Central Sensitization and Its Role in Persistent Pain Among Spondyloarthritis Patients on Biological Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Assessment of Central Sensitization and Pain
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Comparisons Between Patients CSI < 40 and CSI ≥ 40
3.3. Results of Correlation Analysis
3.4. Results of Logistic Regression Analysis (Predictors of CSI ≥ 40)
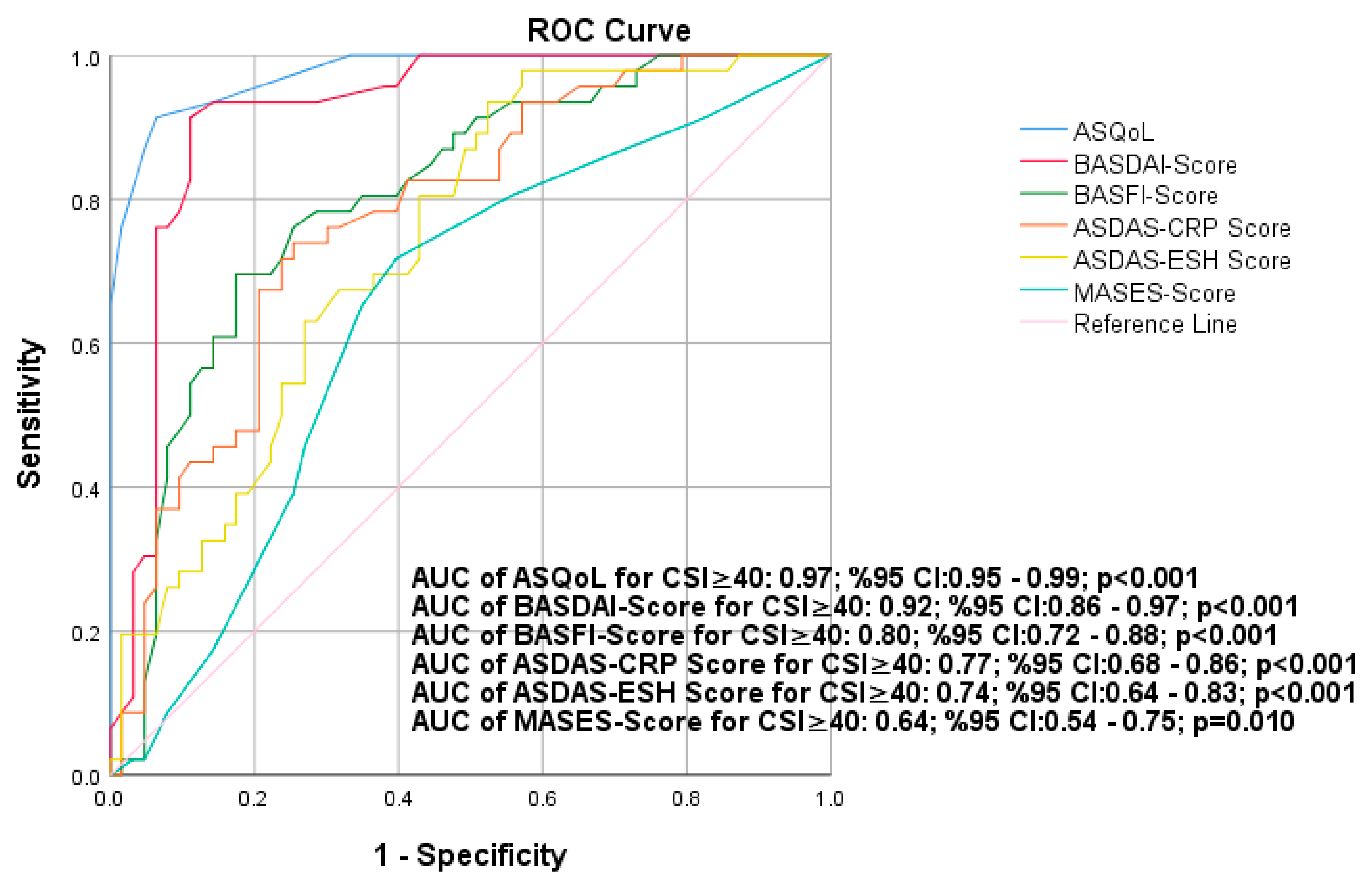
3.5. The Results of ROC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361.e8. [Google Scholar]
- Erturk, M.; Alaca, R.; Tosun, E.; Duruoz, M.T. Evaluation of the Amor and ESSG classification criteria for spondylarthropathies in a Turkish population. Rev. Rhum. Engl. Ed. 1997, 64, 293–300. [Google Scholar] [PubMed]
- Braun, J.; van den Berg, R.; Baraliakos, X.; Boehm, H.; Burgos-Vargas, R.; Collantes-Estevez, E.; Dagfinrud, H.; Dijkmans, B.; Dougados, M.; Emery, P.; et al. 2010 update of the ASAS/EULAR recommendations for the manage- ment of ankylosing spondylitis. Ann. Rheum. Dis. 2011, 70, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Wiąk-Walerowicz, K.; Wielosz, E. Comparison of Ankylosing Spondylitis Disease Activity Score and Bath Ankylosing Spondylitis Disease Activity Index tools in assessment of axial spondyloarthritis activity. Reumatologia 2024, 62, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, Y.; Tu, L.; Wen, X.; Lv, Q.; Liu, B.; Yang, M.; Wu, X.; Zheng, X.; Luo, X.; et al. A guideline on biomarkers in the diagnosis and evaluation in axial spondyloarthritis. Front. Immunol. 2024, 15, 1394148. [Google Scholar] [CrossRef]
- Kieskamp, S.C.; Paap, D.; Carbo, M.J.G.; Wink, F.; Bos, R.; Bootsma, H.; Arends, S.; Spoorenberg, A. Central sensitization has major impact on quality of life in patients with axial spondyloarthritis. Semin. Arthritis Rheum. 2022, 52, 151933. [Google Scholar] [CrossRef]
- Yücel, F.N.; Duruöz, M.T. Central sensitization in axial spondyloarthritis: An explorative study with quantitative sensory testing and clinical scales. Mod. Rheumatol. 2022, 32, 1137–1145. [Google Scholar] [CrossRef]
- Kieskamp, S.C.; Paap, D.; Carbo, M.J.G.; Wink, F.; Bos, R.; Bootsma, H.; Arends, S.; Spoorenberg, A. Central sensitization, illness perception and obesity should be considered when interpreting disease activity in axial spondyloarthritis. Rheumatology 2021, 60, 4476–4485. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Pellegrino, G.; Giorgi, V.; Bongiovanni, S.F.; Varrassi, G.; Di Lascio, S.; Fornasari, D.; Sirotti, S.; Di Carlo, M.; Salaffi, F. Inflammatory or non-inflammatory pain in inflammatory arthritis-How to differentiate it? Best Pract. Res. Clin. Rheumatol. 2024, 38, 101970. [Google Scholar] [CrossRef]
