Hypoabsorption in Bariatric Surgery: Is the Benefit Worth the Risk?
Abstract
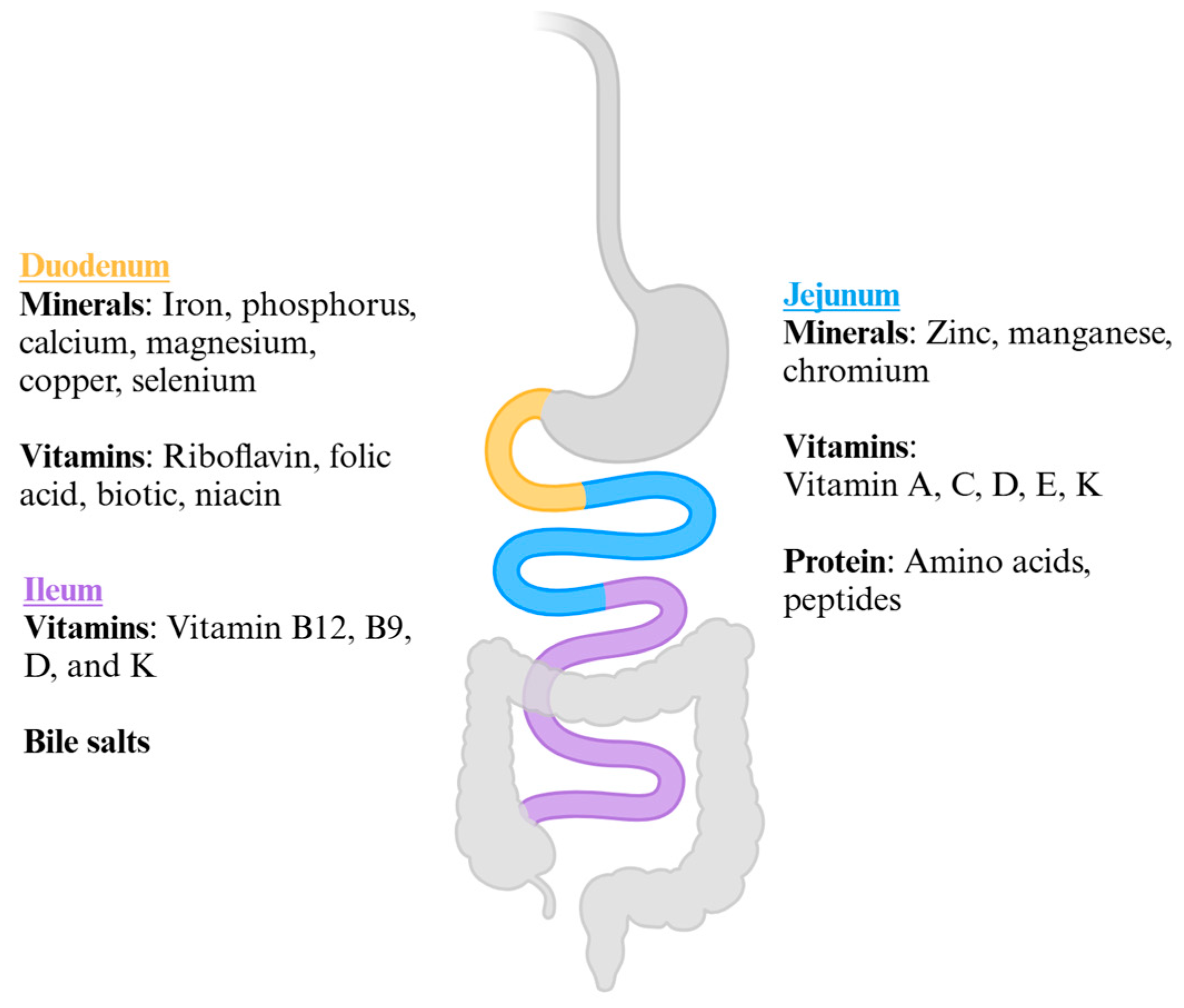
1. Introduction
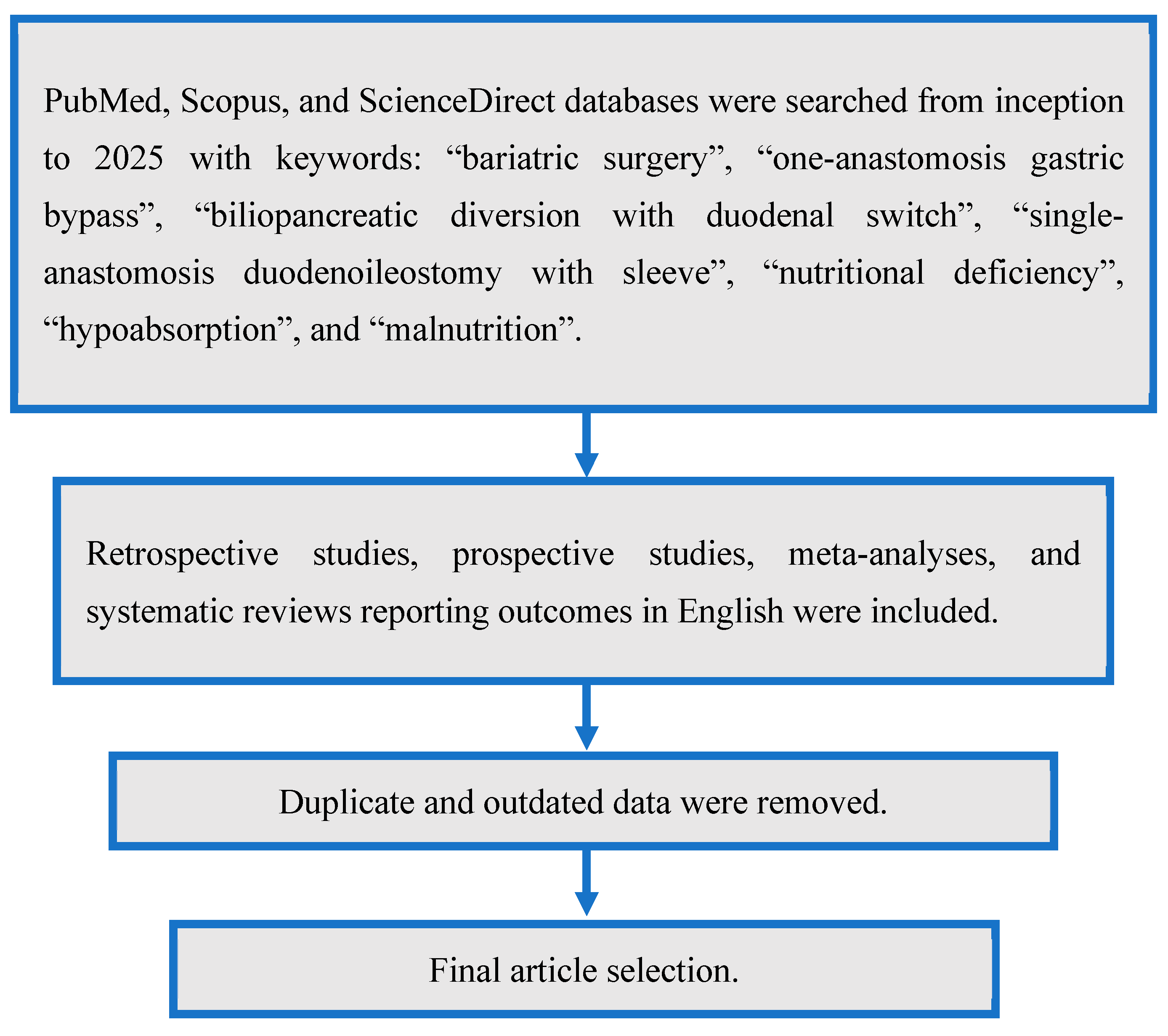
2. Materials and Methods
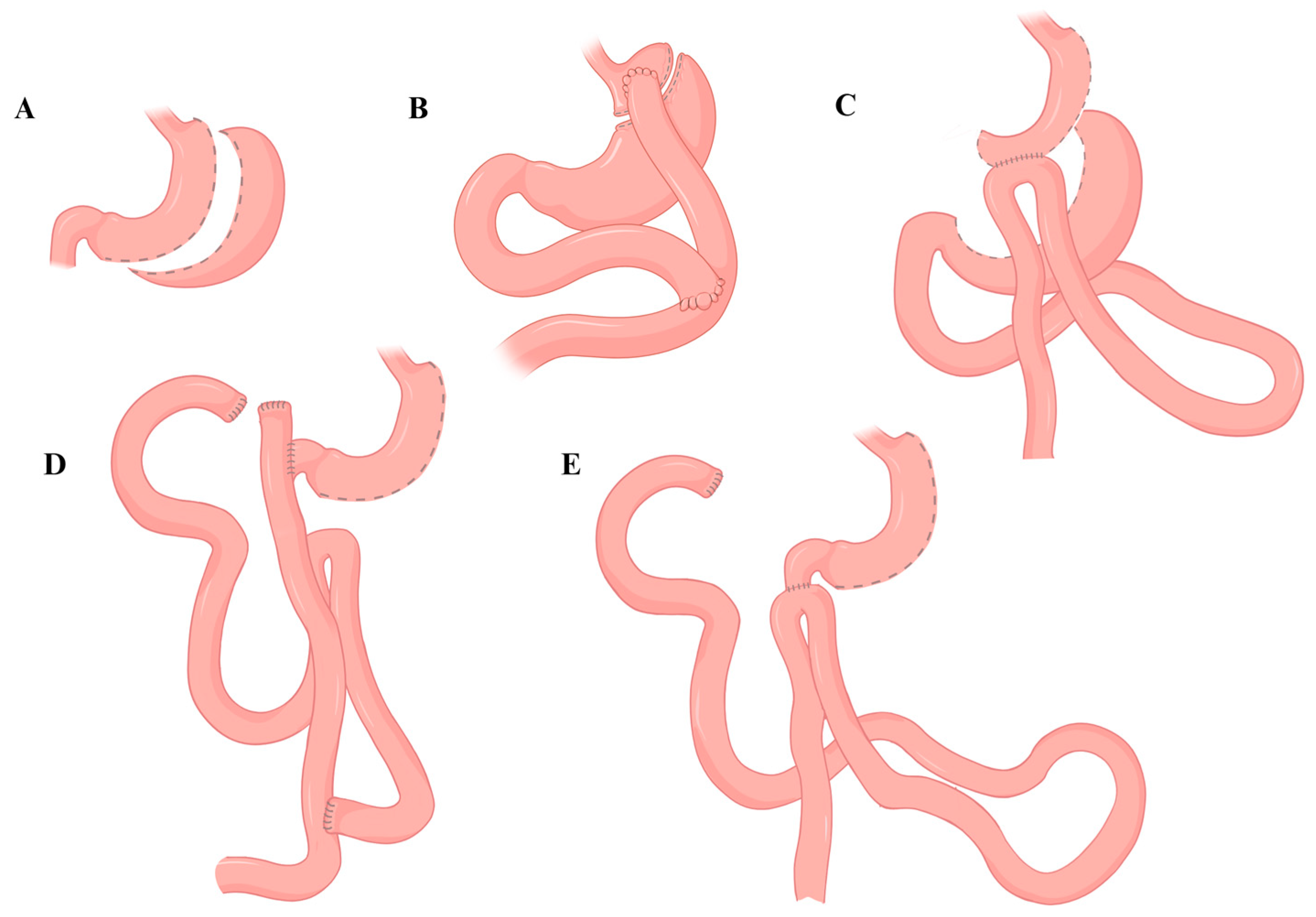
3. One-Anastomosis Gastric Bypass (OAGB)
4. Bilio-Pancreatic Diversion with Duodenal Switch (BPD-DS)
5. Single-Anastomosis Duodenal-Ileal Bypass with Sleeve (SADI-S)
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MBS | Metabolic and bariatric surgery |
| SG | Sleeve gastrectomy |
| RYGB | Roux-en-Y gastric bypass |
| OAGB | One-anastomosis gastric bypass |
| ASMBS | American Society for Metabolic and Bariatric Surgery |
| BPD-DS | Biliopancreatic diversion with duodenal switch |
| SADI-S | Single-anastomosis duodenal switch with sleeve |
| BMI | Body mass index |
| GLP-1 | Glucagon-like peptide-1 |
| %EWL | Percentage excess weight loss |
| %TWL | Percentage total weight loss |
| CC | Common channel |
References
- Zhou, X.D.; Chen, Q.F.; Yang, W.; Zuluaga, M.; Targher, G.; Byrne, C.D.; Valenti, L.; Luo, F.; Katsouras, C.S.; Thaher, O.; et al. Burden of disease attributable to high body mass index: An analysis of data from the Global Burden of Disease Study 2021. eClinicalMedicine 2024, 76, 102848. [Google Scholar] [CrossRef] [PubMed]
- Alsaqaaby, M.S.; Cooney, S.; le Roux, C.W.; Pournaras, D.J. Sex, race, and BMI in clinical trials of medications for obesity over the past three decades: A systematic review. Lancet Diabetes Endocrinol. 2024, 12, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Masrur, M.A.; Manueli Laos, E.G.; Zhang, L.; Acosta, A.; Pirzada, A.; Schlottmann, F. Sleeve Gastrectomy Versus Semaglutide for Weight Loss in a Severely Obese Minority Cohort: A Propensity-Matched Study. Obes. Surg. 2025. [Google Scholar] [CrossRef]
- Sarma, S.; Palcu, P. Weight loss between glucagon-like peptide-1 receptor agonists and bariatric surgery in adults with obesity: A systematic review and meta-analysis. Obesity 2022, 30, 2111–2121. [Google Scholar] [CrossRef]
- Clapp, B.; Badaoui, J.N.; Gamez, J.A.; Vivar, A.; Ghanem, O.M. Reluctance in duodenal switch adoption: An international survey among bariatric surgeons. Surg. Obes. Relat. Dis. 2021, 17, 1760–1765. [Google Scholar] [CrossRef]
- Elmaleh-Sachs, A.; Schwartz, J.L.; Bramante, C.T.; Nicklas, J.M.; Gudzune, K.A.; Jay, M. Obesity Management in Adults: A Review. JAMA 2023, 330, 2000–2015. [Google Scholar] [CrossRef]
- De Luca, M.; Belluzzi, A.; Salminen, P.; Bueter, M.; Pujol-Rafols, J.; Sakran, N.; Stier, C.; Taskin, H.E.; Chiappetta, S.; Carrano, F.M.; et al. Development of the International Federation for Surgery of Obesity and Metabolic Disorders-European Chapter (IFSO-EC) Grade-Based Guidelines on the Surgical Treatment of Obesity Using Multimodal Strategies: Design and Methodological Aspects. J. Clin. Med. 2024, 13, 5106. [Google Scholar] [CrossRef]
- Clapp, B.; Ponce, J.; Corbett, J.; Ghanem, O.M.; Kurian, M.; Rogers, A.M.; Peterson, R.M.; LaMasters, T.; English, W.J. American Society for Metabolic and Bariatric Surgery 2022 estimate of metabolic and bariatric procedures performed in the United States. Surg. Obes. Relat. Dis. 2024, 20, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.; Evans, L.A.; Castillo-Larios, R.; Celik, N.B.; Elli, E.F. One anastomosis gastric bypass as a primary bariatric surgery: MBSAQIP database analysis of short-term safety and outcomes. Surg. Endosc. 2024, 38, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.J.; Park, A.K.; Hutter, M.M. The United States Experience with One Anastomosis Gastric Bypass at MBSAQIP-Accredited Centers. Obes. Surg. 2022, 32, 3239–3247. [Google Scholar] [CrossRef]
- Nakanishi, H.; Mosleh, K.A.; Al-Kordi, M.; Farsi, S.; Chaudhry, S.; Marrero, K.; Scott Davis, S., Jr.; Kermansaravi, M.; Parmar, C.; Clapp, B.; et al. One Anastomosis Gastric Bypass as Revisional Surgery Following Sleeve Gastrectomy: A Systematic Review and Meta-Analysis. Obes. Surg. 2024, 34, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, G.; Bocchinfuso, S.; Jawhar, N.; Gajjar, A.; Betancourt, R.S.; Portela, R.; Ghusn, W.; Ghanem, O.M. Novel Surgical Interventions for the Treatment of Obesity. J. Clin. Med. 2024, 13, 5279. [Google Scholar] [CrossRef]
