Sensory Deficit Following Anterior Cruciate Ligament Reconstruction with Bone–Patellar Tendon–Bone Autograft: Platelet-Rich Fibrin (PRF) Could Provide a Solution
Abstract
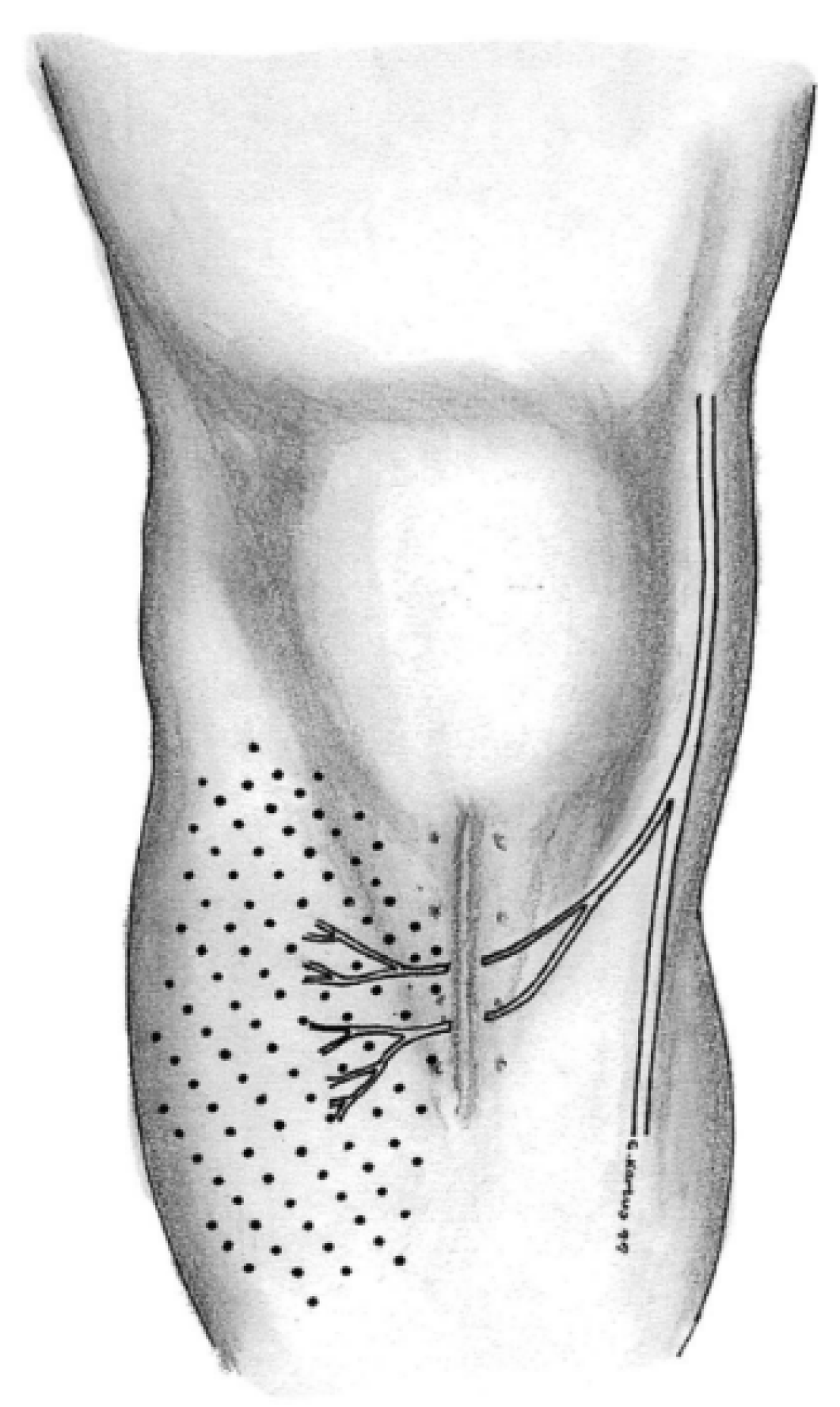
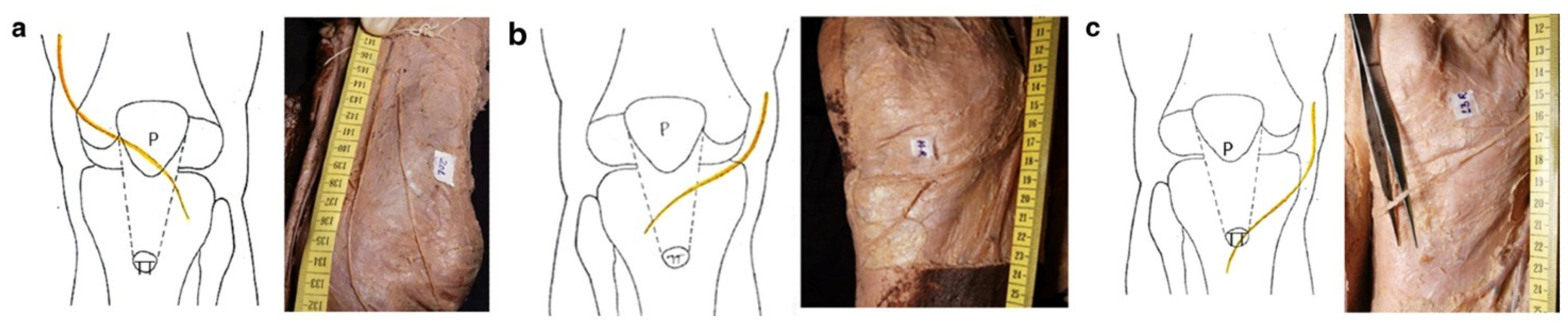
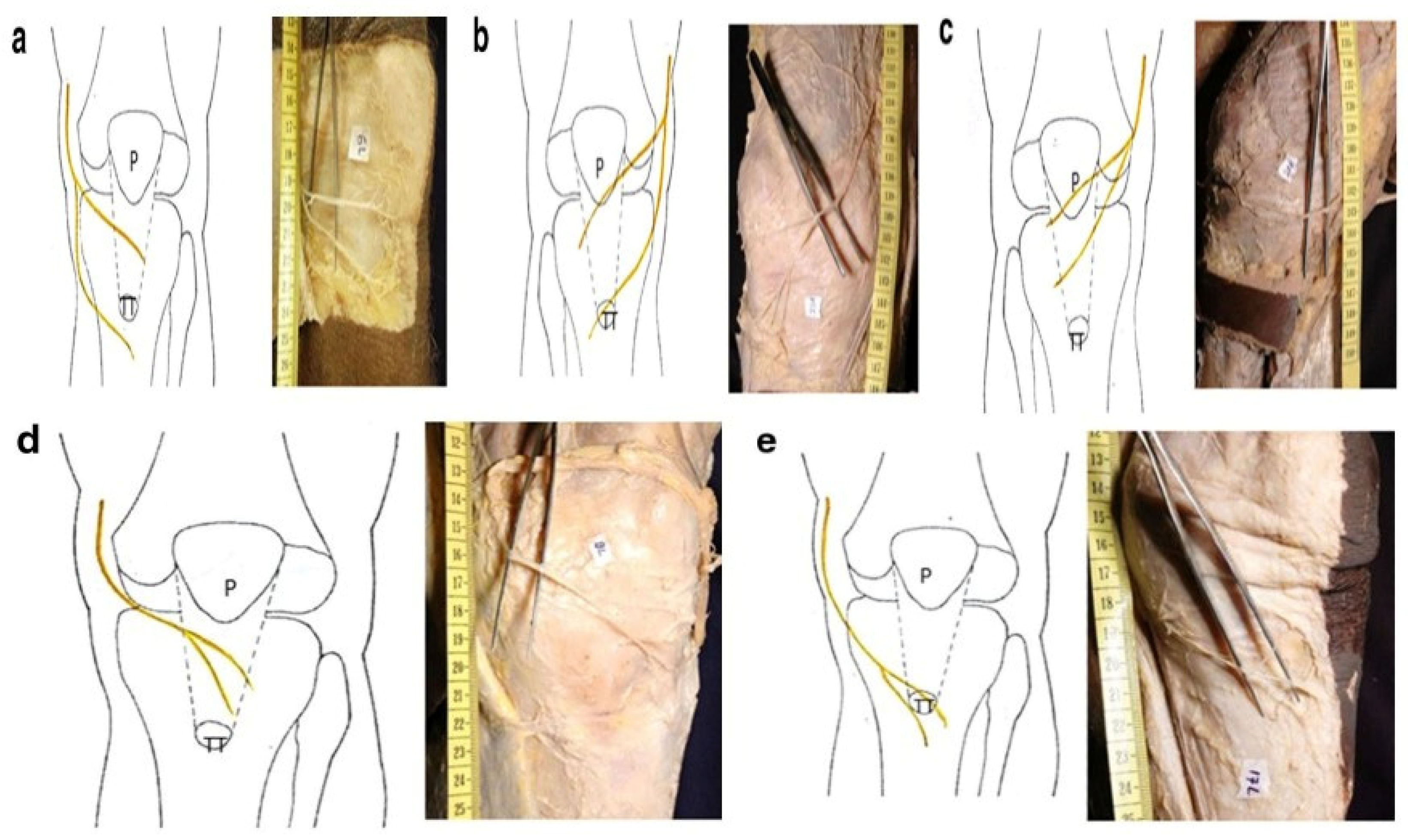
1. Introduction
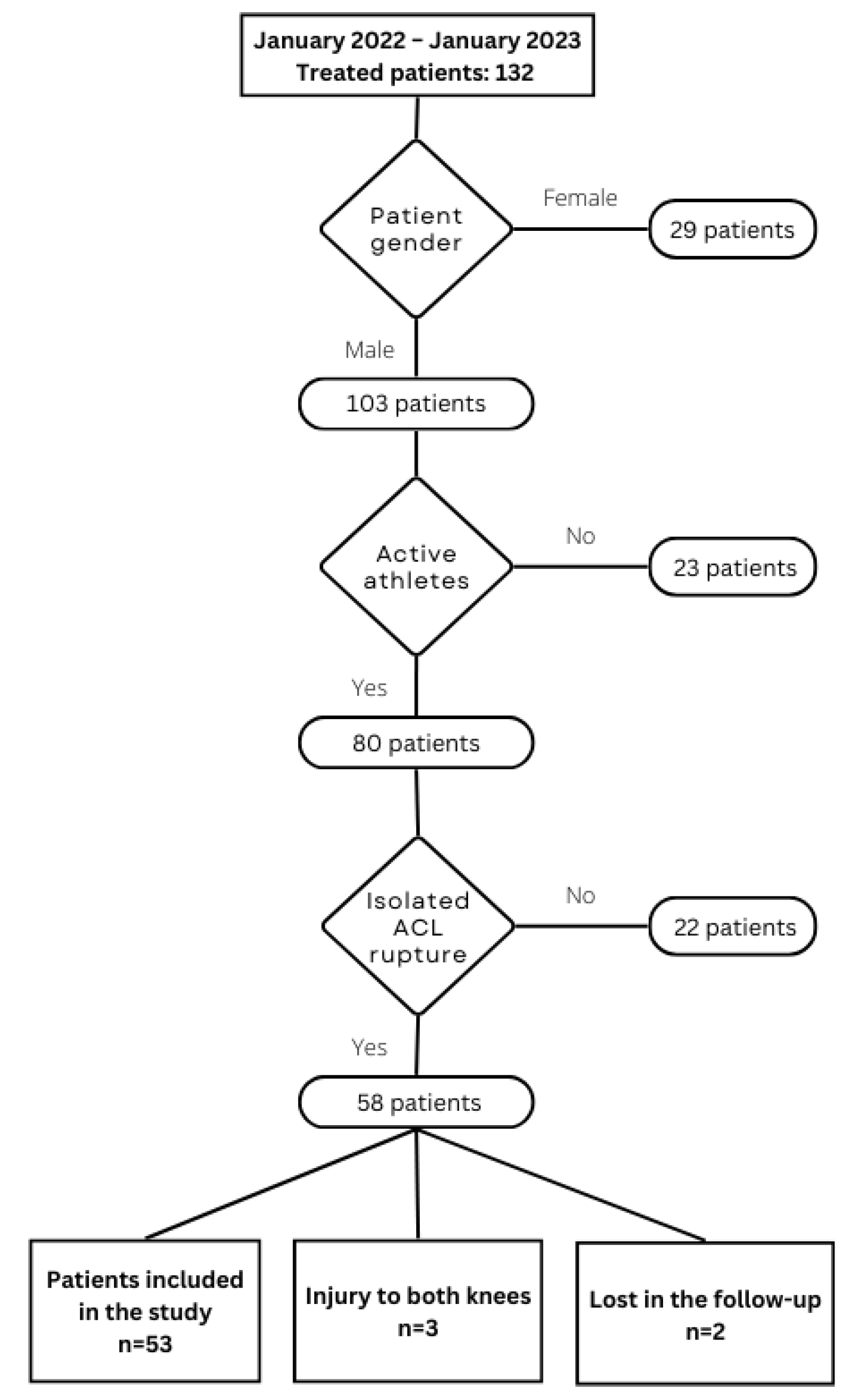
2. Materials and Methods
2.1. Study Background
2.2. Vivostat® Preparation and Surgical Technique
2.3. Postoperative Rehabilitation
2.4. Postoperative Follow-Up
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPTB | Bone–patellar tendon–bone |
| ACL | Anterior cruciate ligament |
| IPBSN | Infrapatellar branch of the saphenous nerve |
| PRF | Linear dichroism |
| PRP | Platelet-rich fibrin |
| IKDC | International Knee Documentation Committee |
| SD | Standard deviation |
| BMI | Body mass index |
References
- Paschos, N.K.; Howell, S.M. Anterior cruciate ligament reconstruction: Principles of treatment. EFORT Open Rev. 2016, 11, 398–408. [Google Scholar] [CrossRef]
