Does Magnesium Affect Sex Hormones and Cardiometabolic Risk Factors in Patients with PCOS? Findings from a Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Protocol
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection and Data Extraction
2.5. Study Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
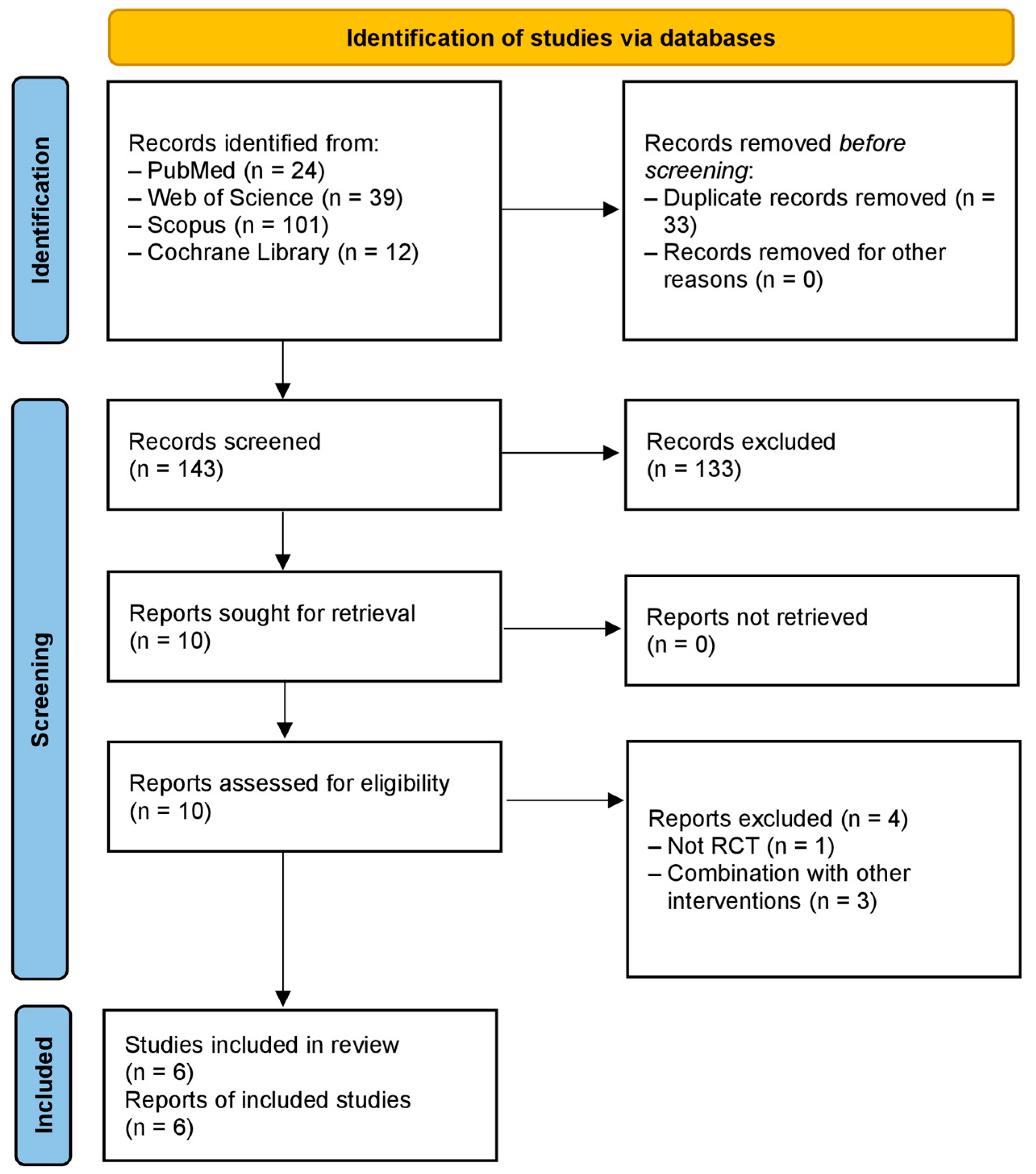
3.1. Summary of Literature Search, Study Selection, and Risk of Bias Assessment
3.2. Characteristics of Included Studies and Risk of Bias Assessment
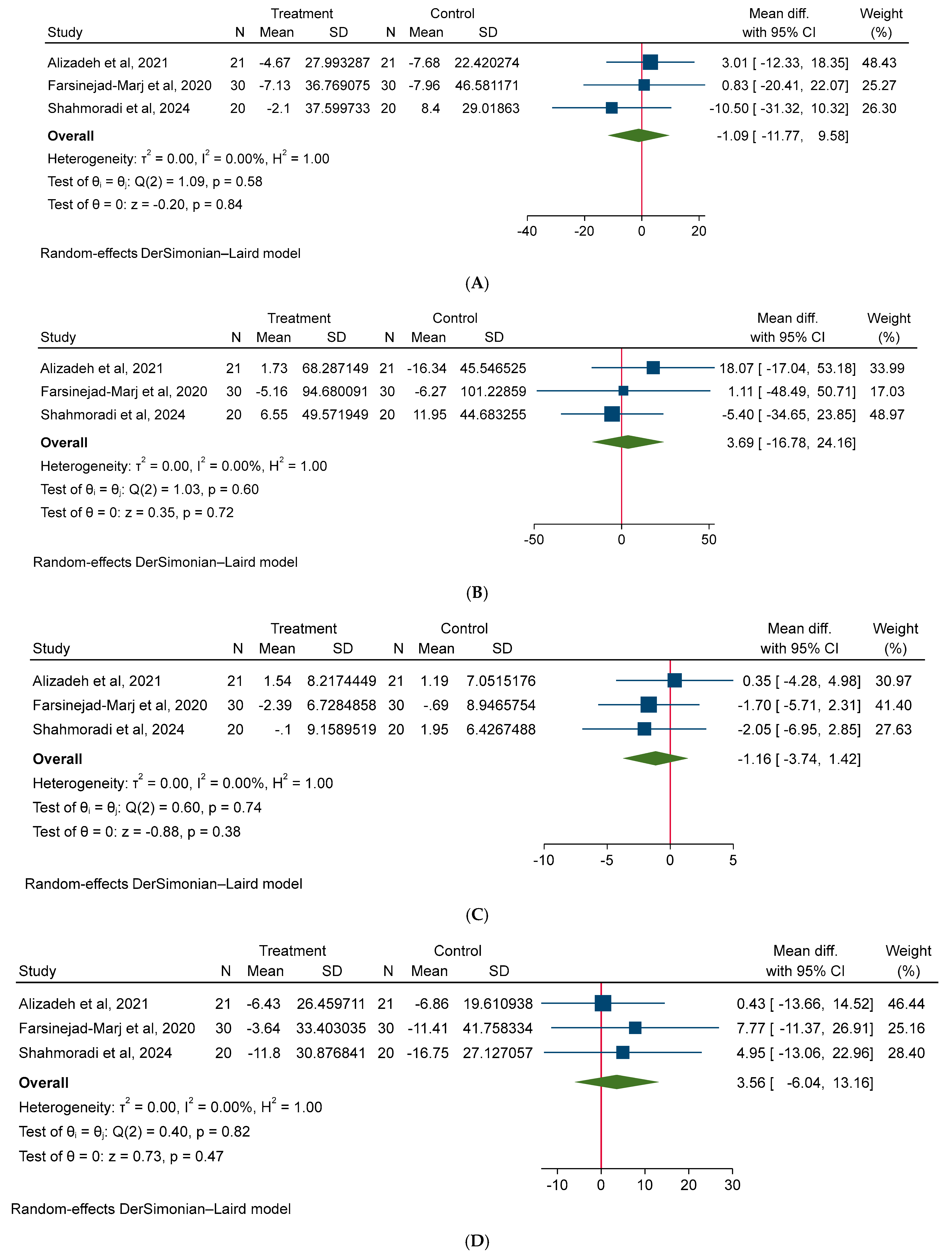
3.3. Effect of Magnesium on Cardiometabolic (Anthropometric, Glycemic, Lipid, and Blood Pressure) Risk Factors
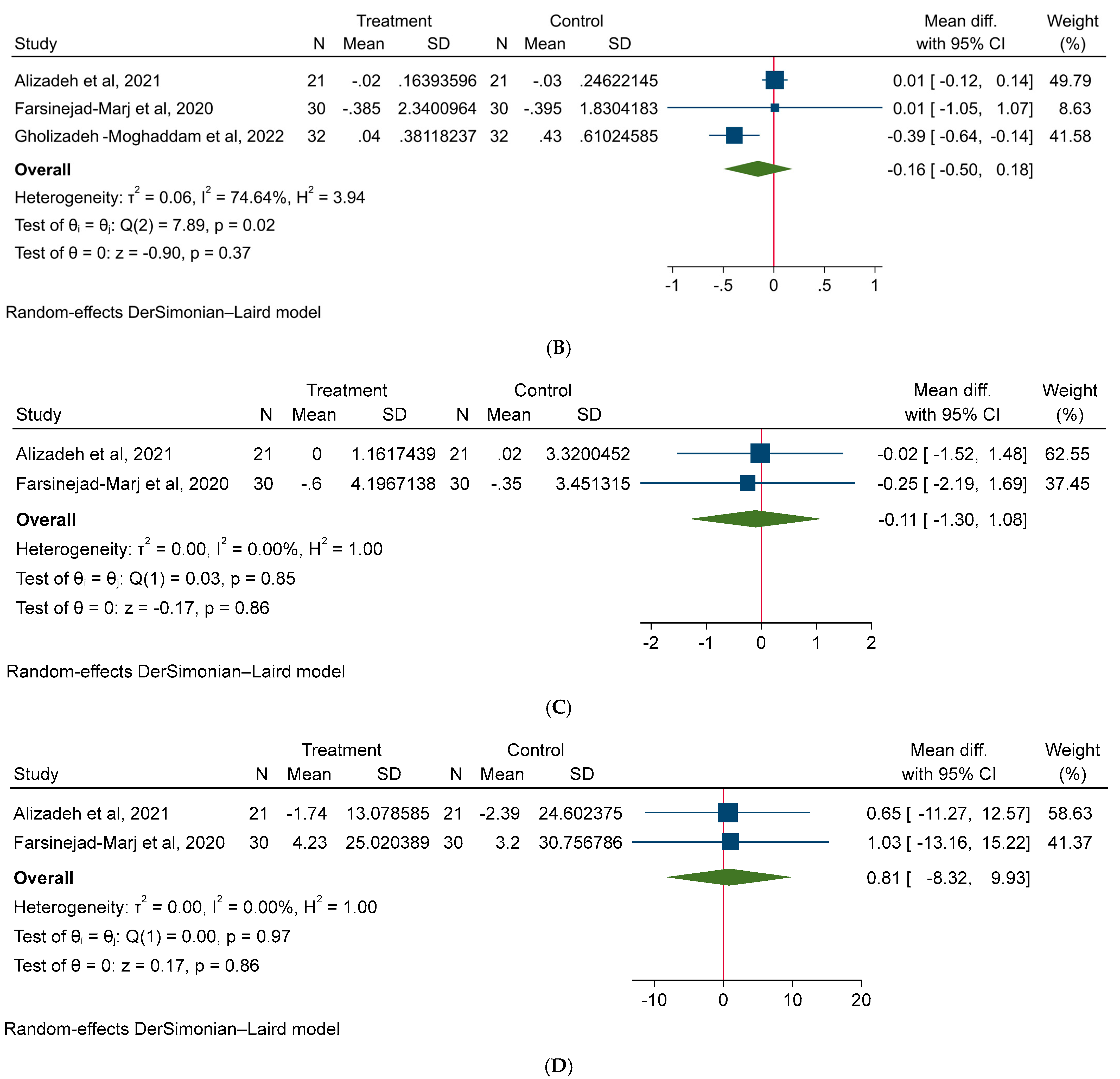
3.4. Effect of Magnesium on Hormonal Factors in PCOS Patients
3.5. Effect of Magnesium on Quality of Life of PCOS Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macut, D.; Bjekić-Macut, J.; Rahelić, D.; Doknić, M. Insulin and the polycystic ovary syndrome. Diabetes Res. Clin. Pract. 2017, 130, 163–170. [Google Scholar] [CrossRef]
- Livadas, S.; Diamanti-Kandarakis, E. Polycystic ovary syndrome: Definitions, phenotypes and diagnostic approach. Front. Horm. Res. 2013, 40, 1–21. [Google Scholar] [CrossRef]
- Goldenberg, N.; Glueck, C. Medical therapy in women with polycystic ovarian syndrome before and during pregnancy and lactation. Minerva Ginecol. 2008, 60, 63–75. [Google Scholar]
- Jones, G.L.; Palep-Singh, M.; Ledger, W.L.; Balen, A.H.; Jenkinson, C.; Campbell, M.J.; Lashen, H. Do South Asian women with PCOS have poorer health-related quality of life than Caucasian women with PCOS? A comparative cross-sectional study. Health Qual. Life Outcomes 2010, 8, 149. [Google Scholar] [CrossRef]
- Benson, S.; Hahn, S.; Tan, S.; Mann, K.; Janssen, O.E.; Schedlowski, M.; Elsenbruch, S. Prevalence and implications of anxiety in polycystic ovary syndrome: Results of an internet-based survey in Germany. Hum. Reprod. 2009, 24, 1446–1451. [Google Scholar] [CrossRef]
- Dokras, A.; Bochner, M.; Hollinrake, E.; Markham, S.; Vanvoorhis, B.; Jagasia, D.H. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet. Gynecol. 2005, 106, 131–137. [Google Scholar] [CrossRef]
