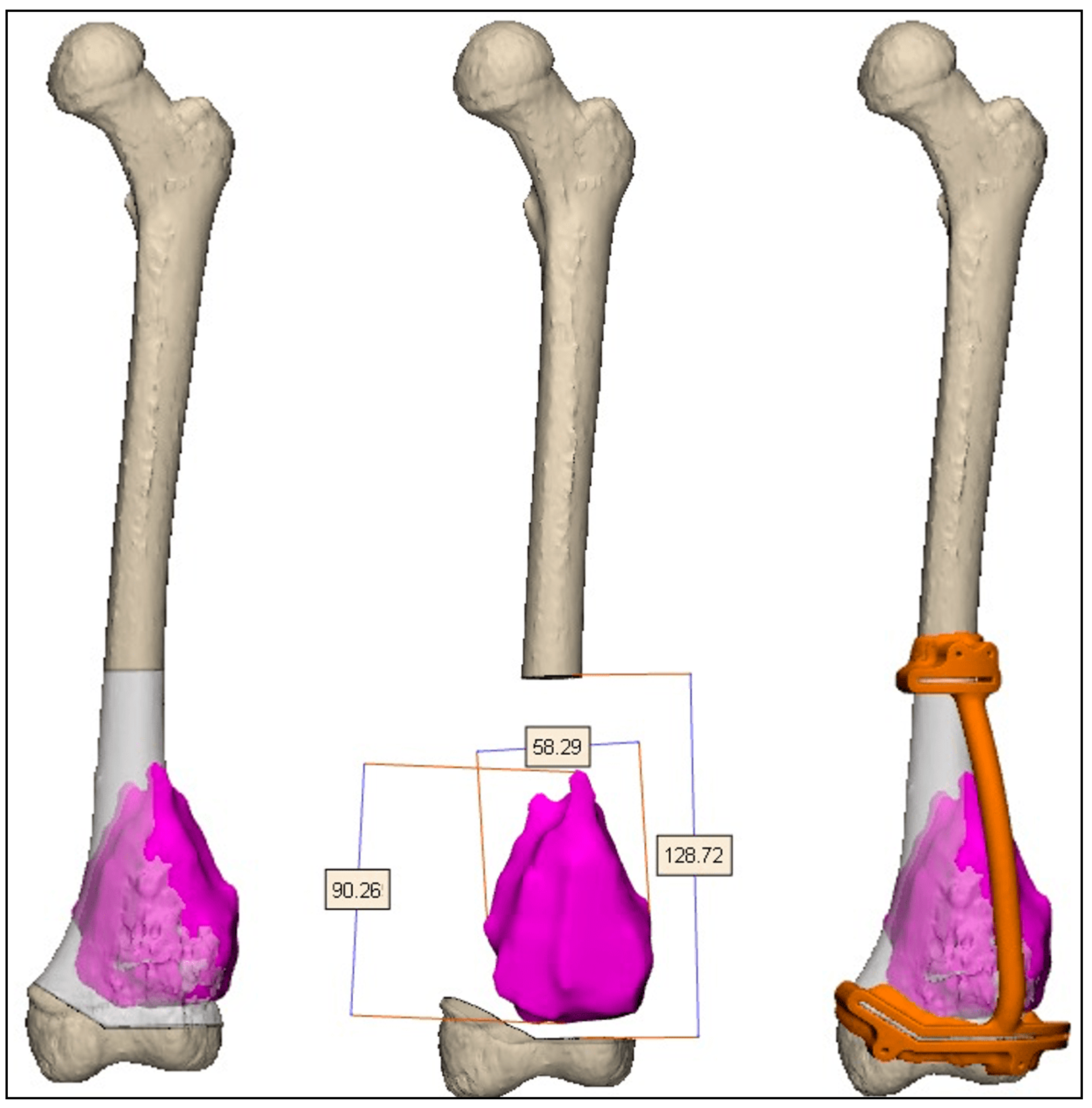
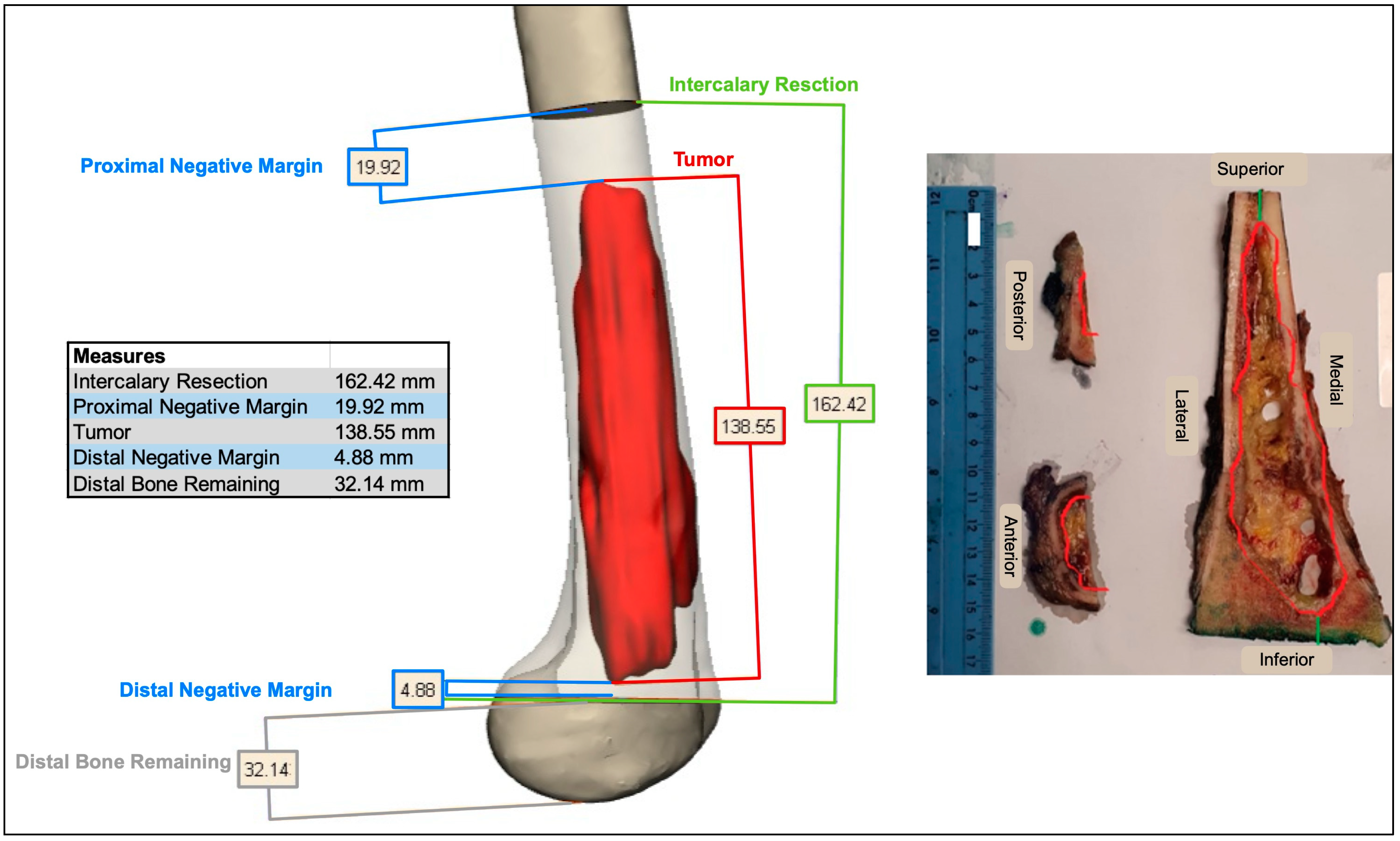
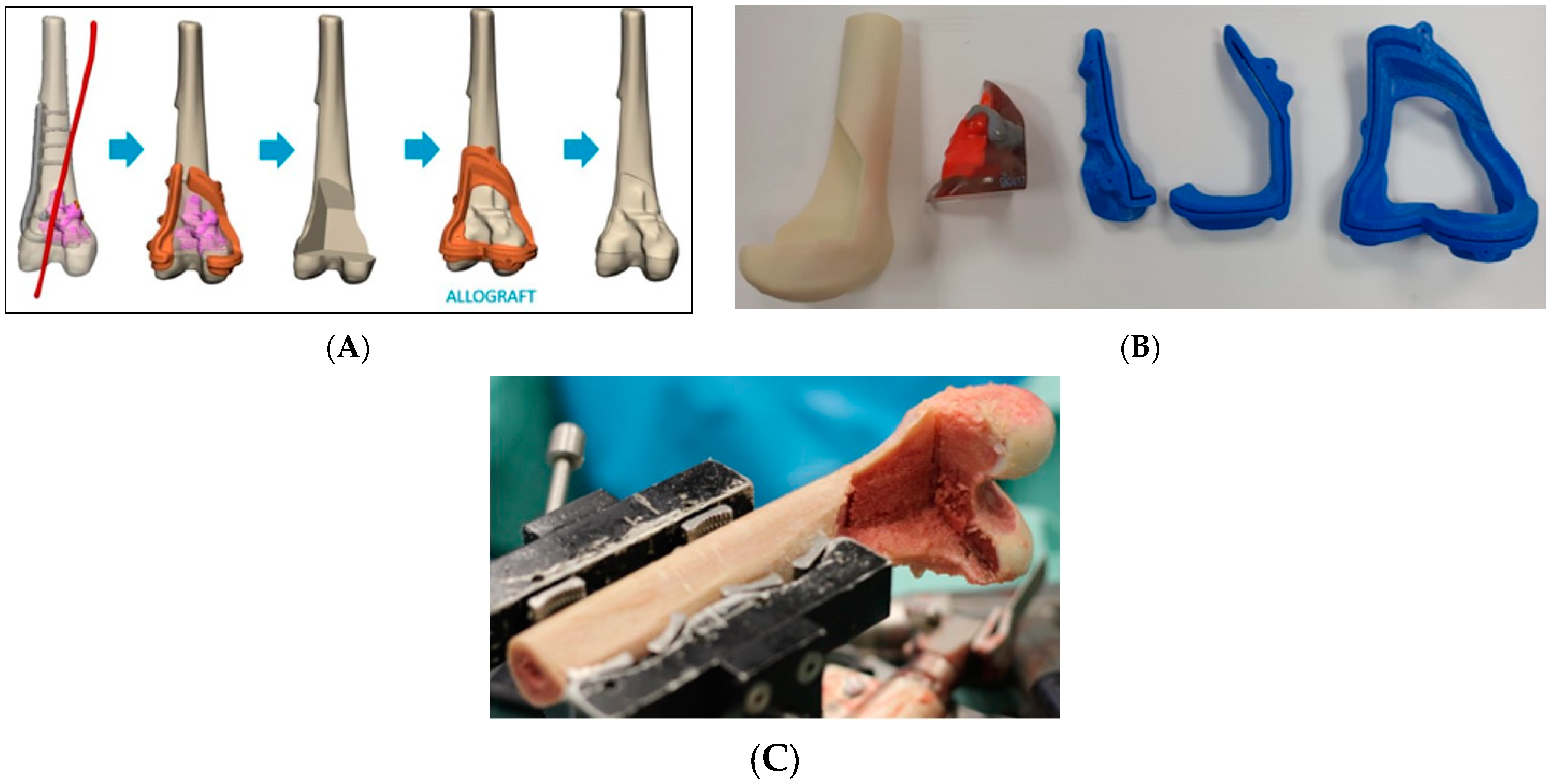
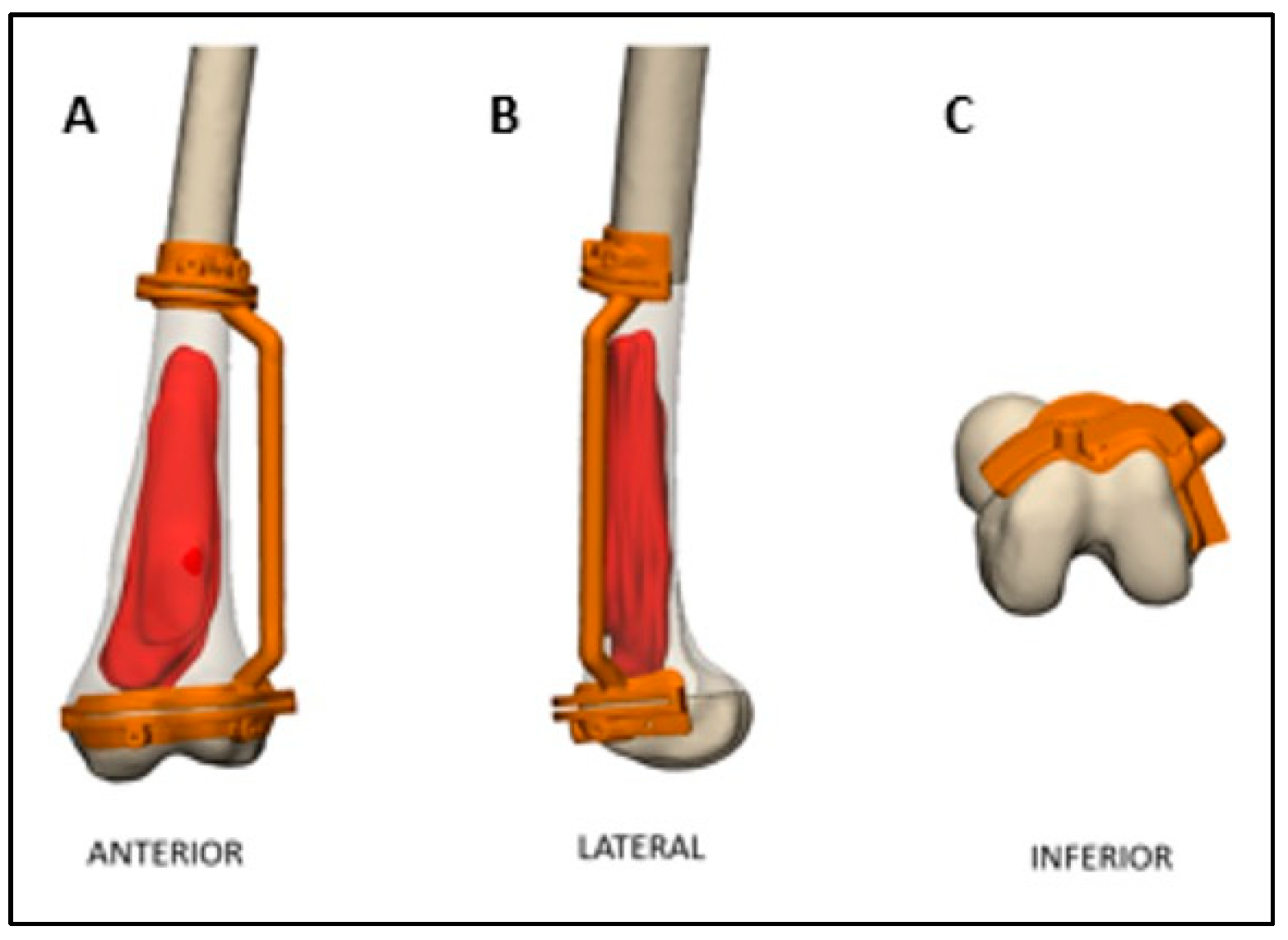
2.2. Preoperative Planning and Simulation
2.3. 3D-Printed Patient-Specific Instruments
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MFH | Malignant fibrous histiocytoma |
| I and D | Irrigation and debridement |
| NED | No evidence of disease |
| AED | Alive with evidence of disease |
| DOD | Death of disease |
| LR | Local recurrence |
| AKA | Above-knee amputation |
References
- Moore, D.D.; Haydon, R.C. Ewing’s Sarcoma of Bone. Cancer Treat. Res. 2014, 162, 93–115. [Google Scholar] [CrossRef]
- Stiller, C.A. International patterns of cancer incidence in adolescents. Cancer Treat. Rev. 2007, 33, 631–645. [Google Scholar] [CrossRef]
- Kelly, C.M.; Wilkins, R.M.; Eckardt, J.J.; Ward, W.G. Treatment of metastatic disease of the tibia. Clin. Orthop. Relat. Res. 2003, 415, S219–S229. [Google Scholar] [CrossRef]
- Fuchs, B.; Ossendorf, C.; Leerapun, T.; Sim, F.H. Intercalary segmental reconstruction after bone tumor resection. Eur. J. Surg. Oncol. 2008, 34, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Nathenson, M.J.; Sausville, E. Looking for answers: The current status of neoadjuvant treatment in localized soft tissue sarcomas. Cancer Chemother. Pharmacol. 2016, 78, 895. [Google Scholar] [CrossRef]