- Kunze, K.N.; Moran, J.; Polce, E.M.; Pareek, A.; Strickland, S.M.; Williams, R.J. Lower donor site morbidity with hamstring and quadriceps tendon autograft compared with bone-patellar tendon-bone autograft after anterior cruciate ligament reconstruction: A systematic review and network meta-analysis of randomized controlled trials. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Hacken, B.A.; Keyt, L.K.; Leland, D.P.; LaPrade, M.D.; Camp, C.L.; Levy, B.A.; Krych, A.J. A Novel Scoring Instrument to Assess Donor Site Morbidity After Anterior Cruciate Ligament Reconstruction With a Patellar Tendon Autograft at 2-Year Follow-up Using Contemporary Graft-Harvesting Techniques. Orthop. J. Sports Med. 2020, 8, 2325967120925482. [Google Scholar] [CrossRef] [PubMed]
- Runer, A.; Keeling, L.; Wagala, N.; Nugraha, H.; Özbek, E.A.; Hughes, J.D.; Musahl, V. Current trends in graft choice for anterior cruciate ligament reconstruction—Part I: Anatomy, biomechanics, graft incorporation and fixation. J. Exp. Orthop. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; McCulloch, P.; Cole, B.J.; Bush-Joseph, C.A.; Bach, B.R. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy 2008, 24, 162–166. [Google Scholar] [CrossRef]
