What Is the Impact of Obesity-Related Comorbidities on the Risk of Premature Aging in Patients with Severe Obesity?: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Endpoints of the Study
2.3. Biological Age Markers
2.4. Assessment of Metabolic Syndrome Components and Comorbidities
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Group
3.2. The Impact of Obesity-Related Comorbidities on Premature Aging
3.3. Telomere Length
3.4. Cognitive Function
3.5. Metabolic Age
3.6. Effects of Individual Obesity-Related Diseases, Including Prediabetes/Diabetes, Hypertension, and Atherogenic Dyslipidemia, on Biological Age Markers
4. Discussion
Strength and Limits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Obesity Federation. The Economic Impact of Overweight & Obesity in 2020 and 2060; World Obesity Federation: London, UK, 2022. [Google Scholar]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The science of Obesity management: An Endocrine Society scientific statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging-Theories, mechanisms, and future prospects. Aging Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vijayan, M.; Bhatti, J.S.; Reddy, P.H. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog. Mol. Biol. Transl. Sci. 2017, 146, 47–94. [Google Scholar]
- Jura, M.; Kozak, L.P. Obesity and related consequences to aging. Age 2016, 38, 23. [Google Scholar] [CrossRef]
- Tzanetakou, I.P.; Katsilambros, N.L.; Benetos, A.; Mikhailidis, D.P.; Perrea, D.N. Is obesity linked to aging?: Adipose tissue and the role of telomeres. Aging Res. Rev. 2012, 11, 220–229. [Google Scholar] [CrossRef]