- Magrey, M.N.; Mease, P.J. Pain in axial spondyloarthritis: More to it than just inflammation. J. Rheumatol. 2021, 48, 1632–1634. [Google Scholar] [CrossRef]
- Scheuren, P.S.; Calvo, M. Exploring neuroinflammation: A key driver in neuropathic pain disorders. Int. Rev. Neurobiol. 2024, 179, 311–338. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; He, Y.; Liu, T.; Yang, T.; Sun, X.; Chen, Y.; Shen, F. Ligustrazine alleviates spinal cord injury-induced neuropathic pain by inhibiting the TLR4/NF-κB signaling pathway. Am. J. Transl. Res. 2024, 16, 3557–3571. [Google Scholar] [CrossRef] [PubMed]
- Volcheck, M.M.; Graham, S.M.; Fleming, K.C.; Mohabbat, A.B.; Luedtke, C.A. Central sensitization, chronic pain, and other symptoms: Better understanding, better management. Clevel. Clin. J. Med. 2023, 90, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; van der Heijde, D.; Landewé, R.; Brandt, J.; Burgos-Vagas, R.; Collantes-Estevez, E.; Dijkmans, B.; Dougados, M.; Khan, M.A.; Leirisalo-Repo, M.; et al. New criteria for inflammatory back pain in patients with chronic back pain: A real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann. Rheum. Dis. 2009, 68, 784–788. [Google Scholar] [CrossRef]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef]
- Düzce Keleş, E.; Birtane, M.; Ekuklu, G.; Kılınçer, C.; Çalıyurt, O.; Taştekin, N.; Is, E.E.; Ketenci, A.; Neblett, R. Validity and reliability of the Turkish version of the central sensitization inventory. Arch. Rheumatol. 2021, 36, 518–526. [Google Scholar] [CrossRef]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in anky- losing spondylitis: The bath ankylosing spondylitis disease activity index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Lukas, C.; Landewé, R.; Sieper, J.; Dougados, M.; Davis, J.; Braun, J.; van der Linden, S.; van der Heijde, D. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2009, 68, 18–24. [Google Scholar] [CrossRef]
- Calin, A.; Garrett, S.; Whitelock, H.; Kennedy, L.G.; O’Hea, J.; Mallorie, P.; Jenkinson, T. A new approach to defining functional ability in ankylosing spondylitis: The development of the bath ankylosing spondylitis functional index. J. Rheumatol. 1994, 21, 2281–2285. [Google Scholar]
- van der Heijde, D.; Landewé, R.; Feldtkeller, E. Proposal of a linear definition of the bath ankylosing spondylitis metrology index (BASMI) and comparison with the 2-step and 10-step definitions. Ann. Rheum. Dis. 2008, 67, 489–493. [Google Scholar] [CrossRef]