- Luciano, A.P.; Honda, R.T.M.; Kamar, A.R.; Franco Filho, N.; Vieira, M.C. Anatomical Study of the Infrapatellar Branch of the Saphenous Nerve in Humans. Rev. Bras. Ortop. 2020, 55, 557–563. [Google Scholar]
- Figueroa, D.; Calvo, R.; Vaisman, A.; Campero, M.; Moraga, C. Injury to the infrapatellar branch of the saphenous nerve in ACL reconstruction with the hamstrings technique: Clinical and electrophysiological study. Knee 2008, 15, 360–363. [Google Scholar] [CrossRef]
- Hardy, A.; Casabianca, L.; Andrieu, K.; Baverel, L.; Noailles, T.; Junior French Arthroscopy Society. Complications following harvesting of patellar tendon or hamstring tendon grafts for anterior cruciate ligament reconstruction: Systematic review of literature. Orthop. Traumatol. Surg. Res. 2017, 103, S245–S248. [Google Scholar] [CrossRef]
- Tifford, C.D.; Spero, L.; Luke, T.; Plancher, K.D. The relationship of the infrapatellar branches of the saphenous nerve to arthroscopy portals and incisions for anterior cruciate ligament surgery. An anatomic study. Am. J. Sports Med. 2000, 28, 562–567. [Google Scholar] [CrossRef]