- Shay, J.W. Telomeres and aging. Curr. Opin. Cell Biol. 2018, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Ridker, P.M. Population Distributions of C-reactive Protein in Apparently Healthy Men and Women in the United States: Implication for Clinical Interpretation. Clin. Chem. 2003, 49, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Fröhlich, M.; Loewel, H.; Helbecque, N.; Woodward, M.; Amouyel, P. Distributions of C-reactive Protein Measured by High-Sensitivity Assays in Apparently Healthy Men and Women from Different Populations in Europe. Clin. Chem. 2003, 49, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Ley, K. Atherosclerosis: A Chronic Inflammatory Disease with an Autoimmune Component. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Chaithanya, V.; Kumar, J.; Leela, K.V.; Murugesan, R.; Angelin, M.; Satheesan, A. Impact of telomere attrition on diabetes mellitus and its complications. Diabetes Epidemiol. Manag. 2023, 12, 100174. [Google Scholar] [CrossRef]
- Zhao, J.; Miao, K.; Wang, H.; Ding, H.; Wang, D.W. Association between telomere length and type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2013, 8, e79993. [Google Scholar] [CrossRef]
- El Hadri, K.; Smith, R.; Duplus, E.; El Amri, C. Inflammation, Oxidative Stress, Senescence in Atherosclerosis: Thioredoxine-1 as an Emerging Therapeutic Target. Int. J. Mol. Sci. 2021, 23, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2022, 13, 1098725. [Google Scholar] [CrossRef]
- Guerville, F.; De Souto Barreto, P.; Ader, I.; Andrieu, S.; Casteilla, L.; Dray, C.; Fazilleau, N.; Guyonnet, S.; Langin, D.; Liblau, R.; et al. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. J. Prev. Alzheimers Dis. 2020, 7, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, F.A.; Bokov, D.O.; Yasin, G.; Vahdat, S.; Abbasalizad-Farhangi, M. Central obesity accelerates leukocyte telomere length (LTL) shortening in apparently healthy adults: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 2119–2128. [Google Scholar] [CrossRef]