- Mahawar, K.K.; Borg, C.-M.; Kular, K.S.; Courtney, M.J.; Sillah, K.; Carr, W.R.J.; Jennings, N.; Madhok, B.; Singhal, R.; Small, P.K. Understanding Objections to One Anastomosis (Mini) Gastric Bypass: A Survey of 417 Surgeons Not Performing this Procedure. Obes. Surg. 2017, 27, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, R. The mini-gastric bypass: Experience with the first 1,274 cases. Obes. Surg. 2001, 11, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Bandlamudi, N.; Holt, G.; Graham, Y.; O’Kane, M.; Singhal, R.; Parmar, C.; Sakran, N.; Mahawar, K.; Pouwels, S.; Potluri, S.; et al. Malnutrition Following One-Anastomosis Gastric Bypass: A Systematic Review. Obes. Surg. 2023, 33, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Currie, A.C.; Askari, A.; Fangueiro, A.; Mahawar, K. Network Meta-Analysis of Metabolic Surgery Procedures for the Treatment of Obesity and Diabetes. Obes. Surg. 2021, 31, 4528–4541. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, E.O.; Liem, R.S.L.; Nienhuijs, S.W.; Greve, J.W.M.; Marang-van de Mheen, P.J. Hospital Variation in Preference for a Specific Bariatric Procedure and the Association with Weight Loss Performance: A Nationwide Analysis. Obes. Surg. 2022, 32, 3589–3599. [Google Scholar] [CrossRef]
- Billeter, A.T.; Fischer, L.; Wekerle, A.L.; Senft, J.; Müller-Stich, B. Malabsorption as a Therapeutic Approach in Bariatric Surgery. Viszeralmedizin 2014, 30, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Abi Mosleh, K.; Belluzzi, A.; Jawhar, N.; Marrero, K.; Al-Kordi, M.; Hage, K.; Ghanem, O.M. Single Anastomosis Duodenoileostomy with Sleeve: A Comprehensive Review of Anatomy, Surgical Technique, and Outcomes. Curr. Obes. Rep. 2024, 13, 121–131. [Google Scholar] [CrossRef]
- Carbajo, M.A.; Luque-de-León, E.; Jiménez, J.M.; Ortiz-de-Solórzano, J.; Pérez-Miranda, M.; Castro-Alija, M.J. Laparoscopic One-Anastomosis Gastric Bypass: Technique, Results, and Long-Term Follow-Up in 1200 Patients. Obes. Surg. 2017, 27, 1153–1167. [Google Scholar] [CrossRef]
- Robert, M.; Espalieu, P.; Pelascini, E.; Caiazzo, R.; Sterkers, A.; Khamphommala, L.; Poghosyan, T.; Chevallier, J.-M.; Malherbe, V.; Chouillard, E.; et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): A multicentre, randomised, open-label, non-inferiority trial. Lancet 2019, 393, 1299–1309. [Google Scholar] [CrossRef]
- Liagre, A.; Debs, T.; Kassir, R.; Ledit, A.; Juglard, G.; Chalret du Rieu, M.; Lazzati, A.; Martini, F.; Petrucciani, N. One Anastomosis Gastric Bypass with a Biliopancreatic Limb of 150 cm: Weight Loss, Nutritional Outcomes, Endoscopic Results, and Quality of Life at 8-Year Follow-Up. Obes. Surg. 2020, 30, 4206–4217. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, K.K.; Parmar, C.; Carr, W.R.J.; Jennings, N.; Schroeder, N.; Small, P.K. Impact of biliopancreatic limb length on severe protein-calorie malnutrition requiring revisional surgery after one anastomosis (mini) gastric bypass. J. Minim. Access Surg. 2018, 14, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zarshenas, N.; Tapsell, L.C.; Batterham, M.; Neale, E.P.; Talbot, M.L. Changes in Anthropometric Measures, Nutritional Indices and Gastrointestinal Symptoms Following One Anastomosis Gastric Bypass (OAGB) Compared with Roux-en-y Gastric Bypass (RYGB). Obes. Surg. 2021, 31, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Kessler, Y.; Adelson, D.; Mardy-Tilbor, L.; Ben-Porat, T.; Szold, A.; Goitein, D.; Sakran, N.; Raziel, A.; Sherf-Dagan, S. Nutritional status following One Anastomosis Gastric Bypass. Clin. Nutr. 2020, 39, 599–605. [Google Scholar] [CrossRef]