- Weber, K.L. What’s new in musculoskeletal oncology. J. Bone Jt. Surg. 2005, 87, 1400–1409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Flanagan, S.J.; Stack, J.P.; McGee, H.M.J.; Dervan, P.; Hurson, B. Imaging of intramedullary tumour spread in osteosarcoma. A comparison of techniques. J. Bone Jt. Surgery. Br. 1991, 73, 998–1001. [Google Scholar] [CrossRef]
- Brigman, B.E.; Hornicek, F.J.; Gebhardt, M.C.; Mankin, H.J. Allografts about the Knee in Young Patients with High-Grade Sarcoma. Clin. Orthop. Relat. Res. 2004, 421, 232–239. [Google Scholar] [CrossRef]
- Manfrini, M.; Gasbarrini, A.; Malaguti, C.; Ceruso, M.; Innocenti, M.; Bini, S.; Capanna, R.; Campanacci, M. Intraepiphyseal resection of the proximal tibia and its impact on lower limb growth. Clin. Orthop. Relat. Res. 1999, 358, 111–119. [Google Scholar] [CrossRef]
- Morgan, H.D.; Cizik, A.M.; Leopold, S.S.; Hawkins, D.S.; Conrad, E.U. Survival of tumor megaprostheses replacements about the knee. Clin. Orthop. Relat. Res. 2006, 450, 39–45. [Google Scholar] [CrossRef]
- Puri, A.; Subin, B.S.; Agarwal, M.G. Fibular centralisation for the reconstruction of defects of the tibial diaphysis and distal metaphysis after excision of bone tumours. J. Bone Jt. Surgery. Br. 2009, 91, 234–239. [Google Scholar] [CrossRef]
- Pala, E.; Trovarelli, G.; Calabrò, T.; Angelini, A.; Abati, C.N.; Ruggieri, P. Survival of modern knee tumor megaprostheses: Failures, functional results, and a comparative statistical analysis. Clin. Orthop. Relat. Res. 2015, 473, 891–899. [Google Scholar] [CrossRef]
