Diagnostic Performance and Interobserver Agreement of the Vesical Imaging–Reporting and Data System (VI-RADS) in Bladder Cancer Staging: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Definitions, Procedural Details, and Clinical Considerations
3.2. Quality Assessment and Methodological Rigor
3.3. Imaging Parameters, Protocols, and Technical Specifications
3.4. Baseline Characteristics of Included Studies
3.5. Statistical Analysis of Diagnostic Performance and Interobserver Agreement
3.6. Investigation of Heterogeneity
3.7. Evidence Certainty Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 10, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Woldu, S.L.; Bagrodia, A.; Lotan, Y. Guideline of Guidelines: Non-Muscle-Invasive Bladder Cancer. BJU Int. 2017, 119, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Egbers, L.; Grotenhuis, A.J.; Aben, K.K.; Witjes, J.A.; Kiemeney, L.A.; Vermeulen, S.H. The Prognostic Value of Family History Among Patients with Urinary Bladder Cancer. Int. J. Cancer 2015, 136, 1117–1124. [Google Scholar] [CrossRef]
- Teleka, S.; Häggström, C.; Nagel, G.; Björkman, K.; Manjer, J. Risk of Bladder Cancer by Disease Severity in Relation to Metabolic Factors and Smoking. Int. J. Cancer 2018, 143, 3071–3082. [Google Scholar] [CrossRef]
- Josephson, D.; Pasin, E.; Stein, J.P. Superficial Bladder Cancer: Part 2. Management. Expert Rev. Anticancer Ther. 2007, 7, 567–581. [Google Scholar] [CrossRef]
- Sherif, A.; Jonsson, M.N.; Wiklund, N.P. Treatment of Muscle-Invasive Bladder Cancer. Expert Rev. Anticancer Ther. 2007, 7, 1279–1283. [Google Scholar] [CrossRef]
- Kim, B.; Semelka, R.C.; Ascher, S.M.; Chalpin, D.B.; Carroll, P.R.; Hricak, H. Bladder Tumour Staging: Comparison of Contrast-Enhanced CT, T1- and T2-Weighted MRI, Dynamic Gadolinium-Enhanced Imaging, and Late Gadolinium-Enhanced Imaging. Radiology 1994, 193, 239–245. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sasaki, S.; Ito, M.; Okada, S.; Takahashi, S.; Kawai, T. Urinary Bladder Cancer: Diffusion-Weighted MR Imaging—Accuracy for Diagnosing T Stage and Estimating Histologic Grade. Radiology 2009, 251, 112–121. [Google Scholar] [CrossRef]
- Panebianco, V.; Barchetti, F.; de Haas, R.J.; Pearson, R.A.; Kennish, S.J.; Giannarini, G.; Catto, J.W. Improving Staging in Bladder Cancer: The Increasing Role of Multiparametric Magnetic Resonance Imaging. Eur. Urol. Focus 2016, 2, 113–121. [Google Scholar] [CrossRef]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R. Multiparametric Magnetic Resonance Imaging for BC: Development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhang, X.; Yu, Y.; Wang, J.; Shi, H. Preliminary Exploration of the Application of Vesical Imaging-Reporting and Data System (VI-RADS) in Post-treatment Patients With Bladder Cancer: A Prospective Single-Center Study. J. Magn. Reson. Imaging 2022, 55, 275–286. [Google Scholar] [CrossRef]
- Ueno, Y.; Takeuchi, M.; Tamada, T.; Sofue, K.; Takahashi, S.; Kamishima, Y.; Hinata, N.; Harada, K.; Fujisawa, M.; Murakami, T. VI-RADS: Multiinstitutional Multireader Diagnostic Accuracy and Interobserver Agreement Study. AJR Am. J. Roentgenol. 2021, 216, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Etxano, J.; Gonzalo, V.; Muñoz, F.; Iriarte, J.I.; Etxezarraga, C.; López, J.I. Vesical Imaging-Reporting and Data System (VI-RADS®): Initial Experience in the Classification of Muscle-Invasive Bladder Cancer. Actas Urol. Esp. 2021, 45, 320–325. [Google Scholar] [CrossRef]