- Alvarez, Y.R.; Pico, M.; Ashokprabhu, N.; Abou-Amro, K.; Bailey, S.; Pung, E.; Oberholster, E.; Quesada, O. Polycystic Ovarian Syndrome: A Risk Factor for Cardiovascular Disease. Curr. Atheroscler. Rep. 2023, 25, 1003–1011. [Google Scholar] [CrossRef]
- Shorakae, S.; Teede, H.; de Courten, B.; Lambert, G.; Boyle, J.; Moran, L.J. The Emerging Role of Chronic Low-Grade Inflammation in the Pathophysiology of Polycystic Ovary Syndrome. Semin. Reprod. Med. 2015, 33, 257–269. [Google Scholar] [CrossRef]
- Artimani, T.; Karimi, J.; Mehdizadeh, M.; Yavangi, M.; Khanlarzadeh, E.; Ghorbani, M.; Asadi, S.; Kheiripour, N. Evaluation of pro-oxidant-antioxidant balance (PAB) and its association with inflammatory cytokines in polycystic ovary syndrome (PCOS). Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2018, 34, 148–152. [Google Scholar] [CrossRef]
- Llanos, P.; Palomero, J. Reactive Oxygen and Nitrogen Species (RONS) and Cytokines—Myokines Involved in Glucose Uptake and Insulin Resistance in Skeletal Muscle. Cells 2022, 11, 4008. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Al-Farsi, Y.M.; Al-Khaduri, M.M.; Saleh, J.; Waly, M.I. Polycystic ovarian syndrome is linked to increased oxidative stress in Omani women. Int. J. Women’s Health 2018, 10, 763–771. [Google Scholar] [CrossRef]
- Okoroh, E.M.; Hooper, W.C.; Atrash, H.K.; Yusuf, H.R.; Boulet, S.L. Prevalence of polycystic ovary syndrome among the privately insured, United States, 2003–2008. Am. J. Obstet. Gynecol. 2012, 207, 299.e1–299.e7. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Deeks, A.A.; Moran, L.J.; Stuckey, B.G.; Wong, J.L.; Norman, R.J.; Costello, M.F. Assessment and management of polycystic ovary syndrome: Summary of an evidence-based guideline. Med. J. Aust. 2011, 195, S65–S112. [Google Scholar] [CrossRef]
- Thomson, R.L.; Buckley, J.D.; Lim, S.S.; Noakes, M.; Clifton, P.M.; Norman, R.J.; Brinkworth, G.D. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 1812–1816. [Google Scholar] [CrossRef]