- Xu, W.L.; Atti, A.R.; Gatz, M.; Pedersen, N.L.; Johansson, B.; Fratiglioni, L. Midlife overweight and obesity increase late-life dementia risk: A population-based twin study. Neurology 2011, 76, 1568–1574. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P., Jr.; Yaffe, K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 2005, 330, 1360. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, J.; Wyleżoł, M.; Major, P.; Budzyński, A.; Binda, A.; Proczko-Stepaniak, M.; Boniecka, I.; Matłok, M.; Sekuła, M.; Kaska, Ł.; et al. Metabolic and bariatric surgery chapter of the association of Polish surgeons. Bariatric and metabolic surgery care standards. Videosurgery Other Miniinvasive Tech. 2020, 15, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- D’Elia, L.F.; Satz, P.; Uchiyama, C.L.; White, T. Color Trails Test; PAR: Odessa, FL, USA, 1996. [Google Scholar]
- Ashendorf, L.; McCaffrey, R.J. Exploring age-related decline on the Wisconsin Card Sorting Test. Clin. Neuropsychol. 2008, 22, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, Y.H.; Chuang, P.N.; Kuo, C.S.; Lu, C.W.; Yang, K.C. The utility of visceral fat level measured by bioelectrical impedance analysis in predicting metabolic syndrome. Obes. Res. Clin. Pract. 2020, 14, 519–523. [Google Scholar] [CrossRef]
- Chen, R.; Zhan, Y.; Pedersen, N.; Fall, K.; Valdimarsdóttir, U.A.; Hägg, S.; Fang, F. Marital status, telomere length and cardiovascular disease risk in a Swedish prospective cohort. Heart 2019, 106, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Gustafson, B.; Kirkland, J.L.; Smith, U. Cellular senescence: At the nexus between ageing and diabetes. Diabetologia 2019, 62, 1835–1841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, Y.S.; Ryu, G.W.; Choi, M. Multiple metabolic comorbidities and their consequences among patients with peripheral arterial disease. PLoS ONE 2022, 17, e0268201. [Google Scholar] [CrossRef]