- Del Giudice, F.; Barchetti, G.; De Berardinis, E.; Pecoraro, M.; Salvo, V.; Simone, G.; Sciarra, A.; Gallucci, M.; Catalano, C.; Panebianco, V. Prospective Assessment of Vesical Imaging Reporting and Data System (VI-RADS) and Its Clinical Impact on the Management of High-Risk Non-Muscle-Invasive Bladder Cancer Patients. Eur. Urol. 2020, 77, 101–109. [Google Scholar] [CrossRef]
- Kim, S.H. Validation of Vesical Imaging Reporting and Data System for Assessing Muscle Invasion in Bladder Tumour. Abdom. Radiol. 2020, 45, 491–498. [Google Scholar] [CrossRef]
- Makboul, M.; Alamleh, H.; Khater, A.; Farghaly, H. Multiparametric MRI in Differentiation Between Muscle Invasive and Non-Muscle Invasive Urinary Bladder Cancer with Vesical Imaging Reporting and Data System (VI-RADS) Application. Br. J. Radiol. 2019, 92, 20190401. [Google Scholar] [CrossRef]
- Barchetti, G.; Simone, G.; Ceravolo, I.; Salvo, V.; Campa, R.; Del Giudice, F.; Gallucci, M.; Catalano, C.; Panebianco, V. Multiparametric MRI of the Bladder: Inter-Observer Agreement and Accuracy with the Vesical Imaging-Reporting and Data System (VI-RADS) at a Single Reference Center. Eur. Radiol. 2019, 29, 5498–5506. [Google Scholar] [CrossRef]
- Wang, H.; Luo, C.; Zhang, F.; Guan, J.; Li, S.; Yao, H.; Chen, Y.; Cheng, Y.; Chen, W. Multiparametric MRI for Bladder Cancer: Validation of VI-RADS for the Detection of Detrusor Muscle Invasion. Radiology 2019, 291, 668–674. [Google Scholar] [CrossRef]
- Ueno, Y.; Tamada, T.; Bist, V.; Reinhold, C.; Miyake, H.; Tanaka, U.; Kitajima, K.; Sugimura, K.; Takahashi, S. Diagnostic Accuracy and Interobserver Agreement for the Vesical Imaging-Reporting and Data System for Muscle-Invasive Bladder Cancer: A Multireader Validation Study. Eur. Urol. 2019, 76, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Tillou, X.; Grardel, E.; Fourmarier, M.; Bernasconi, T.; Demailly, M.; Hakami, F.; Saint, F. Can MRI be Used to Distinguish Between Superficial and Invasive Transitional Cell Bladder Cancer? Prog. Urol. 2008, 18, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; De Berardinis, E.; Barchetti, G.; Simone, G.; Leonardo, C.; Grompone, M.D.; Del Monte, M.; Carano, D.; Gallucci, M.; Catto, J. An Evaluation of Morphological and Functional Multi-Parametric MRI Sequences in Classifying Non-Muscle and Muscle Invasive Bladder Cancer. Eur. Radiol. 2017, 27, 3759–3766. [Google Scholar] [CrossRef]
- Ghafoori, M.; Shakiba, M.; Ghiasi, A.; Asvadi, N.; Hosseini, K.; Alavi, M. Value of MRI in Local Staging of Bladder Cancer. Urol. J. 2013, 10, 866–872. [Google Scholar]
- Del Giudice, F.; Vestri, A.; Fegatelli, D.A.; Hüsch, T.; Belsey, J.; Nair, R.; Skinner, E.C.; Chung, B.I.; Pecoraro, M.; Sciarra, A.; et al. VI-RADS Followed by Photodynamic Transurethral Resection of Non-Muscle-Invasive Bladder Cancer vs White-Light Conventional and Second-resection: The ‘CUT-Less’ Randomised Trial Protocol. BJU Int. 2025, 135, 346–354. [Google Scholar] [CrossRef]
- Kural, S.; Pathak, A.K.; Singh, S.; Jain, G.; Yadav, M.; Agarwal, S.; Kumar, I.; Gupta, M.; Singh, Y.; Kumar, U.; et al. Prospective Assessment of VI-RADS with Muscle Invasion in Urinary Bladder Cancer and Its Implication on Re-Resection/Restaging TURBT Patients. Ann. Surg. Oncol. 2025, 32, 609–618. [Google Scholar] [CrossRef]
- EAU 2023: Clinical Staging for Bladder Cancer: Have Imaging Techniques Reached a Point Where We Can Avoid TURBT—At Initial Diagnosis? After Neoadjuvant Therapy? Available online: https://www.urotoday.com/conference-highlights/eau-annual-congress-2023/eau-2023-bladder-cancer/143145-eau-2023-clinical-staging-for-bladder-cancer-have-imaging-techniques-reached-a-point-where-we-can-avoid-turbt-at-initial-diagnosis-after-neoadjuvant-therapy.html (accessed on 28 February 2025).