- Lim, S.S.; Hutchison, S.K.; Van Ryswyk, E.; Norman, R.J.; Teede, H.J.; Moran, L.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 3, Cd007506. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Artensio, A.C.; Stabile, G.; Volpe, A. Metformin treatment of PCOS during adolescence and the reproductive period. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 121, 3–7. [Google Scholar] [CrossRef]
- Azadi-Yazdi, M.; Karimi-Zarchi, M.; Salehi-Abargouei, A.; Fallahzadeh, H.; Nadjarzadeh, A. Effects of Dietary Approach to Stop Hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: A randomised controlled trial. J. Hum. Nutr. Diet. 2017, 30, 275–283. [Google Scholar] [CrossRef]
- Çıtar Dazıroğlu, M.E.; Acar Tek, N. The effect on inflammation of adherence to the Mediterranean diet in polycystic ovary syndrome. Curr. Nutr. Rep. 2023, 12, 191–202. [Google Scholar] [CrossRef]
- He, J.; Deng, R.; Wei, Y.; Zhang, S.; Su, M.; Tang, M.; Wang, J.; Nong, W.; Lei, X. Efficacy of antioxidant supplementation in improving endocrine, hormonal, inflammatory, and metabolic statuses of PCOS: A meta-analysis and systematic review. Food Funct. 2024, 15, 1779–1802. [Google Scholar] [CrossRef]
- Li, R.; Li, Z.; Huang, Y.; Hu, K.; Ma, B.; Yang, Y. The effect of magnesium alone or its combination with other supplements on the markers of inflammation, OS and metabolism in women with polycystic ovarian syndrome (PCOS): A systematic review. Front. Endocrinol. 2022, 13, 974042. [Google Scholar] [CrossRef]
- Alesi, S.; Ee, C.; Moran, L.J. Nutritional Supplements and Complementary Therapies in Polycystic Ovary Syndrome. Adv. Nutr. 2022, 13, 1243–1266. [Google Scholar] [CrossRef] [PubMed]
- Babapour, M.; Mohammadi, H.; Kazemi, M.; Hadi, A.; Rezazadegan, M.; Askari, G. Associations between serum magnesium concentrations and polycystic ovary syndrome status: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2021, 199, 1297–1305. [Google Scholar] [CrossRef]
- Glasdam, S.M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 73, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morán, M.; Simental Mendía, L.E.; Zambrano Galván, G.; Guerrero-Romero, F. The role of magnesium in type 2 diabetes: A brief based-clinical review. Magnes. Res. 2011, 24, 156–162. [Google Scholar] [CrossRef]