- Valderas, J.M.; Starfield, B.; Roland, M. Multimorbidity’s many challenges: A research priority in the UK. BMJ 2007, 334, 1128. [Google Scholar] [CrossRef]
- Kao, S.L.; Wang, J.H.; Chen, S.C.; Li, Y.Y.; Yang, Y.L.; Lo, R.Y. Impact of Comorbidity Burden on Cognitive Decline: A Prospective Cohort Study of Older Adults with Dementia. Dement. Geriatr. Cogn. Disord. 2021, 50, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Cognitive Function, and Its Relationships with Comorbidities, Physical Activity, and Muscular Strength in Korean Older Adults. Behav. Sci. 2023, 13, 212. [Google Scholar] [CrossRef]
- Fachri, M.; Hatta, M.; Widowati, E.; Akaputra, R.; Dwiyanti, R.; Syukri, A.; Junita, A.R.; Febrianti, A.; Primaguna, M.R. Correlations between comorbidities, chest X-ray findings, and C-Reactive protein level in patients with COVID-19. Ann. Med. Surg. 2022, 77, 103553. [Google Scholar] [CrossRef]
- Eloueyk, A.K.; Alameddine, R.Y.; Osta, B.A.; Awad, D.M. Correlations between serum inflammatory markers and comorbidities in patients with end-stage renal disease. J. Taibah Univ. Med. Sci. 2019, 14, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Viasus, D.; Simonetti, A.F.; Estupiñan-Bohórquez, A.F.; Carratalà, J. Effects of age and comorbidities on serum levels of inflammatory markers in community-acquired pneumonia. Eur. J. Clin. Investig. 2021, 51, e13480. [Google Scholar] [CrossRef]