- Parmar, C.D.; Mahawar, K.K. One Anastomosis (Mini) Gastric Bypass Is Now an Established Bariatric Procedure: A Systematic Review of 12,807 Patients. Obes. Surg. 2018, 28, 2956–2967. [Google Scholar] [CrossRef]
- Jammu, G.S.; Sharma, R. A 7-Year Clinical Audit of 1107 Cases Comparing Sleeve Gastrectomy, Roux-En-Y Gastric Bypass, and Mini-Gastric Bypass, to Determine an Effective and Safe Bariatric and Metabolic Procedure. Obes. Surg. 2016, 26, 926–932. [Google Scholar] [CrossRef]
- Syn, N.L.; Lee, P.C.; Kovalik, J.P.; Tham, K.W.; Ong, H.S.; Chan, W.H.; Tan, C.S.; Lim, C.H. Associations of Bariatric Interventions with Micronutrient and Endocrine Disturbances. JAMA Netw. Open 2020, 3, e205123. [Google Scholar] [CrossRef]
- Shirazi, N.; Beglaibter, N.; Grinbaum, R.; Ahmad, W.A.; Aronis, A. Nutritional Outcomes One Year after One Anastomosis Gastric Bypass Compared to Sleeve Gastrectomy. Nutrients 2022, 14, 2597. [Google Scholar] [CrossRef] [PubMed]
- Voglino, C.; Tirone, A.; Ciuoli, C.; Benenati, N.; Bufano, A.; Croce, F.; Gaggelli, I.; Vuolo, M.L.; Badalucco, S.; Berardi, G.; et al. Controlling Nutritional Status (CONUT) Score and Micronutrient Deficiency in Bariatric Patients: Midterm Outcomes of Roux-en-Y Gastric Bypass Versus One Anastomosis Gastric Bypass/Mini Gastric Bypass. Obes. Surg. 2021, 31, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liang, S.; Yu, X.; Guan, B.; Yang, Q.; Ming, W.-k.; Chen, Y. Change in Mineral Status After Bariatric Surgery: A Meta-analysis. Obes. Surg. 2023, 33, 3907–3931. [Google Scholar] [CrossRef]
- Risstad, H.; Søvik, T.T.; Engström, M.; Aasheim, E.T.; Fagerland, M.W.; Olsén, M.F.; Kristinsson, J.A.; le Roux, C.W.; Bøhmer, T.; Birkeland, K.I. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: A randomized clinical trial. JAMA Surg. 2015, 150, 352–361. [Google Scholar] [CrossRef]
- Nelson, D.W.; Blair, K.S.; Martin, M.J. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs. gastric bypass for morbid obesity. Arch. Surg. 2012, 147, 847–854. [Google Scholar] [CrossRef]
- Sethi, M.; Chau, E.; Youn, A.; Jiang, Y.; Fielding, G.; Ren-Fielding, C. Long-term outcomes after biliopancreatic diversion with and without duodenal switch: 2-, 5-, and 10-year data. Surg. Obes. Relat. Dis. 2016, 12, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Marceau, P.; Biron, S.; Marceau, S.; Hould, F.S.; Lebel, S.; Lescelleur, O.; Biertho, L.; Simard, S.; Kral, J.G. Long-Term Metabolic Outcomes 5 to 20 Years After Biliopancreatic Diversion. Obes. Surg. 2015, 25, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Topart, P.; Becouarn, G.; Sallé, A.; Ritz, P. Biliopancreatic diversion requires multiple vitamin and micronutrient adjustments within 2 years of surgery. Surg. Obes. Relat. Dis. 2014, 10, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Strain, G.W.; Torghabeh, M.H.; Gagner, M.; Ebel, F.; Dakin, G.F.; Connolly, D.; Goldenberg, E.; Pomp, A. Nutrient Status 9 Years After Biliopancreatic Diversion with Duodenal Switch (BPD/DS): An Observational Study. Obes. Surg. 2017, 27, 1709–1718. [Google Scholar] [CrossRef]
- Finno, P.; Osorio, J.; García-Ruiz-de-Gordejuela, A.; Casajoana, A.; Sorribas, M.; Admella, V.; Serrano, M.; Marchesini, J.B.; Ramos, A.C.; Pujol-Gebellí, J. Single Versus Double-Anastomosis Duodenal Switch: Single-Site Comparative Cohort Study in 440 Consecutive Patients. Obes. Surg. 2020, 30, 3309–3316. [Google Scholar] [CrossRef]
- Salame, M.; Teixeira, A.F.; Lind, R.; Abi Mosleh, K.; Ghanem, M.; Jawad, M.A.; Kendrick, M.L.; Ghanem, O.M. Effect of Limb Length on Weight Loss Outcomes Following Biliopancreatic Diversion with Duodenal Switch: A Multi-Centered Study. Obes. Surg. 2025, 35, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Papadia, F.S.; Adami, G.; Razzetta, A.; Florenzano, A.; Longo, G.; Rubartelli, A.; Carlini, F.; De Cian, O.; Camerini, G. Biliopancreatic diversion for severe obesity: Long-term weight maintenance and occurrence of nutritional complications are two facets of the same coin. Br. J. Surg. 2024, 111, znae058. [Google Scholar] [CrossRef]