- Cianni, L.; Taccari, F.; Bocchi, M.B.; Micheli, G.; Sangiorgi, F.; Ziranu, A.; Fantoni, M.; Maccauro, G.; Vitiello, R. Characteristics and Epidemiology of Megaprostheses Infections: A Systematic Review. Healthcare 2024, 12, 1283. [Google Scholar] [CrossRef]
- DiGioia, A.M.; Jaramaz, B.; Plakseychuk, A.Y.; Moody, J.E.; Nikou, C.; LaBarca, R.S.; Levison, T.J.; Picard, F. Comparison of a mechanical acetabular alignment guide with computer placement of the socket. J. Arthroplast. 2002, 17, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Benady, A.; Meyer, S.J.; Golden, E.; Dadia, S.; Levy, G.K. Patient-specific Ti-6Al-4V lattice implants for critical-sized load-bearing bone defects reconstruction. Mater. Des. 2023, 226, 111605. [Google Scholar] [CrossRef]
- Sternheim, A.; Daly, M.; Qiu, J.; Weersink, R.; Chan, H.; Jaffray, D.; Irish, J.C.; Ferguson, P.C.; Wunder, J.S. Navigated pelvic osteotomy and tumor resection: A study assessing the accuracy and reproducibility of resection planes in Sawbones and cadavers. J. Bone Jt. Surg. Am. 2015, 97, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Puri, A.; Gulia, A.; Reddy, K. Joint-sparing or physeal-sparing diaphyseal resections: The challenge of holding small fragments. Clin. Orthop. Relat. Res. 2010, 468, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pollock, R.; Cannon, S.R.; Briggs, T.W.R.; Skinner, J.; Blunn, G. A knee-sparing distal femoral endoprosthesis using hydroxyapatite-coated extracortical plates. Preliminary results. J. Bone Jt. Surgery. Br. 2006, 88, 1367–1372. [Google Scholar] [CrossRef]