- Brunelli, D.T.; Boldrini, V.O.; Bonfante, I.L.P.; Duft, R.G.; Mateus, K.; Costa, L.; Chacon-Mikahil, M.P.; Teixeira, A.M.; Farias, A.S.; Cavaglieri, C.R. Obesity Increases Gene Expression of Markers Associated with Immunosenescence in Obese Middle-Aged Individuals. Front. Immunol. 2022, 13, 806400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical Effects of Obesity on T-Cell Function During Tumor Progression and PD-1 Checkpoint Blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Tchkonia, T.; LeBrasseur, N.K.; Chini, E.N.; Xu, M.; Kirkland, J.L. Cellular senescence in type 2 diabetes: A therapeutic opportunity. Diabetes 2015, 64, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.; Hong, R.; Teo, L.L.; Tan, R.S.; Koh, A.S. Atherosclerotic cardiovascular disease in aging and the role of advanced cardiovascular imaging. NPJ Cardiovasc. Health 2024, 1, 11. [Google Scholar] [CrossRef]
- Gruna, L.K.; Teixeira, N.R., Jr.; von Mengden, L.; de Bastiani, M.A.; Parisi, M.M.; Bortoline, R.; Lavandoski, P.; Pierdoná, V.; Alves, L.B.; Moreira, J.C.; et al. TRF1 as a major contributor for telomeres’ shortening in the context of Obesity. Free. Radic. Biol. Med. 2018, 129, 286–295. [Google Scholar] [CrossRef]
- Trubnikova, O.A.; Mamontova, A.S.; Tarasova, I.V.; Maleva, O.V.; Kuzmina, A.A.; Barbarash, O.L. Association of cognitive impairments with carbohydrate and lipid metabolic parameters in type 2 diabetes mellitus patients undergoing coronary bypass surgery. Ter. Arkhiv 2015, 87, 69–75. [Google Scholar] [CrossRef]
- Herghelegiu, A.M.; Nacu, R.M.; Prada, G.I. Metabolic parameters and cognitive function in a cohort of older diabetic patients. Aging Clin. Exp. Res. 2016, 28, 1105–1112. [Google Scholar] [CrossRef]