- Aasheim, E.T.; Björkman, S.; Søvik, T.T.; Engström, M.; Hanvold, S.E.; Mala, T.; Olbers, T.; Bøhmer, T. Vitamin status after bariatric surgery: A randomized study of gastric bypass and duodenal switch. Am. J. Clin. Nutr. 2009, 90, 15–22. [Google Scholar] [CrossRef]
- Søvik, T.T.; Aasheim, E.T.; Taha, O.; Engström, M.; Fagerland, M.W.; Björkman, S.; Kristinsson, J.; Birkeland, K.I.; Mala, T.; Olbers, T. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: A randomized trial. Ann. Intern. Med. 2011, 155, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Magee, C.J.; Barry, J.; Brocklehurst, J.; Javed, S.; Macadam, R.; Kerrigan, D.D. Outcome of laparoscopic duodenal switch for morbid obesity. Br. J. Surg. 2011, 98, 79–84. [Google Scholar] [CrossRef]
- Surve, A.; Cottam, D.; Medlin, W.; Richards, C.; Belnap, L.; Horsley, B.; Cottam, S.; Cottam, A. Long-term outcomes of primary single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). Surg. Obes. Relat. Dis. 2020, 16, 1638–1646. [Google Scholar] [CrossRef]
- Pennestrì, F.; Sessa, L.; Prioli, F.; Salvi, G.; Gallucci, P.; Ciccoritti, L.; Greco, F.; De Crea, C.; Raffaelli, M. Single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S): Experience from a high-bariatric volume center. Langenbecks Arch. Surg. 2022, 407, 1851–1862. [Google Scholar] [CrossRef]
- Yashkov, Y.; Bordan, N.; Torres, A.; Malykhina, A.; Bekuzarov, D. SADI-S 250 vs. Roux-en-Y Duodenal Switch (RY-DS): Results of 5-Year Observational Study. Obes. Surg. 2021, 31, 570–579. [Google Scholar] [CrossRef]
- Gebellí, J.P.; Lazzara, C.; de Gordejuela, A.G.R.; Nora, M.; Pereira, A.M.; Sánchez-Pernaute, A.; Osorio, J.; Sobrino, L.; García, A.J.T. Duodenal Switch vs. Single-Anastomosis Duodenal Switch (SADI-S) for the Treatment of Grade IV Obesity: 5-Year Outcomes of a Multicenter Prospective Cohort Comparative Study. Obes. Surg. 2022, 32, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, L.; Li, S.; Liu, Y.; Zhang, Z.; Xiao, M.; Zhang, Z.; Wei, Z.; Cui, L.; Jiang, T. Evaluation study of single-anastomosis duodenal-ileal bypass with sleeve gastrectomy in the treatment of Chinese obese patients based on efficacy and nutrition. Sci. Rep. 2024, 14, 6522. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pernaute, A.; Herrera MÁ, R.; Ferré, N.P.; Rodríguez, C.S.; Marcuello, C.; Pañella, C.; Antoñanzas, L.L.; Torres, A.; Pérez-Aguirre, E. Long-Term Results of Single-Anastomosis Duodeno-ileal Bypass with Sleeve Gastrectomy (SADI-S). Obes. Surg. 2022, 32, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Surve, A.; Rao, R.; Cottam, D.; Rao, A.; Ide, L.; Cottam, S.; Horsley, B. Early Outcomes of Primary SADI-S: An Australian Experience. Obes. Surg. 2020, 30, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, M.; García-Caballero, M.; Toledano, M.; Osorio, D.; García-Lanza, C.; Carmona, J.A. One-Anastomosis Gastric Bypass by Laparoscopy: Results of the First 209 Patients. Obes. Surg. 2005, 15, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Abdelhalim, M.A.; Mahawar, K.K.; Boyle, M.; Carr, W.R.J.; Jennings, N.; Small, P.K. Management of super–super obese patients: Comparison between one anastomosis (mini) gastric bypass and Roux-en-Y gastric bypass. Surg. Endosc. 2017, 31, 3504–3509. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.D.; Bryant, C.; Luque-de-Leon, E.; Peraglie, C.; Prasad, A.; Rheinwalt, K.; Musella, M. One Anastomosis Gastric Bypass in Morbidly Obese Patients with BMI ≥ 50 kg/m2: A Systematic Review Comparing It with Roux-En-Y Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2019, 29, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.D.; Zakeri, R.; Mahawar, K. A Systematic Review of One Anastomosis/Mini Gastric Bypass as a Metabolic Operation for Patients with Body Mass Index ≤ 35 kg/m2. Obes. Surg. 2020, 30, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Kermansaravi, M.; Parmar, C.; Chiappetta, S.; Shahabi, S.; Abbass, A.; Abbas, S.I.; Abouzeid, M.; Antozzi, L.; Asghar, S.T.; Bashir, A.; et al. Patient Selection in One Anastomosis/Mini Gastric Bypass—An Expert Modified Delphi Consensus. Obes. Surg. 2022, 32, 2512–2524. [Google Scholar] [CrossRef]