- Wang, B.; Hao, Y.; Pu, F.; Jiang, W.; Shao, Z. Computer-aided designed, three dimensional-printed hemipelvic prosthesis for peri-acetabular malignant bone tumour. Int. Orthop. 2018, 42, 687–694. [Google Scholar] [CrossRef]
- Lin, C.-L.; Fang, J.-J.; Lin, R.-M. Resection of giant invasive sacral schwannoma using image-based customized osteotomy tools. Eur. Spine J. 2016, 25, 4103–4107. [Google Scholar] [CrossRef]
- Wong, K.; Kumta, S.; Sze, K.; Wong, C. Use of a patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Comput. Aided Surg. 2012, 17, 284–293. [Google Scholar] [CrossRef]
- Liu, W.; Shao, Z.; Rai, S.; Hu, B.; Wu, Q.; Hu, H.; Zhang, S.; Wang, B. Three-dimensional-printed intercalary prosthesis for the reconstruction of large bone defect after joint-preserving tumor resection. J. Surg. Oncol. 2020, 121, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.A.; Gaur, B.; Sagar, S.; Chetana, M.S.; Naik, S.; Khambati, B.; Natrajan, S.; Ghyar, R.; Bhallamudi, R. Guidelines to Design Custom 3D Printed Jig for Orthopaedic Surgery. Smart Innov. Syst. Technol. 2021, 223, 585–594. [Google Scholar] [CrossRef]
- Lal, H.; Patralekh, M.K. 3D printing and its applications in orthopaedic trauma: A technological marvel. J. Clin. Orthop. Trauma. 2018, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Benady, A.; Meyer, J.S.; Ran, Y.; Mor, Y.; Gurel, R.; Rumack, N.; Golden, E.; Gortzak, Y.; Segal, O.; Merose, O.; et al. Intercalary and geographic lower limb tumor resections with the use of 3D printed Patient Specific Instruments—When less is more. J. Orthop. 