- Beijert, I.J.; Hentschel, A.E.; Bründl, J.; Compérat, E.M.; Plass, K.; Rodríguez, O.; Subiela Henríquez, J.D.; Hernández, V.; de la Peña, E.; Alemany, I.; et al. Prognosis of Primary Papillary Ta Grade 3 Bladder Cancer in the Non-Muscle-Invasive Spectrum. Eur. Urol. Oncol. 2023, 6, 214–221. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, S.M.; Kim, B.; Park, J.; Jeong, B.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y. Quantitation of Bladder Cancer for the Prediction of Muscle Layer Invasion as a Complement to the Vesical Imaging-Reporting and Data System. Eur. Radiol. 2021, 31, 1656–1666. [Google Scholar] [CrossRef]
- Ghanshyam, K.; Taneja, K.; Elhence, P.; Pandey, H.; Elhence, P.A.; Goel, A. Validation of Vesical Imaging Reporting and Data System Score for the Diagnosis of Muscle-Invasive Bladder Cancer: A Prospective Cross-Sectional Study. Asian J. Urol. 2022, 9, 467–472. [Google Scholar] [CrossRef]
- Aziz, A.; Shariat, S.F.; Roghmann, F.; Brookman-May, S.; Stief, C.G.; Rink, M.; Chun, F.K.; Fisch, M.; Novotny, V.; Froehner, M. Prediction of Cancer-Specific Survival After Radical Cystectomy in pT4a Urothelial Carcinoma of the Bladder: Development of a Tool for Clinical Decision-Making. BJU Int. 2016, 117, 272–279. [Google Scholar] [CrossRef]
- Moschini, M.; D’Andrea, D.; Korn, S.; Irmak, Y.; Soria, F.; Compérat, E.; Shariat, S.F. Characteristics and Clinical Significance of Histological Variants of Bladder Cancer. Nat. Rev. Urol. 2017, 14, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Sankhwar, S.; Goel, A.; Kumar, M.; Purkait, B.; Aeron, R. Grading of Complications of Transurethral Resection of Bladder Tumor Using Clavien-Dindo Classification System. Indian J. Urol. 2016, 32, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, S.W.E.; Veenboer, P.W.; Wessels, F.J.; Meijer, R.P. Diagnostic Accuracy of Multiparametric MRI for Local Staging of Bladder Cancer: A Systematic Review and Meta-Analysis. Urology 2020, 145, 22–29. [Google Scholar] [CrossRef]