- Pall, D.; Kiss, I.; Katona, E. Importance of ambulatory blood pressure monitoring in adolescent hypertension. Kidney Blood Press. Res. 2012, 35, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Settakis, G.; Pall, D.; Molnar, C.; Katona, E.; Bereczki, D.; Fulesdi, B. Hyperventilation-induced cerebrovascular reactivity among hypertensive and healthy adolescents. Kidney Blood Press. Res. 2006, 29, 306–311. [Google Scholar] [CrossRef]
- Gatto, N.M.; Henderson, V.W.; St John, J.A.; McCleary, C.; Hodis, H.N.; Mack, W.J. Metabolic syndrome and cognitive function in healthy middle-aged and older adults without diabetes. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2008, 15, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, M.; Ropele, S.; Petrovic, K.; Pluta-Fuerst, A.; Homayoon, N.; Enzinger, C.; Grazer, A.; Katschnig, P.; Schwingenschuh, P.; Berghold, A.; et al. Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care 2010, 33, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K. Metabolic syndrome and cognitive disorders: Is the sum greater than its parts? Alzheimer Dis. Assoc. Disord. 2007, 21, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhao, W.; Lu, M.; Zhang, X.; Zhang, P.; Xin, Z.; Sun, R.; Tian, W.; Cardoso, M.A.; Yang, J.; et al. Relationship between Central Obesity and the incidence of Cognitive Impairment and Dementia from Cohort Studies Involving 5,060,687 Participants. Neurosci. Biobehav. Rev. 2021, 130, 301–313. [Google Scholar] [CrossRef]
| Parameter | O+C | O−C | HC |
|---|---|---|---|
| Total, n | 78 | 21 | 30 |
| Mean age, years (M ± SD) | 43.33 ± 10.49 | 38.4 ± 8.51 | 44.5 ± 8.55 |
| Sex (female), n (%) | 27 (34.6) | 7 (33.3) | 10 (33.3) |
| Education | |||
| primary, n (%) | 1 (1.3) *& | 0 (0) | 0 (0) |
| vocational, n (%) | 3 (3.84) *& | 3 (14.3) & | 0 (0) |
| secondary, n (%) | 35 (44.88) *& | 4 (19.0) & | 0 (0) |
| higher education, n (%) | 39 (50) & | 14 (66.7) & | 30 (100) |