- Szczuko, M.; Skowronek, M.; Zapałowska-Chwyć, M.; Starczewski, A. Quantitative assessment of nutrition in patients with polycystic ovary syndrome (PCOS). Rocz. Panstw. Zakl. Hig. 2016, 67, 419–426. [Google Scholar] [PubMed]
- Asemi, Z.; Esmaillzadeh, A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: A randomized controlled clinical trial. Horm. Metab. Res. 2015, 47, 232–238. [Google Scholar] [CrossRef]
- Parazzini, F.; Di Martino, M.; Pellegrino, P. Magnesium in the gynecological practice: A literature review. Magnes. Res. 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Goluch-Koniuszy, Z.S. Nutrition of women with hair loss problem during the period of menopause. Menopause Rev. 2016, 15, 56–61. [Google Scholar] [CrossRef]
- Chandrasekaran, N.C.; Weir, C.; Alfraji, S.; Grice, J.; Roberts, M.S.; Barnard, R.T. Effects of magnesium deficiency--more than skin deep. Exp. Biol. Med. 2014, 239, 1280–1291. [Google Scholar] [CrossRef]
- Heidary, Z.; Khalili, H. Effect of Magnesium Loading Dose on Insulin Resistance in Patients With Stress-Induced Hyperglycemia: A Randomized Clinical Trial. J. Intensive Care Med. 2020, 35, 687–693. [Google Scholar] [CrossRef]
- Veronese, N.; Watutantrige-Fernando, S.; Luchini, C.; Solmi, M.; Sartore, G.; Sergi, G.; Manzato, E.; Barbagallo, M.; Maggi, S.; Stubbs, B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1354–1359. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Jaquez-Chairez, F.O.; Rodríguez-Morán, M. Magnesium in metabolic syndrome: A review based on randomized, double-blind clinical trials. Magnes. Res. 2016, 29, 146–153. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Chiti, H.; Tavakolizadeh, M.; Hatami, R.; Motamed, N.; Ghaemi, M. The Effect of Magnesium Supplementation on Insulin Resistance and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Clinical Trial. Biol. Trace Elem. Res. 2024, 202, 941–946. [Google Scholar] [CrossRef]