2022, 32, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kiss, S.; Terebessy, T.; Szöke, G.; Kiss, J.; Antal, I.; Szendröi, M. Epiphysis preserving resection of malignant proximal tibial tumours. Int. Orthop. 2013, 37, 99–104. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Wan, S.L.; Sakayama, K.; Yamamoto, N.; Nishida, H.; Tomita, K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J. Bone Jt. Surgery. Br. 2005, 87, 218–225. [Google Scholar] [CrossRef]
- Houdek, M.T.; Rose, P.S.; Milbrandt, T.A.; Stans, A.A.; Moran, S.L.; Sim, F.H. Comparison of Pediatric Intercalary Allograft Reconstructions with and without a Free Vascularized Fibula. Plast. Reconstr. Surg. 2018, 142, 1065–1071. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yamamoto, N.; Hayashi, K.; Matsubara, H.; Kimura, H.; Miwa, S.; Higuchi, T.; Abe, K.; Taniguchi, Y.; Tsuchiya, H. Growth of epiphysis after epiphyseal-preservation surgery for childhood osteosarcoma around the knee joint. BMC Musculoskelet. Disord. 2018, 19, 185. [Google Scholar] [CrossRef]
- Benevenia, J.; Kirchner, R.; Patterson, F.; Beebe, K.; Wirtz, D.C.; Rivero, S.; Palma, M.; Friedrich, M.J. Outcomes of a Modular Intercalary Endoprosthesis as Treatment for Segmental Defects of the Femur, Tibia, and Humerus. Clin. Orthop. Relat. Res. 2016, 474, 539–548. [Google Scholar] [CrossRef]