| Prediabetes/diabetes, n (%) | 47 (60.3) | - | - |
| Hypertension, n (%) | 36 (46.2) | - | - |
| Atherosclerosis, n (%) | 34 (43.6) | - | - |
| Arthritis, n (%) | 5 (6.4) | - | - |
| Psoriasis, n (%) | 5 (6.4) | - | - |
| PCOS, n (%) | 2 (2.6) | - | - |
| Nonalcoholic fatty liver disease, n (%) | 3 (3.8) | - | - |
| Hypothyroidism, n (%) | 13 (16.7) | - | - |
| Graves-basedov disease, n (%) | 3 (3.00) | - | - |
| Obstructive sleep apnea, n (%) | 2 (2.6) | - | - |
| Asthma, n (%) | 5 (6.4) | - | - |
| COPD, n (%) | 1 (1.3) | - | - |
| Duration of obesity, years (M ± SD) | 20.31 ± 11.2 | 20.31 ± 10.02 | - |
| BMI (kg/m2), (M ± SD) | 44.98 ± 6.06 & | 44.34 ± 6.3 & | 23.06± 3.13 |
| Muscle mass, kg (M ± SD) | 65.45 ± 14.13 & | 64.83 ± 11.13 & | 50.88 ± 12.21 |
| Percentage of body fat, % (M ± SD) | 46.15 ± 6.83 & | 45.6± 7.24 & | 24.3 ± 7.97 |
| Visceral fat index (n), M ± SD | 19 ± 8 & | 18 ± 7 & | 5 ± 3 |
| Basal metabolic rate (kcal) | 2201 ± 328 & | 2179 ± 481 & | 1578 ± 362 |
| Kruskal–Wallis Test Parameters | Pairwise Comparisons a | |
|---|---|---|
| Chronological age | H(2) = 5.88, p = 0.053 | HC vs. O+C: p > 0.999 HC vs. O−C: p = 0.057 O+C vs. O−C: p = 0.124 |
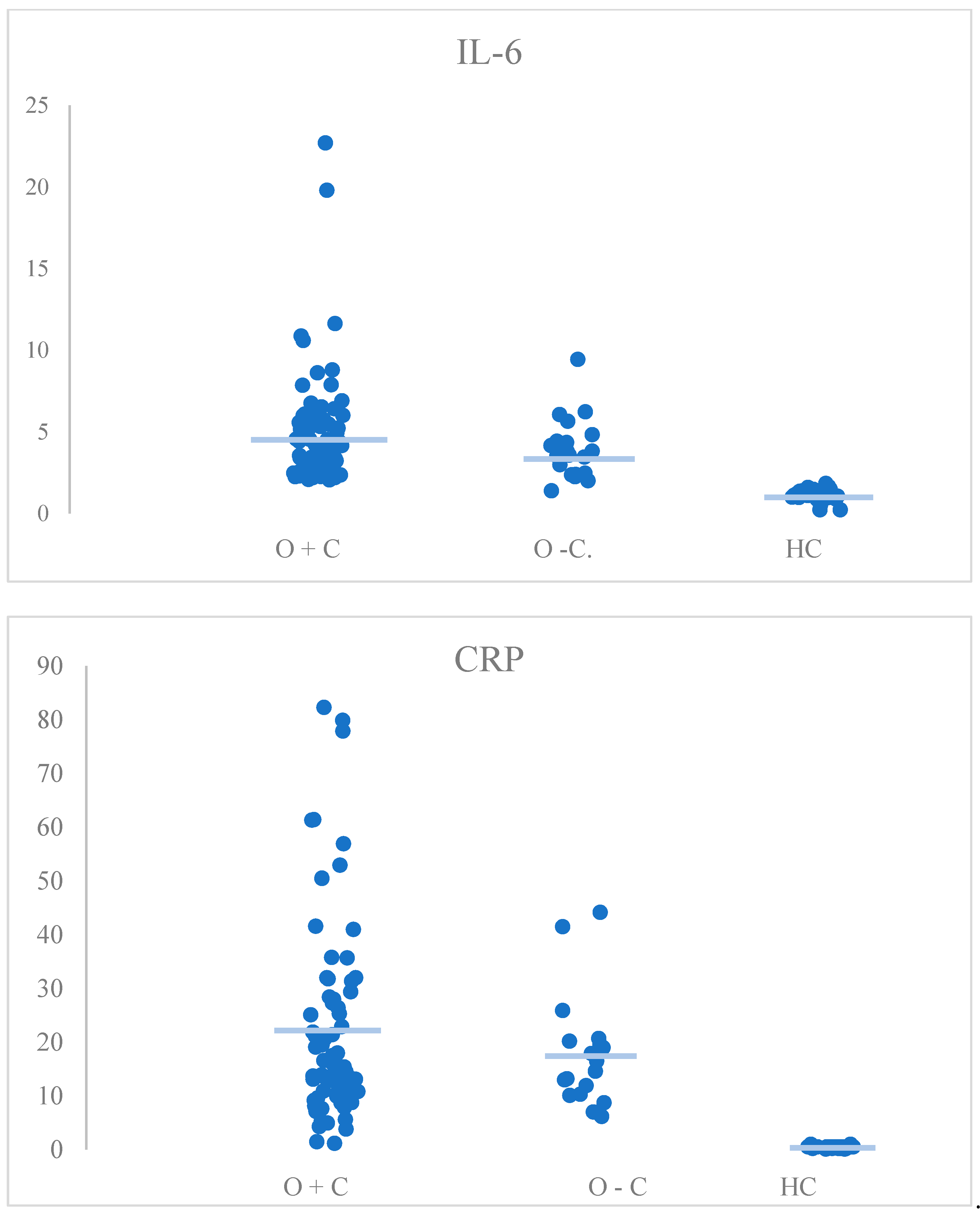
| CRP | H(2) = 68.11, p < 0.001 | HC vs. O+C: p < 0.001 * HC vs. O−C: p < 0.001 * O+C vs. O−C: p > 0.999 |
| IL-6 | H(2) = 68.25, p < 0.001 | HC vs. O+C: p < 0.001 * HC vs. O−C: p < 0.001 * O+C vs. O−C: p > 0.999 |
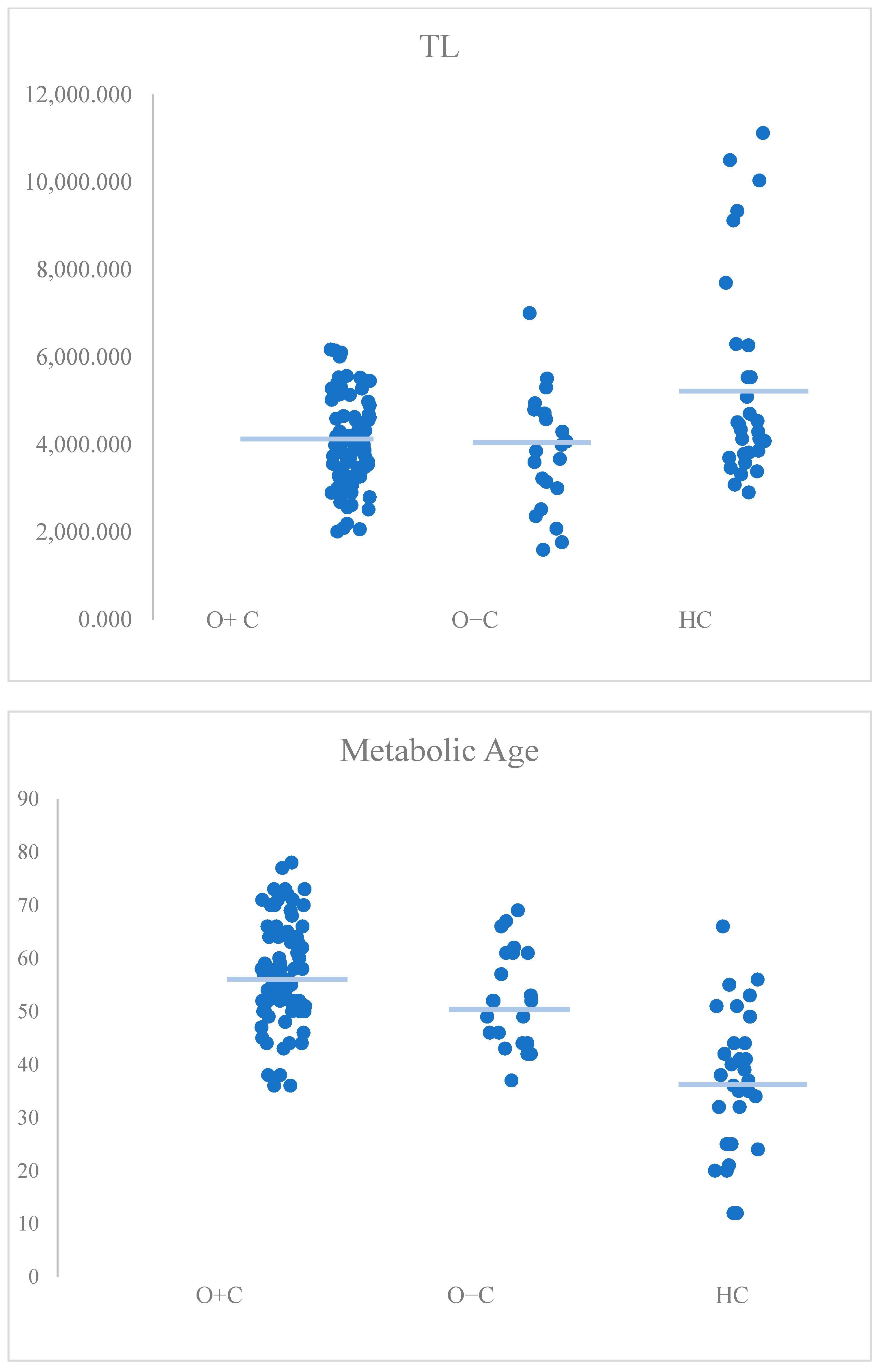
| Telomere length | H(2) = 8.28, p = 0.016 | HC vs. O+C: p = 0.028 * HC vs. O−C: p = 0.043 * O+C vs. O−C: p > 0.999 |
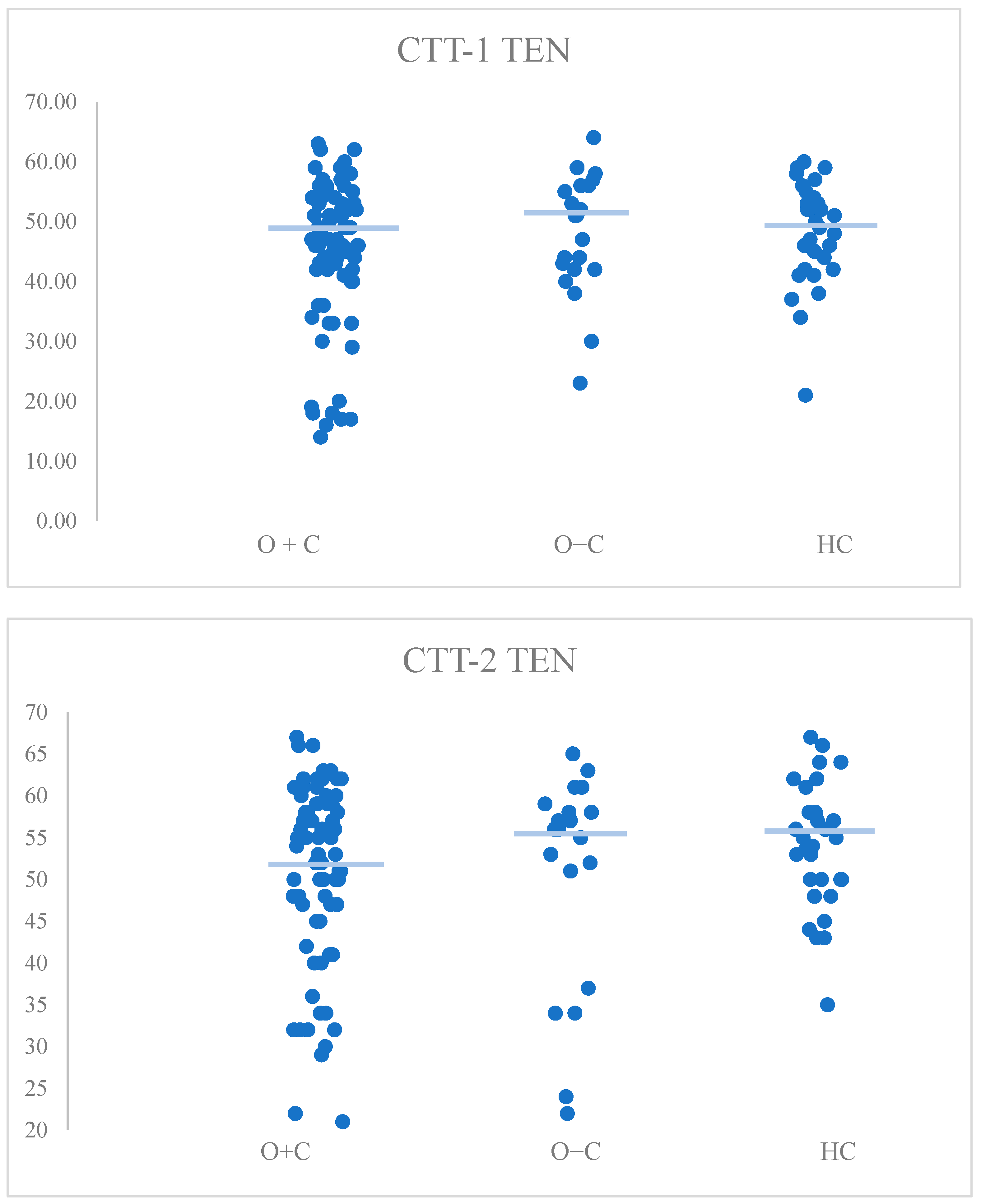
| CTT1-TEN | H(2) = 1.08, p = 0.584 | HC vs. O+C: p > 0.999 HC vs. O−C: p > 0.999 O+C vs. O−C: p > 0.999 |