- Level, L.; Rojas, A.; Piñango, S.; Avariano, Y. One anastomosis gastric bypass vs. Roux-en-Y gastric bypass: A 5-year follow-up prospective randomized trial. Langenbecks Arch. Surg. 2021, 406, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Stoica, L.; Dobrescu, A.; Isaic, A.; Verdeş, G.; Tarţa, C.; Lazăr, F. Metabolic and Hormonal Changes after Sleeve Gastrectomy and Mini Gastric Bypass in a Rat Model of Induced Type 2 Diabetes Mellitus and Obesity. Chirurgia 2019, 114, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Wang, W.; Lee, Y.C.; Huang, M.T.; Ser, K.H.; Chen, J.C. Laparoscopic mini-gastric bypass: Experience with tailored bypass limb according to body weight. Obes. Surg. 2008, 18, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, X.; Fu, C.; Han, L.; Xie, M.; Ouyang, S. Efficacy and Safety of One Anastomosis Gastric Bypass Versus Roux-en-Y Gastric Bypass for Obesity: A Meta-analysis and Systematic Review. Obes. Surg. 2023, 33, 611–622. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Tzovaras, G. One Anastomosis Gastric Bypass Versus Roux-en-Y Gastric Bypass for Morbid Obesity: An Updated Meta-Analysis. Obes. Surg. 2019, 29, 2721–2730. [Google Scholar] [CrossRef]
- Bhandari, M.; Nautiyal, H.K.; Kosta, S.; Mathur, W.; Fobi, M. Comparison of one-anastomosis gastric bypass and Roux-en-Y gastric bypass for treatment of obesity: A 5-year study. Surg. Obes. Relat. Dis. 2019, 15, 2038–2044. [Google Scholar] [CrossRef]
- Jia, D.; Tan, H.; Faramand, A.; Fang, F. One Anastomosis Gastric Bypass Versus Roux-en-Y Gastric Bypass for Obesity: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Obes. Surg. 2020, 30, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Yu, P.J.; Wang, W.; Chen, T.C.; Wei, P.L.; Huang, M.T. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: A prospective randomized controlled clinical trial. Ann. Surg. 2005, 242, 20–28. [Google Scholar] [CrossRef]
- Uhe, I.; Douissard, J.; Podetta, M.; Chevallay, M.; Toso, C.; Jung, M.K.; Meyer, J. Roux-en-Y gastric bypass, sleeve gastrectomy, or one-anastomosis gastric bypass? A systematic review and meta-analysis of randomized-controlled trials. Obesity 2022, 30, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Delko, T.; Kraljević, M.; Lazaridis, I.I.; Köstler, T.; Jomard, A.; Taheri, A.; Lutz, T.A.; Osto, E.; Zingg, U. Laparoscopic Roux-Y-gastric bypass versus laparoscopic one-anastomosis gastric bypass for obesity: Clinical & metabolic results of a prospective randomized controlled trial. Surg. Endosc. 2024, 38, 3875–3886. [Google Scholar] [PubMed]
- Parikh, M.; Eisenberg, D.; Johnson, J.; El-Chaar, M. American Society for Metabolic and Bariatric Surgery review of the literature on one-anastomosis gastric bypass. Surg. Obes. Relat. Dis. 2018, 14, 1088–1092. [Google Scholar] [CrossRef]
- Wickremasinghe, A.C.; Leang, Y.J.; Johari, Y.; Laurie, C.; Nadebaum, D.; Yue, H.; Yap, K.S.; Hebbard, G.S.; Brown, W.A.; Burton, P.R. Modified One Anastomosis Gastric Bypass Following Sleeve Gastrectomy for Severe Reflux and Delayed Gastric Emptying: A Prospective Trial with Clinical and Physiological Outcome Measures. Obes. Surg. 2024, 34, 2940–2953. [Google Scholar] [CrossRef]
- Guirat, A.; Addossari, H.M. One Anastomosis Gastric Bypass and Risk of Cancer. Obes. Surg. 2018, 28, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Via, M.A.; Mechanick, J.I. Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Update. Curr. Obes. Rep. 2017, 6, 286–296. [Google Scholar] [CrossRef]