- Aponte-Tinao, L.; Ayerza, M.A.; Muscolo, L.D.; Farfalli, G.L. Survival, recurrence, and function after epiphyseal preservation and allograft reconstruction in osteosarcoma of the knee. Clin. Orthop. Relat. Res. 2015, 473, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Guder, W.K.; Hardes, J.; Gosheger, G.; Nottrott, M.; Streitbürger, A. Ultra-short stem anchorage in the proximal tibial epiphysis after intercalary tumor resections: Analysis of reconstruction survival in four patients at a mean follow-up of 56 months. Arch. Orthop. Trauma Surg. 2017, 137, 481–488. [Google Scholar] [CrossRef]
- Yu, X.; Xu, M.; Xu, S.; Song, R. Long-term outcomes of epiphyseal preservation and reconstruction with inactivated bone for distal femoral osteosarcoma of children. Orthop. Surg. 2012, 4, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Donati, D.; Di Liddo, M.; Zavatta, M.; Manfrini, M.; Bacci, G.; Picci, P.; Capanna, R.; Mercuri, M. Massive bone allograft reconstruction in high-grade osteosarcoma. Clin. Orthop. Relat. Res. 2000, 377, 186–194. [Google Scholar] [CrossRef]
- Allograft Reconstruction After Proximal Tibial Resection for Bone Tumors. An Analysis of Function and Outcome Comparing allograft and Prosthetic Reconstructions—PubMed n.d. Available online: https://pubmed.ncbi.nlm.nih.gov/8194221/ (accessed on 23 September 2024).
- The Use of Hemipelvic Allografts or Autoclaved Grafts for Reconstruction After Wide Resections of Malignant Tumors of the Pelvis—PubMed n.d. Available online: https://pubmed.ncbi.nlm.nih.gov/1548259/ (accessed on 23 September 2024).