| CTT2-TEN | H(2) = 2.7, p = 0.260 | HC vs. O+C: p = 0.327 HC vs. O−C: p > 0.999 O+C vs. O−C: p > 0.999 |
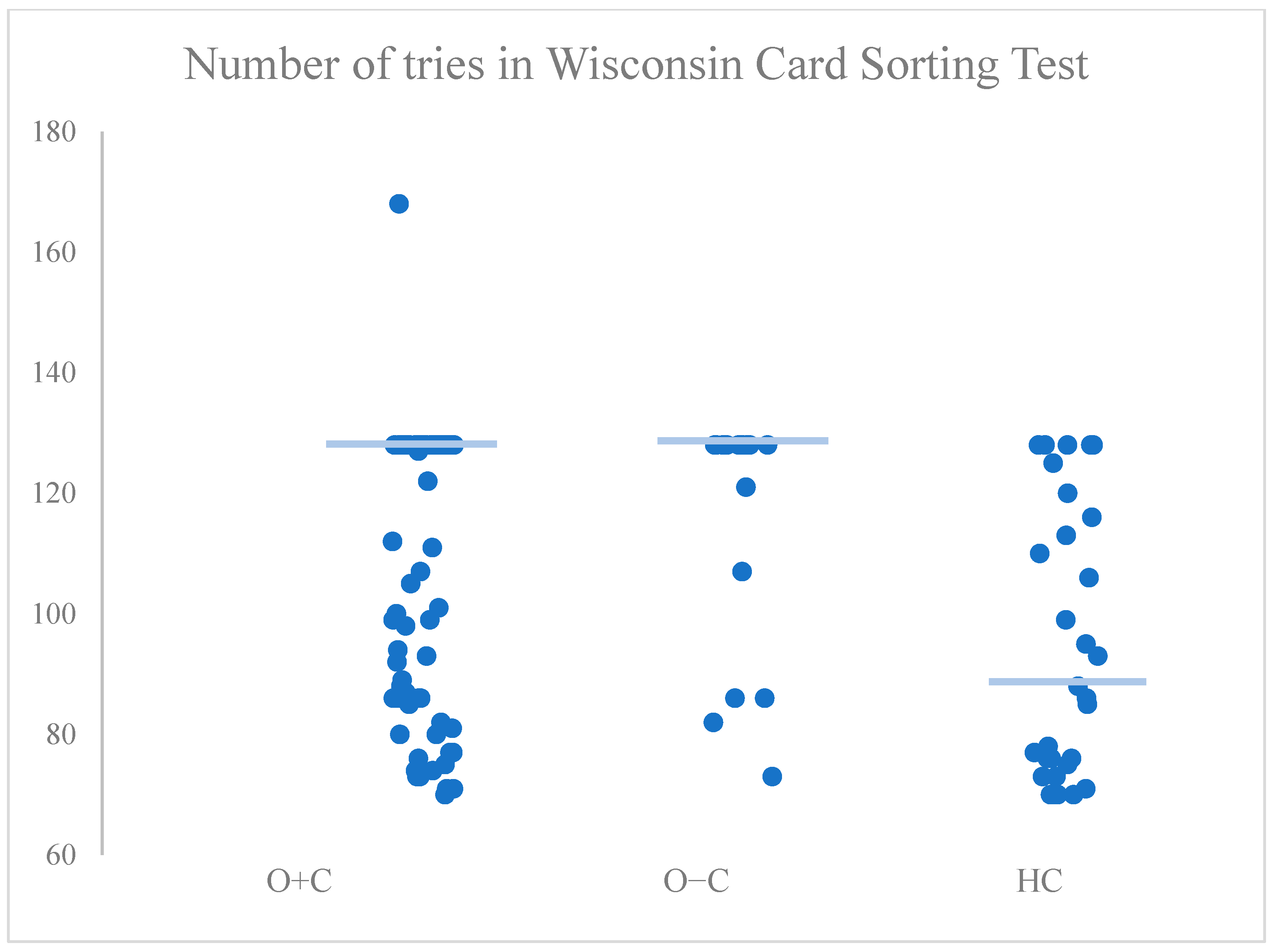
| Number of trials | H(2) = 15.52, p < 0.001 | HC vs. O+C: p = 0.005 * HC vs. O−C: p = 0.001 * O+C vs. O−C: p = 0.369 |
| Metabolic age | H(2) = 42.05, p < 0.001 | HC vs. O+C: p < 0.001 * HC vs. O−C: p = 0.001 * O+C vs. O−C: p = 0.525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, A.; Zapała, B.; Gorostowicz, A.; Kawa, I.; Ciszek, K.; Tylec, P.; Cyranka, K.; Sierocki, W.; Wysocki, M.; Major, P. What Is the Impact of Obesity-Related Comorbidities on the Risk of Premature Aging in Patients with Severe Obesity?: A Cross-Sectional Study. Medicina 2025, 61, 293. https://doi.org/10.3390/medicina61020293
Dudek A, Zapała B, Gorostowicz A, Kawa I, Ciszek K, Tylec P, Cyranka K, Sierocki W, Wysocki M, Major P. What Is the Impact of Obesity-Related Comorbidities on the Risk of Premature Aging in Patients with Severe Obesity?: A Cross-Sectional Study. Medicina. 2025; 61(2):293. https://doi.org/10.3390/medicina61020293
Chicago/Turabian StyleDudek, Alicja, Barbara Zapała, Aleksandra Gorostowicz, Ilona Kawa, Karol Ciszek, Piotr Tylec, Katarzyna Cyranka, Wojciech Sierocki, Michał Wysocki, and Piotr Major. 2025. "What Is the Impact of Obesity-Related Comorbidities on the Risk of Premature Aging in Patients with Severe Obesity?: A Cross-Sectional Study" Medicina 61, no. 2: 293. https://doi.org/10.3390/medicina61020293
APA StyleDudek, A., Zapała, B., Gorostowicz, A., Kawa, I., Ciszek, K., Tylec, P., Cyranka, K., Sierocki, W., Wysocki, M., & Major, P. (2025). What Is the Impact of Obesity-Related Comorbidities on the Risk of Premature Aging in Patients with Severe Obesity?: A Cross-Sectional Study. Medicina, 61(2), 293. https://doi.org/10.3390/medicina61020293