- Aleman, R.; Lo Menzo, E.; Szomstein, S.; Rosenthal, R.J. Efficiency and risks of one-anastomosis gastric bypass. Ann. Transl. Med. 2020, 8 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Aills, L.; Blankenship, J.; Buffington, C.; Furtado, M.; Parrott, J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg. Obes. Relat. Dis. 2008, 4 (Suppl. S5), S73–S108. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Pacheco, D.; Izaola, O.; Terroba, M.C.; Cuellar, L.; Cabezas, G. Micronutrient status in morbidly obese women before bariatric surgery. Surg. Obes. Relat. Dis. 2013, 9, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Salgado, W., Jr.; Modotti, C.; Nonino, C.B.; Ceneviva, R. Anemia and iron deficiency before and after bariatric surgery. Surg. Obes. Relat. Dis. 2014, 10, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Krzizek, E.C.; Brix, J.M.; Herz, C.T.; Kopp, H.P.; Schernthaner, G.H.; Schernthaner, G.; Ludvik, B. Prevalence of Micronutrient Deficiency in Patients with Morbid Obesity Before Bariatric Surgery. Obes. Surg. 2018, 28, 643–648. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, M.; Parretti, H.M.; Pinkney, J.; Welbourn, R.; Hughes, C.A.; Mok, J.; Walker, N.; Thomas, D.; Devin, J.; Coulman, K.D.; et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery-2020 update. Obes. Rev. 2020, 21, e13087. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- Biertho, L.; Lebel, S.; Marceau, S.; Hould, F.S.; Julien, F.; Biron, S. Biliopancreatic Diversion with Duodenal Switch: Surgical Technique and Perioperative Care. Surg. Clin. N. Am. 2016, 96, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Sudan, R.; Jacobs, D.O. Biliopancreatic diversion with duodenal switch. Surg. Clin. N. Am. 2011, 91, 1281–1293. [Google Scholar] [CrossRef]
- Hess, D.S.; Hess, D.W.; Oakley, R.S. The biliopancreatic diversion with the duodenal switch: Results beyond 10 years. Obes. Surg. 2005, 15, 408–416. [Google Scholar] [CrossRef]
- Biertho, L.; Simon-Hould, F.; Marceau, S.; Lebel, S.; Lescelleur, O.; Biron, S. Current Outcomes of Laparoscopic Duodenal Switch. Ann. Surg. Innov. Res. 2016, 10, 1. [Google Scholar] [CrossRef]
- Elias, K.; Webb, D.L.; Diaz Tartera, H.O.; Hellström, P.M.; Sundbom, M. Impact of biliopancreatic diversion with duodenal switch on glucose homeostasis and gut hormones and their correlations with appetite. Surg. Obes. Relat. Dis. 2022, 18, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Bloom, S.R. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver 2012, 6, 10–20. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Clemente-Postigo, M.; Tinahones, F.J. Metabolic and Endocrine Consequences of Bariatric Surgery. Front. Endocrinol. 2019, 10, 626. [Google Scholar] [CrossRef]
- Li, W.; Baraboi, E.-D.; Cluny, N.L.; Roy, M.-C.; Samson, P.; Biertho, L. Malabsorption plays a major role in the effects of the biliopancreatic diversion with duodenal switch on energy metabolism in rats. Surg. Obes. Relat. Dis. 2015, 11, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Sudan, R.; Maciejewski, M.L.; Wilk, A.R.; Nguyen, N.T.; Ponce, J.; Morton, J.M. Comparative effectiveness of primary bariatric operations in the United States. Surg. Obes. Relat. Dis. 2017, 13, 826–834. [Google Scholar] [CrossRef]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Prachand, V.N.; Davee, R.T.; Alverdy, J.C. Duodenal switch provides superior weight loss in the super-obese (BMI > or =50 kg/m2) compared with gastric bypass. Ann. Surg. 2006, 244, 611–619. [Google Scholar] [PubMed]
- Biertho, L.; Lebel, S.; Marceau, S.; Hould, F.S.; Lescelleur, O.; Marceau, P.; Biron, S. Laparoscopic sleeve gastrectomy: With or without duodenal switch? A consecutive series of 800 cases. Dig. Surg. 2014, 31, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.E.; Thornley, C.J.; Blackstone, R.P. Outcomes in Bariatric and Metabolic Surgery: An Updated 5-Year Review. Curr. Obes. Rep. 2020, 9, 380–389. [Google Scholar] [CrossRef]
- Park, C.H.; Nam, S.J.; Choi, H.S.; Kim, K.O.; Kim, D.H.; Kim, J.W.; Sohn, W.; Yoon, J.H.; Jung, S.H.; Hyun, Y.S.; et al. Comparative Efficacy of Bariatric Surgery in the Treatment of Morbid Obesity and Diabetes Mellitus: A Systematic Review and Network Meta-Analysis. Obes. Surg. 2019, 29, 2180–2190. [Google Scholar] [CrossRef]