- da Silva, M.C.; Pecoraro, M.; Pisciotti, M.L.; Dehghanpour, A.; Forookhi, A.; Lucciola, S.; Bicchetti, M.; Messina, E.; Catalano, C.; Panebianco, V. The Learning Curve in Bladder MRI Using VI-RADS Assessment Score During an Interactive Dedicated Training Program. Eur. Radiol. 2022, 32, 7494–7503. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Tramanzoli, P.; Castellani, D.; De Stefano, V.; Brocca, C.; Nedbal, C.; Chiacchio, G.; Galosi, A.B.; Da Silva, R.D.; Teoh, J.Y.; Tiong, H.Y. Radiomics vs Radiologist in Bladder and Renal Cancer: Results from a Systematic Review. Cent. Eur. J. Urol. 2023, 76, 12–19. [Google Scholar] [CrossRef]
- Eryuruk, U.; Tasdemir, M.N.; Aslan, S. Comparison of the Diagnostic Performance of Biparametric and Multiparametric MRI in Detecting Muscle Invasion of Bladder Cancer Located at the Ureteral Orifice. Abdom. Radiol. 2023, 48, 3174–3182. [Google Scholar] [CrossRef]
- Caglic, I.; Panebianco, V.; Vargas, H.A. MRI of Bladder Cancer: Local and Nodal Staging. J. Magn. Reson. Imaging 2020, 52, 1041–1059. [Google Scholar] [CrossRef]
- El-Fawakry, R.M.; Hamed, E.M.; Metwally, M.I. Diagnostic Accuracy and Discriminative Power of Biparametric Versus Multiparametric MRI in Predicting Muscle-Invasive Bladder Cancer. Eur. J. Radiol. 2022, 156, 110503. [Google Scholar]

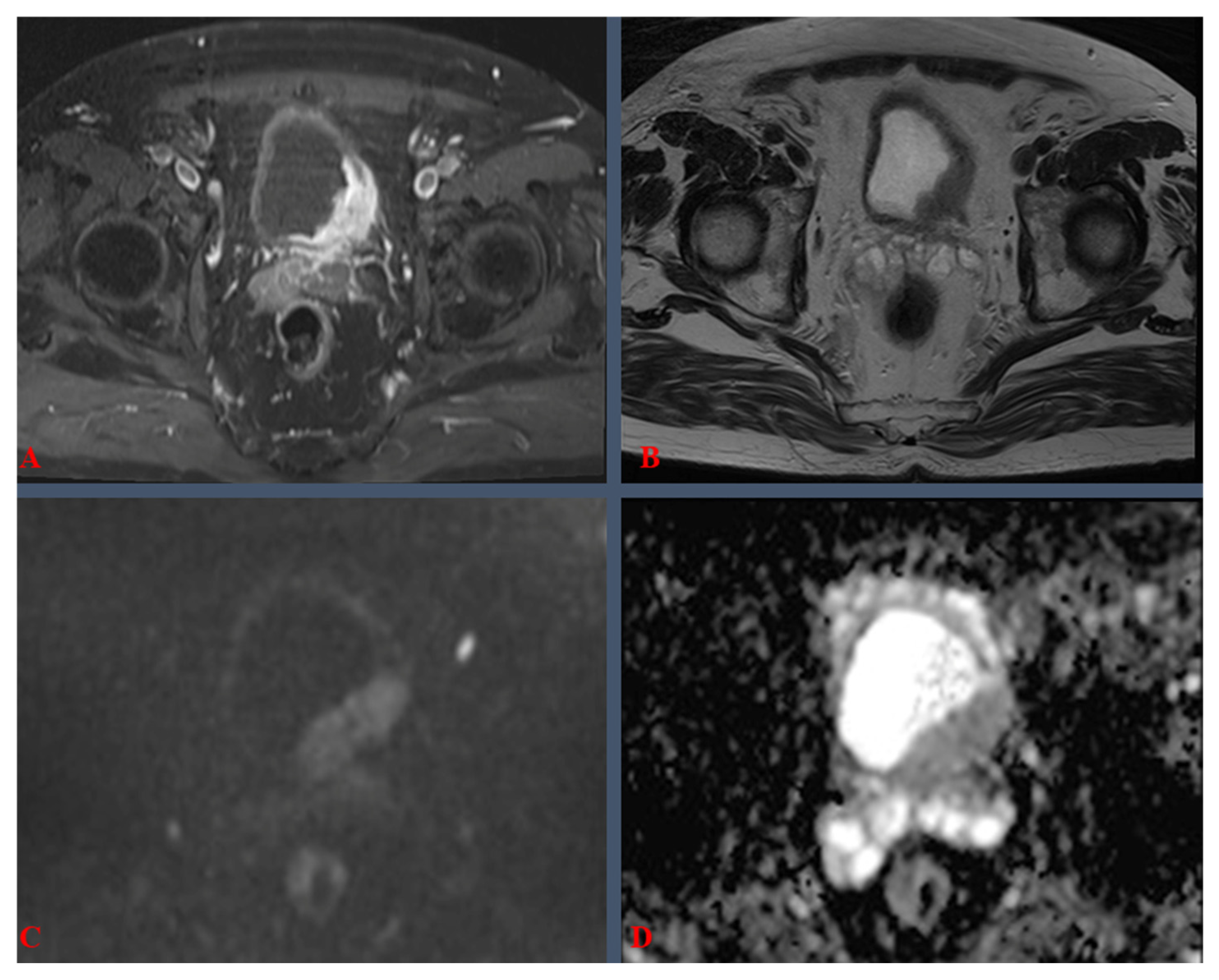
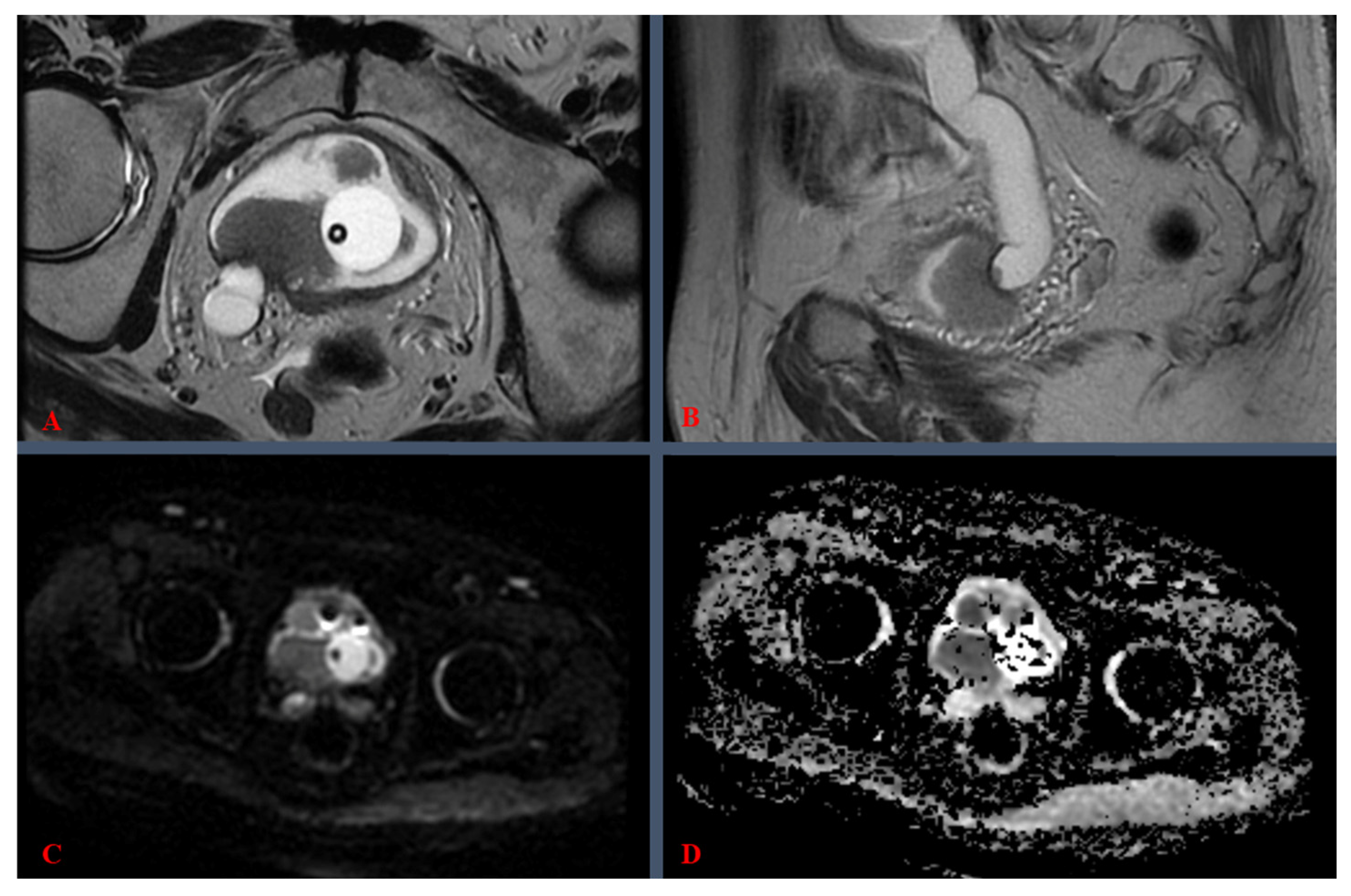
| VI-RADS 1 Score | Clinical Meaning |
|---|---|
| 1 | Muscle invasion is highly unlikely; Lesion < 1 cm. |