- Farsinejad-Marj, M.; Azadbakht, L.; Mardanian, F.; Saneei, P.; Esmaillzadeh, A. Clinical and Metabolic Responses to Magnesium Supplementation in Women with Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2020, 196, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Muneyyirci-Delale, O.; Kaplan, J.; Joulak, I.; Yang, L.; Von Gizycki, H.; Nacharaju, V.L. Serum free fatty acid levels in PCOS patients treated with glucophage, magnesium oxide and spironolactone. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2013, 29, 474–477. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Christ, J.P.; Cedars, M.I. Current guidelines for diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Bmj 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Alizadeh, M.; Karandish, M.; Jafarabadi, M.A.; Heidari, L.; Nikbakht, R.; Rezaei, H.B.; Mousavi, R. Metabolic and hormonal effects of melatonin and/or magnesium supplementation in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Nutr. Metab. 2021, 18, 57. [Google Scholar] [CrossRef]
- Gholizadeh-Moghaddam, M.; Ghasemi-Tehrani, H.; Askari, G.; Jaripur, M.; Clark, C.C.; Rouhani, M.H. Effect of magnesium supplementation in improving hyperandrogenism, hirsutism, and sleep quality in women with polycystic ovary syndrome: A randomized, placebo-controlled clinical trial. Health Sci. Rep. 2023, 6, e1013. [Google Scholar] [CrossRef]
- Jaripur, M.; Ghasemi-Tehrani, H.; Askari, G.; Gholizadeh-Moghaddam, M.; Clark, C.C.; Rouhani, M.H. The effects of magnesium supplementation on abnormal uterine bleeding, alopecia, quality of life, and acne in women with polycystic ovary syndrome: A randomized clinical trial. Reprod. Biol. Endocrinol. 2022, 20, 110. [Google Scholar] [CrossRef]
- Mousavi, R.; Alizadeh, M.; Asghari Jafarabadi, M.; Heidari, L.; Nikbakht, R.; Babaahmadi Rezaei, H.; Karandish, M. Effects of melatonin and/or magnesium supplementation on biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2022, 200, 1010–1019. [Google Scholar] [CrossRef]
- Baptiste, C.G.; Battista, M.C.; Trottier, A.; Baillargeon, J.P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2010, 122, 42–52. [Google Scholar] [CrossRef]