- Thompson, R.C.; A Pickvance, E.; Garry, D. Fractures in large-segment allografts. J. Bone Jt. Surg. Am. 1993, 75, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Hornicek, F.J.; Gebhardt, M.C.; Tomford, W.W.; Sorger, J.I.; Zavatta, M.; Menzner, J.P.; Mankin, H.J. Factors affecting nonunion of the allograft-host junction. Clin. Orthop. Relat. Res. 2001, 382, 87–98. [Google Scholar] [CrossRef]
- Capanna, R.; Campanacci, D.A.; Belot, N.; Beltrami, G.; Manfrini, M.; Innocenti, M.; Ceruso, M. A new reconstructive technique for intercalary defects of long bones: The association of massive allograft with vascularized fibular autograft. Long-term results and comparison with alternative techniques. Orthop. Clin. N. Am. 2007, 38, 51–60. [Google Scholar] [CrossRef]

| Patient # | Age at Surgery | Sex | Pathology/Diagnosis | Site | Follow-Up (Months) | MSTS93 (%) |
|---|---|---|---|---|---|---|
| 1 | 34 | Female | Osteosarcoma | Femur | 102 | 93.3 |
| 2 | 9 | Male | Osteosarcoma | Femur | 93 | 96.6 |
| 3 | 16 | Male | Ewing | Tibia | 89 | 86.6 |
| 4 | 6 | Male | Ewing | Femur | 86 | N/A |
| 5 | 44 | Male | MFH of Bone | Tibia | 85 | 26.6 |
| 6 | 20 | Female | Osteosarcoma | Femur | 83 | 76.6 |
| 7 | 12 | Female | Ewing | Femur | 78 | 96.6 |
| 8 | 17 | Male | Osteosarcoma | Femur | 76 | 90 |
| 9 | 18 | Male | Osteosarcoma | Femur | 76 | 83.3 |
| 10 | 9 | Male | Osteosarcoma | Femur | 66 | 43.3 |
| 11 | 20 | Female | Chondrosarcoma | Femur | 65 | N/A |
| 12 | 25 | Male | Osteosarcoma | Femur | 62 | 63.3 |
| 13 | 7 | Female | Osteosarcoma | Femur | 50 | 70 |
| 14 | 16 | Female | Osteosarcoma | Femur | 50 | 93.3 |
| 15 | 9 | Male | Ewing | Tibia | 47 | N/A |
| 16 | 34 | Female | Atypical Cartilaginous Tumor | Femur | 47 | 90 |
| 17 | 12 | Male | Osteosarcoma | Femur | 41 | 90 |
| 18 | 20 | Male | Ewing | Tibia | 37 | 93.3 |
| 19 | 17 | Female | Osteosarcoma | Femur | 35 | N/A |
| 20 | 14 | Male | Osteosarcoma | Femur | 21 | 50 |
| 21 | 14 | Female | Osteosarcoma | Femur | 21 | 80 |
| 22 | 56 | Male | Chondrosarcoma | Tibia | 13 | 86.6 |
| 23 | 59 | Male | Chondrosarcoma | Femur | 12 | N/A |
| Patient # | Resection | Reconstruction | Short-Term Complications | Long-Term Complications | LLD (cm) |
|---|---|---|---|---|---|
| 1 | Geographic | Allograft | None | None | |
| 2 | Intercalary | Vascularized Fibula and Allograft | None | None | 6 |
| 3 | Intercalary | Vascularized Fibula and Allograft | None | None | 2 |
| 4 | Intercalary | Allograft | None | None | 8.5 |
| 5 | Intercalary | Printed Cage Reconstruction | None | None | |
| 6 | Geographic | Allograft | None | Re-grafting due to non-union | |
| 7 | Geographic | Allograft | None | None | |
| 8 | Intercalary | Vascularized Fibula and Allograft | None | Re-grafting and re-fixation due to non-union | |
| 9 | Intercalary | Vascularized Fibula and Allograft | None | None | |
| 10 | Intercalary | Vascularized Fibula and Allograft | None | None | |
| 11 | Geographic | Allograft | None | Revision to megaprosthesis due to LR | |
| 12 | Geographic | Allograft | None | None | |
| 13 | Intercalary | Vascularized Fibula and Allograft | None | None | 2 |
| 14 | Intercalary | Vascularized Fibula and Allograft | Infection of soft tissues—antibiotics | None | 1.5 |