- Kartus, J.; Ejerhed, L.; Sernert, N.; Brandsson, S.; Karlsson, J. Comparison of Traditional and Subcutaneous Patellar Tendon Harvest: A Prospective Study of Donor Site-Related Problems After Anterior Cruciate Ligament Reconstruction Using Different Graft Harvesting Techniques. Am. J. Sports Med. 2000, 28, 328–335. [Google Scholar] [CrossRef]
- Dunaway, D.J.; Steensen, R.N.; Wiand, W.; Dopirak, R.M. The sartorial branch of the saphenous nerve: Its anatomy at the joint line of the knee. Arthroscopy 2005, 21, 547–551. [Google Scholar] [CrossRef]
- Warren, L.F.; Marshall, J.L. The supporting structures and layers on the medial side of the knee: An anatomical analysis. J. Bone Jt. Surg. Am. 1979, 61, 56–62. [Google Scholar] [CrossRef]
- Kalthur, S.G.; Sumalatha, S.; Nair, N.; Pandey, A.K.; Sequeria, S.; Shobha, L. Anatomic study of infrapatellar branch of saphenous nerve in male cadavers. Ir. J. Med. Sci. 2015, 184, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, J.; Habash, M.; Ghobrial, B.; Alnajjar, R.; Ellanti, P. Current Status and Advancements in Platelet-Rich Plasma Therapy. Cureus 2023, 15, e47176. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Beyzadeoglu, T.; Pehlivanoglu, T.; Yildirim, K.; Buldu, H.; Tandogan, R.; Tuzun, U. Does the Application of Platelet-Rich Fibrin in Anterior Cruciate Ligament Reconstruction Enhance Graft Healing and Maturation? A Comparative MRI Study of 44 Cases. Orthop. J. Sports Med. 2020, 8, 2325967120902013. [Google Scholar] [CrossRef]
- Bayer, A.; Lammel, J.; Tohidnezhad, M.; Lippross, S.; Behrendt, P.; Klüter, T.; Harder, J. The Antimicrobial Peptide Human Beta-Defensin-3 Is Induced by Platelet-Released Growth Factors in Primary Keratinocytes. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Skarpas, G.A. Arthrozheal®, a Bioactive Fibrin Scaffold for Joint Cartilage, Tendon and Soft Tissue Lesions. Latest Results and Application Perspectives. 2022. Available online: https://surgicaltechnology.com/41-Orthopaedic-Surgery.htm#1636 (accessed on 21 April 2025).