- Heuft-Dorenbosch, L.; Spoorenberg, A.; van Tubergen, A.; Landewé, R.; van der Tempel, H.; Mielants, H.; Dougados, M.; van der Heijde, D. Assessment of enthesitis in ankylosing spondylitis. Ann. Rheum. Dis. 2003, 62, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Doward, L.; Spoorenberg, A.; Cook, S.; Whalley, D.; Helliwell, P.; Kay, L.; McKenna, S.; Tennant, A.; van der Heijde, D.; Chamberlain, M. Devel- opment of the ASQoL: A quality of life instrument specific to ankylosing spondylitis. Ann. Rheum. Dis 2003, 62, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Lampa, J. Pain without inflammation in rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101439. [Google Scholar] [CrossRef] [PubMed]
- Pathan, E.M.I.; Inman, R.D. Pain in spondyloarthritis: A neuro-immune interaction. Best Pract. Res. Clin. Rheumatol. 2017, 31, 830–845. [Google Scholar] [CrossRef]
- Şaş, S.; Cengiz, G.; Kaplan, H. The effect of central sensitization on disease activity measures, quality of life and clinical parameters in axial spondyloarthritis: A cross-sectional study. J. Rheum. Dis. 2023, 30, 176–184. [Google Scholar] [CrossRef]
- Bedaiwi, M.; Sari, I.; Thavaneswaran, A.; Ayearst, R.; Haroon, N.; Inman, R.D. Fatigue in Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis: Analysis from a Longitudinal Observation Cohort. J. Rheumatol. 2015, 42, 2354–2360. [Google Scholar] [CrossRef]
- Druce, K.L.; McBeth, J. Central sensitization predicts greater fatigue independently of musculoskeletal pain. Rheumatology 2019, 58, 1923–1927. [Google Scholar] [CrossRef]
- Hemington, K.S.; Wu, Q.; Kucyi, A.; Inman, R.D.; Davis, K.D. Abnormal inter-network functional connectivity in chronic pain and its relationship to clinical symptoms. Brain Struct. Funct. 2016, 221, 4203.e19. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Sariyildiz, A.; Coskun Benlidayi, I.; Turk, I.; Zengin Acemoglu, S.S.; Unal, I. Biopsychosocial factors should be considered when evaluating central sensitization in axial spondyloarthritis. Rheumatol. Int. 2023, 43, 923–932. [Google Scholar] [CrossRef]
- Nijs, J.; Leysen, L.; Vanlauwe, J.; Logghe, T.; Ickmans, K.; Polli, A.; Mal- fliet, A.; Coppieters, I.; Huysmans, E. Treatment of central sensitization in patients with chronic pain: Time for change? Expert Opin. Pharmacother. 2019, 20, 1961–1970. [Google Scholar] [CrossRef]
| All Patients (n = 120) | CSI < 40 (n = 71) | CSI ≥ 40 (n = 49) | p Value | ||
|---|---|---|---|---|---|
| Age (years) (mean ± SD) | 43.9 SD 9.24 | 45.02 SD 8.53 | 42.26 SD 10.05 | 0.108 | t |
| Female, gender, n (%) | 49 (%40.8) | 25 (%35.2) | 24 (%49.0) | 0.132 | X2 |
| Male, gender, n (%) | 71 (%59.2) | 46 (%74.8) | 25 (%51.0) | 0.132 | X2 |
| Marital status, married, n (%) | 103 (%85.8) | 62 (%87.3) | 41 (%83.7) | 0.573 | X2 |
| Education of high school and above, n (%) | 62 (%52.5) | 39 (%54.9) | 24 (%49) | 0.521 | X2 |
| Current smoker, n (%) | 17 (%14.2) | 10 (%14.1) | 7 (%14.3) | 0.590 | X2 |