- Shirazi, F.K.H.; Khodamoradi, Z.; Jeddi, M. Insulin resistance and high molecular weight adiponectin in obese and non-obese patients with Polycystic Ovarian Syndrome (PCOS). BMC Endocr. Disord. 2021, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mamaghani, M.; Saghafi-Asl, M.; Pirouzpanah, S.; Aliasgharzadeh, A.; Aliashrafi, S.; Rezayi, N.; Mehrzad-Sadaghiani, M. Association of insulin resistance with lipid profile, metabolic syndrome, and hormonal aberrations in overweight or obese women with polycystic ovary syndrome. J. Health Popul. Nutr. 2015, 33, 157–167. [Google Scholar]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metab. Clin. Exp. 2019, 92, 108–120. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 38. [Google Scholar] [CrossRef]
- Layegh, P.; Mousavi, Z.; Farrokh Tehrani, D.; Parizadeh, S.M.; Khajedaluee, M. Insulin resistance and endocrine-metabolic abnormalities in polycystic ovarian syndrome: Comparison between obese and non-obese PCOS patients. Int. J. Reprod. Biomed. 2016, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J. Clinical review 124: Diabetic dyslipidemia: Causes and consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Hama, S.; Liu, Y.; Siahmansur, T.; Schofield, J.; Syed, A.A.; France, M.; Pemberton, P.; Adam, S.; Ho, J.H.; et al. Effect of Roux-en-Y Bariatric Surgery on Lipoproteins, Insulin Resistance, and Systemic and Vascular Inflammation in Obesity and Diabetes. Front. Immunol. 2017, 8, 1512. [Google Scholar] [CrossRef]
- Duleba, A.J.; Dokras, A. Is PCOS an inflammatory process? Fertil. Steril. 2012, 97, 7–12. [Google Scholar] [CrossRef]
- Costello, M.F.; Shrestha, B.; Eden, J.; Johnson, N.P.; Sjoblom, P. Metformin versus oral contraceptive pill in polycystic ovary syndrome: A Cochrane review. Hum. Reprod. 2007, 22, 1200–1209. [Google Scholar] [CrossRef]
- Hyderali, B.N.; Mala, K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 15–22. [Google Scholar] [CrossRef]
- González, F. Inflammation in Polycystic Ovary Syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids 2012, 77, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gupta, V. Assessment of Serum Elements Concentration and Polycystic Ovary Syndrome (PCOS): Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2022, 200, 4582–4593. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochemist. Rev. 2003, 24, 47–66. [Google Scholar]