| 2 | Muscle invasion is unlikely; Lesion > 1 cm. |
| 3 | Muscle invasion cannot be excluded. |
| 4 | Muscle invasion is likely; Interruption and invasion of low-signal muscle layer. |
| 5 | Muscle invasion is very likely; Beyond-bladder invasion is very likely; Extension of lesion into perivesical fat and surroundings. |
| Study | Institution Type | Field Strength | T2WI Slice Thickness (mm) | DWI B-Values (s/mm2) | DCE Temporal Resolution (s) | Mean ADC (×10−3 mm2/s) for MIBC |
|---|---|---|---|---|---|---|
| Cao B. et al., (2022) [13] | Single center | 3.0 T | 3/4 * | 0, 800, 1000 | 8 | 0.85 |
| Ueno Y. et al., (2021) [14] | Multi-center | 1.5 T/3.0 T | 3–4 | 0, 1000 | 12 | NR |
| Etxano J. et al., (2021) [15] | Single center | 1.5 T | 4 | 0, 800 | 18 | 0.91 |
| Del Giudice F. et al., (2020) [16] | Single center | 3.0 T | 3 | 0, 1000, 2000 | 7 | 0.87 |
| Kim S.H. et al., (2020) [17] | Single center | 3.0 T | 3 | 0, 1000 | 12 | 0.89 |
| Makboul M. et al., (2019) [18] | Single center | 1.5 T | 4 | 0, 800 | 16 | 0.82 |
| Barchetti G. et al., (2019) [19] | Single center | 3.0 T | 3 | 0, 1000 | 9 | 0.86 |
| Wang H. et al., (2019) [20] | Single center | 3.0 T | 3 | 0, 1000 | 13 | 0.84 |
| Ueno Y. et al., (2019) [21] | Single center | 3.0 T | 3 | 0, 1000 | 11 | 0.88 |
| Study | Sensitivity | Specificity | Cohen κ (K) | AUROC | Patients |
|---|---|---|---|---|---|
| Cao B. et al., (2022) [13] | 85% | 94.3% | 0.70 | 0.901 | 73 |
| Ueno Y. et al., (2021) [14] | 74.1% | 94.1% | 0.55 | 0.87 | 91 |
| Etxano J. et al., (2021) [15] | 91.7% | 87.5% | 0.55 | 0.963 | 18 |
| Del Giudice F., et al. (2020) [16] | 91.9% | 91.1% | 0.81 | 0.94 | 231 |
| Kim S.H. et al., (2020) [17] | 94.6% | 43.9% | 0.85 | - | 297 |