| 15 | Intercalary | Allograft | None | Capanna surgery due to allograft fracture | |
| 16 | Geographic | Allograft | None | None | |
| 17 | Intercalary | Vascularized Fibula and Allograft | None | Re-grafting and re-fixation due to non-union | 3 |
| 18 | Intercalary | Printed Cage Reconstruction | None | None | 2 |
| 19 | Intercalary | Printed Cage Reconstruction | None | I and D due to suspected infection | |
| 20 | Intercalary | Printed Cage Reconstruction | None | None | |
| 21 | Intercalary | Printed Cage Reconstruction | None | None | |
| 22 | Intercalary | Allograft | None | None | |
| 23 | Intercalary | Printed Cage Reconstruction | None | None |
| Patient # | Margins | Tissue Margin | Necrosis | Oncologic Event | Oncologic Status |
|---|---|---|---|---|---|
| 1 | R0 | Bone | N/A | None | NED |
| 2 | R0 | Bone + Soft | 87 | None | NED |
| 3 | R1 | Bone + Soft | 40 | None | NED |
| 4 | R1 | N/A | 100 | None | NED |
| 5 | R0 | Bone | 95 | LR + lung metastases | DOD |
| 6 | R1 | Bone + Soft | N/A | None | NED |
| 7 | R0 | Bone | 100 | None | NED |
| 8 | R0 | Bone | 99 | Lobectomy due to lung metastases | NED |
| 9 | R0 | Bone + Soft | 96 | None | NED |
| 10 | R0 | Bone | 100 | AKA due to LR + lobectomy due to lung metastases | NED |
| 11 | R1 | Bone + Soft | N/A | LR | NED |
| 12 | R1 | Bone + Soft | 78 | MTS | AED |
| 13 | R1 | Bone + Soft | 91 | Lung metastases | AED |
| 14 | R0 | Bone + Soft | 99 | None | NED |
| 15 | R1 | Bone + Soft | 94 | None | NED |
| 16 | R0 | Bone + Soft | N/A | None | NED |
| 17 | R0 | Bone + Suspected Soft | 100 | None | NED |
| 18 | R0 | Bone + Soft | N/A | None | NED |
| 19 | R0 | Bone | 100 | None | NED |
| 20 | R0 | Bone | 99 | MTS | AED |
| 21 | R0 | Bone + Soft | 93 | Lobectomy due to lung metastases | NED |
| 22 | R0 | Bone | N/A | None | NED |
| 23 | R0 | Bone | N/A | None | NED |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benady, A.; Yehiel, N.; Segal, O.; Merose, O.; Sterenheim, A.; Sher, O.; Efrima, B.; Golden, E.; Gortzak, Y.; Dadia, S. Knee-Sparing Resection and Reconstruction Surgery for Bone Sarcoma Using 3D-Surgical Approach: Average of 5-Year Follow-Up. Medicina 2025, 61, 476. https://doi.org/10.3390/medicina61030476
Benady A, Yehiel N, Segal O, Merose O, Sterenheim A, Sher O, Efrima B, Golden E, Gortzak Y, Dadia S. Knee-Sparing Resection and Reconstruction Surgery for Bone Sarcoma Using 3D-Surgical Approach: Average of 5-Year Follow-Up. Medicina. 2025; 61(3):476. https://doi.org/10.3390/medicina61030476
Chicago/Turabian StyleBenady, Amit, Noy Yehiel, Ortal Segal, Omri Merose, Amir Sterenheim, Osnat Sher, Ben Efrima, Eran Golden, Yair Gortzak, and Solomon Dadia. 2025. "Knee-Sparing Resection and Reconstruction Surgery for Bone Sarcoma Using 3D-Surgical Approach: Average of 5-Year Follow-Up" Medicina 61, no. 3: 476. https://doi.org/10.3390/medicina61030476
APA StyleBenady, A., Yehiel, N., Segal, O., Merose, O., Sterenheim, A., Sher, O., Efrima, B., Golden, E., Gortzak, Y., & Dadia, S. (2025). Knee-Sparing Resection and Reconstruction Surgery for Bone Sarcoma Using 3D-Surgical Approach: Average of 5-Year Follow-Up. Medicina, 61(3), 476. https://doi.org/10.3390/medicina61030476