- Topart, P.; Becouarn, G.; Ritz, P. Weight loss is more sustained after biliopancreatic diversion with duodenal switch than Roux-en-Y gastric bypass in superobese patients. Surg. Obes. Relat. Dis. 2013, 9, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Laurenius, A.; Taha, O.; Maleckas, A.; Lönroth, H.; Olbers, T. Laparoscopic biliopancreatic diversion/duodenal switch or laparoscopic Roux-en-Y gastric bypass for super-obesity-weight loss versus side effects. Surg. Obes. Relat. Dis. 2010, 6, 408–414. [Google Scholar] [CrossRef]
- Skogar, M.L.; Sundbom, M. Weight loss and effect on co-morbidities in the long-term after duodenal switch and gastric bypass: A population-based cohort study. Surg. Obes. Relat. Dis. 2020, 16, 17–23. [Google Scholar] [CrossRef]
- Maroun, J.; Li, M.; Oyefule, O.; Badaoui, J.E.; McKenzie, T.; Kendrick, M.; Kellogg, T.; Ghanem, O.M. Ten year comparative analysis of sleeve gastrectomy, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch in patients with BMI ≥ 50 kg/m2. Surg. Endosc. 2022, 36, 4946–4955. [Google Scholar] [CrossRef]
- Lange, J.; Königsrainer, A. Malnutrition as a Complication of Bariatric Surgery—A Clear and Present Danger? Visc. Med. 2019, 35, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt-Silva, R.; Neves, J.S.; Pedro, J.; Guerreiro, V.; Ferreira, M.J.; Salazar, D.; Souteiro, P.; Magalhães, D.; Oliveira, S.C.; Queirós, J.; et al. Comparative Effectiveness of Different Bariatric Procedures in Super Morbid Obesity. Obes. Surg. 2019, 29, 281–291. [Google Scholar] [CrossRef]
- Vahibe, A.; Aizpuru, M.J.; Sarr, M.G.; Mundi, M.S.; Vierkant, R.A.; McKenzie, T.; Abu Dayyeh, B.K.; Ghanem, O.M. Safety and Efficacy of Revisional Surgery as a Treatment for Malnutrition after Bariatric Surgery. J. Am. Coll. Surg. 2023, 236, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Lind, R.P.; Salame, M.; Kendrick, M.; Ghanem, M.; Jawad, M.A.; Ghanem, O.M.; Teixeira, A.F. Management of Malnutrition and Hepatic Impairment After Duodenal Switch. Obes. Surg. 2024, 34, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pernaute, A.; Rubio Herrera, M.A.; Pérez-Aguirre, E.; García Pérez, J.C.; Cabrerizo, L.; Díez Valladares, L.; Fernández, C.; Talavera, P.; Torres, A. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: Proposed technique. Obes. Surg. 2007, 17, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Kallies, K.; Rogers, A.M. American Society for Metabolic and Bariatric Surgery updated statement on single-anastomosis duodenal switch. Surg. Obes. Relat. Dis. 2020, 16, 825–830. [Google Scholar] [CrossRef]
- Salama, A.F.; Baazaoui, J.; Shahid, F.; Singh, R.; Torres, A.J.; Bashah, M.M. Comparative analysis of 5-year efficacy and outcomes of single anastomosis procedures as revisional surgery for weight regain following sleeve gastrectomy. Surg. Endosc. 2023, 37, 7548–7555. [Google Scholar] [CrossRef] [PubMed]
- Hage, K.; Teixeira, A.F.; Surve, A.; Lind, R.; Jawad, M.A.; Ghanem, M.; Abi Mosleh, K.; Kendrick, M.L.; Cottam, D.; Ghanem, O.M. Single anastomosis duodenal switch versus Roux-en-Y gastric bypass in patients with BMI ≥ 50 kg/m2: A multi-centered comparative analysis. Surg. Endosc. 2024, 38, 2657–2665. [Google Scholar] [CrossRef]
- Verhoeff, K.; Mocanu, V.; Jogiat, U.; Forbes, H.; Switzer, N.J.; Birch, D.W.; Karmali, S. Patient Selection and 30-Day Outcomes of SADI-S Compared to RYGB: A Retrospective Cohort Study of 47,375 Patients. Obes. Surg. 2022, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clapp, B.; Corbett, J.; Jordan, M.; Portela, R.; Ghanem, O.M. Single-anastomosis duodenoileal bypass with sleeve in the United States: A first comparative safety analysis of the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database. Surg. Obes. Relat. Dis. 2023, 19, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Surve, A.; Cottam, D.; Sanchez-Pernaute, A.; Torres, A.; Roller, J.; Kwon, Y.; Mourot, J.; Schniederjan, B.; Neichoy, B.; Enochs, P.; et al. The incidence of complications associated with loop duodeno-ileostomy after single-anastomosis duodenal switch procedures among 1328 patients: A multicenter experience. Surg. Obes. Relat. Dis. 2018, 14, 594–601. [Google Scholar] [CrossRef]