| Hypertension, n (%) | 4 (%3.3) | 4 (%5.6) | 0 | 0.144 | * |
| Diabetes mellitus, n (%) | 2 (%1.7) | 2 (%2.8) | 0 | 0.513 | * |
| Weight (kg) (mean ± SD) | 77.2 SD 11.4 | 78.9 SD 11.3 | 74.8 SD 11.3 | 0.057 | t |
| Height (cm) | 170 (165–178) | 170 (165–178) | 170 (165–175) | 0.363 | m |
| BMI (kg/m2) | 26,6 (24–29.1) | 25.8 (23.9–29.3) | 26.7 (24.1–28.6) | 1.000 | m |
| Obesity (BMI ≥ 30 kg/m2), n (%) | 32 (%26.7) | 18 (%25.4) | 14 (%28.6) | 0.695 | X2 |
| Radiographic axSpA, n (%) | 87 (%72.5) | 51 (%71.8) | 36 (%73.5) | 0.843 | X2 |
| Non-radiographic axSpA, n (%) | 33 (%27.5) | 20 (%28.2) | 13 (%26.5) | 0.843 | X2 |
| Morning stiffness duration (minute) | 36 (12–60) | 24 (12–36) | 72 (36–84) | <0.001 | m |
| Hip involvement n (%) | 29 (%24,2) | 15 (%21.1) | 14 (%28.6) | 0.349 | X2 |
| History of uveitis n (%) | 15 (%12.5) | 9 (%12.7) | 6 (%12.2) | 0.944 | X2 |
| History of IBD, n (%) | 8 (%6.7) | 2 (%2.8) | 6 (%12.2) | 0.062 | * |
| History of psoriasis, n (%) | 14 (%11.7) | 7 (%9.9) | 7 (%14.3) | 0.458 | X2 |
| Family history of SpA, n (%) | 29 (%24.2) | 18 (%25.4) | 11 (%22.4) | 0.714 | X2 |
| Symptoms duration (month) | 162 (114–219) | 170 (135–230) | 140 (98–205) | 0.024 | m |
| Disease duration (month) | 129 (92–172) | 134 (105–167) | 110 (80–176) | 0.046 | m |
| Time delay in diagnosis (month) | 20 (12.5–38) | 25 (13–48) | 15 (12–25) | 0.035 | m |
| CRP (mg/dL) | 4.1 (3.0–10) | 4.18 (3.0–9.17) | 3.90 (3.02–10.0) | 0.571 | m |
| ESR (mm/h) | 20 (6–36) | 20 (6–34) | 21 (6–37) | 0.687 | m |
| Use of biological drugs duration (month) | 74 (54–100) | 75 (54–100) | 66 (58–98) | 0.781 | m |
| Biologic drugs used | |||||
| Adalimumab, n (%) | 34 (%28.3) | 17 (%23.9) | 17 (%34.7) | 0.201 | X2 |
| Golimumab, n (%) | 32 (%26.7) | 22 (%31) | 10 (%20.4) | 0.198 | X2 |
| Infliximab, n (%) | 5 (%4.2) | 1 (%1.4) | 4 (%8.2) | 0.069 | * |
| Etanercept, n (%) | 16 (%13.3) | 13 (%18.3) | 3 (%6.1) | 0.054 | X2 |
| Sertolizumab, n (%) | 10 (%8.3) | 6 (%8.5) | 4 (%8.2) | 0.955 | * |
| Sekukinumab, n (%) | 23 (%19.2) | 12 (%16.9) | 11 (%22.4) | 0.448 | X2 |
| Patients using ≥ 3 biological drugs, n (%) | 13 (%10.8) | 1 (%1.4) | 12 (%24.5) | <0.001 | X2 |
| All Patients (n = 120) | CSI < 40 (n = 71) | CSI ≥ 40 (n = 49) | p Value | ||
|---|---|---|---|---|---|
| Disease-related clinical variables | |||||
| CSI Part A (mean ± SD) | 35.44 SD 16.25 | 23.83 SD 7.87 | 52.27 SD 8.71 | <0.001 | t |
| BASDAI score | 3.4 (2.5–5.4) | 2.6 (1.8–3.4) | 5.5 (5.0–6.6) | <0.001 | m |
| ASDAS-CRP (mean ± SD) | 2.80 SD 0.79 | 2.50 SD 0.71 | 3.24 SD 0.70 | <0.001 | t |
| ASDAS-ESR (mean ± SD) | 2.85 SD 0.85 | 2.55 SD 0.82 | 3.30 SD 0.69 | <0.001 | t |
| BASFI score | 3.5 (2.1–5.8) | 2.4 (1.6–3.8) | 5.7 (4.0–6.4) | <0.001 | m |