- Noronha, J.L.; Matuschak, G.M. Magnesium in critical illness: Metabolism, assessment, and treatment. Intensive Care Med. 2002, 28, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Afshar Ebrahimi, F.; Foroozanfard, F.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The Effects of Magnesium and Zinc Co-Supplementation on Biomarkers of Inflammation and Oxidative Stress, and Gene Expression Related to Inflammation in Polycystic Ovary Syndrome: A Randomized Controlled Clinical Trial. Biol. Trace Elem. Res. 2018, 184, 300–307. [Google Scholar] [CrossRef]
- Talebi, S.; Miraghajani, M.; Hosseini, R.; Mohammadi, H. The Effect of Oral Magnesium Supplementation on Inflammatory Biomarkers in Adults: A Comprehensive Systematic Review and Dose-response Meta-analysis of Randomized Clinical Trials. Biol. Trace Elem. Res. 2022, 200, 1538–1550. [Google Scholar] [CrossRef]
- Sharifi, F.; Mazloomi, S.; Hajihosseini, R.; Mazloomzadeh, S. Serum magnesium concentrations in polycystic ovary syndrome and its association with insulin resistance. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2012, 28, 7–11. [Google Scholar] [CrossRef]
- Shokrpour, M.; Asemi, Z. The Effects of Magnesium and Vitamin E Co-Supplementation on Hormonal Status and Biomarkers of Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2019, 191, 54–60. [Google Scholar] [CrossRef] [PubMed]






| Study | Country | n | Magnesium Dose (mg/d) | Magnesium Duration (wk) | Age (Years) | BMI (kg/m2) | Main Outcomes * | ||
|---|---|---|---|---|---|---|---|---|---|
| Magnesium | Control | Magnesium | Control | ||||||
| Alizadeh et al., 2021 [42] Mousavi et al., 2022 * [45] | Iran | 84 | 250 | 8 | 25.57 ± 4.88 | 26.2 ± 5.72 | 27.99 ± 3.22 | 26.94 ± 3.83 | ↓Testosterone, ↓WC, ↔BMI, ↔FBS, ↔Insulin, ↔HOMA-IR, ↔TC, ↔TG, ↔HDL, ↔LDL, ↔SHBG, ↔Hirsutism, ↔MDA, ↔TAC, ↔TNF-α, ↔hs-CRP |
| Farsinejad-Marj et al., 2020 [35] | Iran | 60 | 250 | 8 | 26.32 ± 3.92 | 26 ± 5.06 | 27.8 ± 5.07 | 27.72 ± 5.40 | ↓BMI, ↔Weight, ↔WC, ↔SBP, ↔DBP, ↔FBS, ↔Insulin, ↔QUICKI, ↔HOMA-IR, ↔HOMA-B, ↔TC, ↔TG, ↔HDL, ↔LDL, ↔FSH, ↑LH, ↔FAI, ↓Testosterone, ↔SHBG, ↑DHEA |