- Choudhury, A.; Jena, A.; Jearth, V.; Dutta, A.K.; Makharia, G.; Dutta, U.; Goenka, M.; Kochhar, R.; Sharma, V. Vitamin B12 deficiency and use of proton pump inhibitors: A systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 479–487. [Google Scholar] [CrossRef]
- Bobrowicz, M.; Pachucki, J.; Popow, M. Hypomagnesaemia leading to parathyroid dysfunction, hypocalcaemia, and hypokalaemia as a complication of long-term treatment with a proton pump inhibitor—A literature review. Endokrynol. Pol. 2024, 75, 359–365. [Google Scholar] [CrossRef] [PubMed]
| Bariatric Procedure | First Author | Publication Year | Type of Article | Outcomes |
|---|---|---|---|---|
| One-anastomosis Gastric Bypass (OAGB) | Bandlamudi, N. [15] | 2023 | Systematic Review |
|
| Carbajo, M.A. [20] | 2017 | Retrospective Study |
| |
| Robert, M. [21] | 2019 | Randomized Controlled Trial |
| |
| Liagre, A. [22] | 2020 | Retrospective Study |
| |
| Mahawar, K.K. [23] | 2018 | Observational Study and Review |
| |
| Zarshenas, N. [24] | 2021 | Retrospective Study |
| |
| Kessler, Y. [25] | 2020 | Prospective Study |
| |
| Parmar, C.D. [26] | 2018 | Systematic Review |
| |
| Jammu, G.S. [27] | 2016 | Prospective Study |
| |
| Syn, N.L. [28] | 2020 | Prospective Study |
| |
| Shirazi, N. [29] | 2022 | Retrospective Study |
| |
| Voglino, C. [30] | 2021 | Retrospective Study |
| |
| Cao, L. [31] | 2023 | Meta-Analysis |
| |
| Biliopancreatic Diversion with Duodenal Switch (BPD-DS) | Risstad, H. [32] | 2015 | Randomized Controlled Trial |
|
| Nelson, D.W. [33] | 2012 | Retrospective Study |
| |
| Sethi, M. [34] | 2016 | Retrospective Study |
| |
| Marceau, P. [35] | 2015 | Retrospective Study |
| |
| Topart, P. [36] | 2014 | Retrospective Study |
| |
| Strain, G.W. [37] | 2017 | Retrospective Study |
| |
| Finno, P. [38] | 2020 | Retrospective Study |
| |
| Salame, M. [39] | 2025 | Retrospective Study |
| |
| Papadia, F.S. [40] | 2024 | Retrospective Study |
| |
| Aasheim, E.T. [41] | 2009 | Randomized Controlled Trial |
| |
| Søvik, T.T. [42] | 2011 | Randomized Controlled Trial |
| |
| Magee, C.J. [43] | 2011 | Retrospective Study |
| |
| Single-anastomosis Duodenoileostomy with Sleeve (SADI-S) | Surve, A. [44] | 2020 | Retrospective Study |
|
| Pennestrì, F. [45] | 2022 | Retrospective Study |
| |
| Yashkov, Y. [46] | 2021 | Retrospective Study |
| |
| Gebellí, J.P. [47] | 2022 | Retrospective Study |
| |
| Hu, L. [48] | 2024 | Retrospective Study |
| |
| Sánchez-Pernaute, A. [49] | 2022 | Retrospective Study |
| |
| Surve, A. [50] | 2020 | Retrospective Study |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedalqader, T.; Jawhar, N.; Gajjar, A.; Portela, R.; Perrotta, G.; El Ghazal, N.; Laplante, S.J.; Ghanem, O.M. Hypoabsorption in Bariatric Surgery: Is the Benefit Worth the Risk? Medicina 2025, 61, 398. https://doi.org/10.3390/medicina61030398
Abedalqader T, Jawhar N, Gajjar A, Portela R, Perrotta G, El Ghazal N, Laplante SJ, Ghanem OM. Hypoabsorption in Bariatric Surgery: Is the Benefit Worth the Risk? Medicina. 2025; 61(3):398. https://doi.org/10.3390/medicina61030398
Chicago/Turabian StyleAbedalqader, Tala, Noura Jawhar, Aryan Gajjar, Ray Portela, Gerardo Perrotta, Nour El Ghazal, Simon J. Laplante, and Omar M. Ghanem. 2025. "Hypoabsorption in Bariatric Surgery: Is the Benefit Worth the Risk?" Medicina 61, no. 3: 398. https://doi.org/10.3390/medicina61030398
APA StyleAbedalqader, T., Jawhar, N., Gajjar, A., Portela, R., Perrotta, G., El Ghazal, N., Laplante, S. J., & Ghanem, O. M. (2025). Hypoabsorption in Bariatric Surgery: Is the Benefit Worth the Risk? Medicina, 61(3), 398. https://doi.org/10.3390/medicina61030398