| Makboul M., et al., (2019) [18] | 78% | 88% | 0.82 | 0.83 | 50 |
| Barchetti G., et al., (2019) [19] | 82% | 89% | 0.731 | 0.926 | 75 |
| Wang H. et al., (2019) [20] | 87.1% | 96.5% | 0.92 | 0.94 | 340 |
| Ueno Y. et al., (2019) [21] | 88% | 77% | 0.85 | 0.9 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesiu, A.; Novacescu, D.; Latcu, S.; Bardan, R.; Cumpanas, A.; Zara, F.; Buciu, V.; Caprariu, R.; Cut, T.G.; Stana, A.H. Diagnostic Performance and Interobserver Agreement of the Vesical Imaging–Reporting and Data System (VI-RADS) in Bladder Cancer Staging: A Systematic Review. Medicina 2025, 61, 469. https://doi.org/10.3390/medicina61030469
Nesiu A, Novacescu D, Latcu S, Bardan R, Cumpanas A, Zara F, Buciu V, Caprariu R, Cut TG, Stana AH. Diagnostic Performance and Interobserver Agreement of the Vesical Imaging–Reporting and Data System (VI-RADS) in Bladder Cancer Staging: A Systematic Review. Medicina. 2025; 61(3):469. https://doi.org/10.3390/medicina61030469
Chicago/Turabian StyleNesiu, Alexandru, Dorin Novacescu, Silviu Latcu, Razvan Bardan, Alin Cumpanas, Flavia Zara, Victor Buciu, Radu Caprariu, Talida Georgiana Cut, and Ademir Horia Stana. 2025. "Diagnostic Performance and Interobserver Agreement of the Vesical Imaging–Reporting and Data System (VI-RADS) in Bladder Cancer Staging: A Systematic Review" Medicina 61, no. 3: 469. https://doi.org/10.3390/medicina61030469
APA StyleNesiu, A., Novacescu, D., Latcu, S., Bardan, R., Cumpanas, A., Zara, F., Buciu, V., Caprariu, R., Cut, T. G., & Stana, A. H. (2025). Diagnostic Performance and Interobserver Agreement of the Vesical Imaging–Reporting and Data System (VI-RADS) in Bladder Cancer Staging: A Systematic Review. Medicina, 61(3), 469. https://doi.org/10.3390/medicina61030469