| Gholizadeh-Moghaddam et al., 2022 [43] Jaripur et al., 2022 * [44] | Iran | 64 | 250 | 10 | 31.69 ± 5.41 | 32.44 ± 6.42 | 26.89 ± 4.68 | 26.63 ± 4.06 | ↔Life quality, ↔Hirsutism, ↔Sleep Quality, ↔Testosterone, ↔DHEA |
| Shahmoradi et al., 2024 [34] | Iran | 40 | 250 | 20 | 27.45 ± 5.04 | 29.00 ± 4.24 | 29.73 ± 6.44 | 30.61 ± 5.03 | ↔BMI, ↔Weight, ↔WC, ↔HC, ↔SBP, ↔DBP, ↓FBS, ↓Insulin, ↓HOMA-IR, ↓TC, ↔TG, ↑HDL, ↓LDL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Zaid, A.; Alzayed, M.M.; Albahrani, S.J.; Almaqhawi, A.; Al Shaikh, M.A.; Baradwan, S.; Almudiheem, N.A.; Abuzaid, M.; Adly, H.M.; Saleh, S.A.K.; et al. Does Magnesium Affect Sex Hormones and Cardiometabolic Risk Factors in Patients with PCOS? Findings from a Systematic Review and Meta-Analysis. Medicina 2025, 61, 280. https://doi.org/10.3390/medicina61020280
Abu-Zaid A, Alzayed MM, Albahrani SJ, Almaqhawi A, Al Shaikh MA, Baradwan S, Almudiheem NA, Abuzaid M, Adly HM, Saleh SAK, et al. Does Magnesium Affect Sex Hormones and Cardiometabolic Risk Factors in Patients with PCOS? Findings from a Systematic Review and Meta-Analysis. Medicina. 2025; 61(2):280. https://doi.org/10.3390/medicina61020280
Chicago/Turabian StyleAbu-Zaid, Ahmed, Mooza M. Alzayed, Suha Jafar Albahrani, Abdullah Almaqhawi, Mona Ahmed Al Shaikh, Saeed Baradwan, Nawaf Abdulaziz Almudiheem, Mohammed Abuzaid, Heba M. Adly, Saleh A. K. Saleh, and et al. 2025. "Does Magnesium Affect Sex Hormones and Cardiometabolic Risk Factors in Patients with PCOS? Findings from a Systematic Review and Meta-Analysis" Medicina 61, no. 2: 280. https://doi.org/10.3390/medicina61020280
APA StyleAbu-Zaid, A., Alzayed, M. M., Albahrani, S. J., Almaqhawi, A., Al Shaikh, M. A., Baradwan, S., Almudiheem, N. A., Abuzaid, M., Adly, H. M., Saleh, S. A. K., & Alomar, O. (2025). Does Magnesium Affect Sex Hormones and Cardiometabolic Risk Factors in Patients with PCOS? Findings from a Systematic Review and Meta-Analysis. Medicina, 61(2), 280. https://doi.org/10.3390/medicina61020280