| BASMI score | 3.05 (2.0–4.9) | 3.0 (2.0–4.5) | 3.4 (2.0–5.0) | 0.957 | m |
| MASES score | 4.0 (2.0–7.0) | 3.0 (1.0–6.0) | 5.0 (3.0–7.0) | 0.005 | m |
| ASQoL score | 6 (4–11) | 5 (3–6) | 12 (10–13) | <0.001 | m |
| VAS-fatigue score | 5 (3–7) | 40 (30–50) | 70 (60–80) | <0.001 | m |
| VAS-pain score | 40 (30–60) | 30 (20–40) | 60 (50–80) | <0.001 | m |
| PtGA score | 55 (40–70) | 50 (30–70) | 60 (50–70) | 0.005 | m |
| PGA score | 50 (30–60) | 50 (30–60) | 50 (50–60) | 0.017 | m |
| Laboratory findings | |||||
| 25-hydroxy-vitamin D [25(OH)D] (ng/mL) | 20 (15.9–25.4) | 20.0 (16.5–26.8) | 19.9 (15.0–25.0) | 0.248 | m |
| CSI-A r | |
|---|---|
| Age, years | −0.172 |
| Disease duration, month | −0.164 |
| Symptoms duration, month | −0.186 * |
| BASDAI score | 0.774 ** |
| ASDAS-CRP | 0.538 ** |
| ASDAS-ESR | 0.479 ** |
| ASDAS-CRP | 0.538 ** |
| BASFI score | 0.509 ** |
| BASMI score | −0.047 |
| MASES score | 0.305 ** |
| ASQoL score | 0.839 ** |
| VAS-fatigue score | 0.666 ** |
| VAS-pain score | 0.630 ** |
| PtGA score | 0.374 ** |
| PGA score | 0.337 ** |
| ESR, mm/h | 0.054 |
| CRP, mg/dL | 0.025 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Patients using ≥3 biological drugs | 22.703 (2.840–181.456) | 0.003 | 85.173 (0.657–11,038.403) | 0.073 |
| BASDAI score | 3.943 (2.504–6.210) | <0.001 | 2.221 (1.134–4.350) | 0.020 |
| ASDAS-CRP | 4.323 (2.241–8.340) | <0.001 | ||
| ASDAS-ESR | 3.529 (1.948–6.394) | <0.001 | ||
| BASFI score | 1.792 (1.428–2.247) | <0.001 | ||
| MASES score | 1.164 (1.029–1.316) | 0.016 | ||
| ASQoL score | 3.165 (2.034–4.924) | <0.001 | 3.030 (1.755–5.230) | <0.001 |
| VAS-fatigue score | 1.072 (1.046–1.100) | <0.001 | ||
| VAS-pain score | 1.077 (1.050–1.106) | <0.001 | ||
| PtGA score | 1.028 (1.009–1.048) | 0.004 | ||
| PGA score | 1.023 (1.004–1.043) | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öz, N.; Özer, A.; Duruöz, M.T. Central Sensitization and Its Role in Persistent Pain Among Spondyloarthritis Patients on Biological Treatments. Medicina 2025, 61, 319. https://doi.org/10.3390/medicina61020319
Öz N, Özer A, Duruöz MT. Central Sensitization and Its Role in Persistent Pain Among Spondyloarthritis Patients on Biological Treatments. Medicina. 2025; 61(2):319. https://doi.org/10.3390/medicina61020319
Chicago/Turabian StyleÖz, Nuran, Aygün Özer, and Mehmet Tuncay Duruöz. 2025. "Central Sensitization and Its Role in Persistent Pain Among Spondyloarthritis Patients on Biological Treatments" Medicina 61, no. 2: 319. https://doi.org/10.3390/medicina61020319
APA StyleÖz, N., Özer, A., & Duruöz, M. T. (2025). Central Sensitization and Its Role in Persistent Pain Among Spondyloarthritis Patients on Biological Treatments. Medicina, 61(2), 319. https://doi.org/10.3390/medicina61020319