- Lichtenfels, M.; Colomé, L.; Sebben, A.D.; Braga-Silva, J. Effect of platelet rich plasma and platelet rich fibrin on sciatic nerve regeneration in a rat model. Microsurgery 2013, 33, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhu, Q.; Liu, X.; Huang, X.; He, C.; Jiang, L.; Quan, D.; Zhou, X.; Zhu, Z. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J. Tissue Eng. Regen. Med. 2016, 10, 428–436. [Google Scholar] [CrossRef]
- Lambrichts, I.; Wolfs, E.; Bronckaers, A.; Gervois, P.; Vangansewinkel, T. The Effect of Leukocyte- and Platelet-Rich Fibrin on Central and Peripheral Nervous System Neurons—Implications for Biomaterial Applicability. Int. J. Med. Sci. 2023, 24, 14314. [Google Scholar] [CrossRef]
- Kuffler, D.P.; Reyes, O.; Sosa, I.J.; Santiago-Figueroa, J. Neurological Recovery Across a 12-cm-Long Ulnar Nerve Gap Repaired 3.25 Years Post Trauma: Case Report. Neurosurgery 2011, 69, E1321–E1326. [Google Scholar] [CrossRef]
- Dodd, R.A.; Cornwell, R.; Holm, N.E.; Garbarsch, A.; Hollingsbee, D.A. The Vivostat application system: A comparison with conventional fibrin sealant application systems. Technol. Health Care 2002, 10, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Flato, R.; Wascher, J.; Watson, R.; Salminen, M.; O’Brien, D.; Ciccotti, M. Incidence and Characterization of Hypoesthesia in the Distribution of the Infrapatellar Branch of the Saphenous Nerve after Anterior Cruciate Ligament Reconstruction: A Prospective Study of Patient-Reported Numbness. J. Knee Surg. 2018, 31, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Barié, A.; Sprinckstub, T.; Huber, J.; Jaber, A. Quadriceps tendon vs. patellar tendon autograft for ACL reconstruction using a hardware-free press-fit fixation technique: Comparable stability, function and return-to-sport level but less donor site morbidity in athletes after 10 years. Arch. Orthop. Trauma. Surg. 2020, 140, 1465–1474. [Google Scholar] [CrossRef]
- Cervellin, M.; de Girolamo, L.; Bait, C.; Denti, M.; Volpi, P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: A randomized, controlled clinical study. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kovindha, K.; Ganokroj, P.; Lertwanich, P.; Vanadurongwan, B. Quantifying anterior knee pain during specific activities after using the bone-patellar tendon-bone graft for arthroscopic anterior cruciate ligament reconstruction. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2019, 15, 6–12. [Google Scholar] [CrossRef]
- Tsuda, E.; Okamura, Y.; Ishibashi, Y.; Otsuka, H.; Toh, S. Techniques for Reducing Anterior Knee Symptoms after Anterior Cruciate Ligament Reconstruction Using a Bone-Patellar Tendon-Bone Autograft. Am. J. Sports Med. 2001, 29, 450–456. [Google Scholar] [CrossRef]
- Mishra, A.K.; Fanton, G.S.; Dillingham, M.F.; Carver, T.J. Patellar tendon graft harvesting using horizontal incisions for anterior cruciate ligament reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 1995, 11, 749–752. [Google Scholar] [CrossRef]
- Gaudot, F.; Leymarie, J.B.; Drain, O.; Boisrenoult, P.; Charrois, O.; Beaufils, P. Double-incision mini-invasive technique for BTB Harvesting: Its superiority in reducing anterior knee pain following ACL reconstruction. Orthop. Traumatol. Surg. Res. 2009, 95, 28–35. [Google Scholar] [CrossRef]


| Characteristics | Total | Vivostat | Standard | Test |
|---|---|---|---|---|
| Gender | ||||
| Male | 53 (100%) | 24 (100%) | 29 (100%) | |
| Age (years) | ns * | |||
| Mean (SD) | 27.13 (8.47) | 26.54 (9.55) | 27.62 (7.6) | |
| Median (Range) | 25 (17–52) | 24 (17–52) | 26 (17–44) | |
| BMI (kg/m2) | ns * | |||
| Mean (SD) | 25.68 (2.56) | 25.48 (2.43) | 25.84 (2.7) | |
| Median (Range) | 24.97 (21.61–35.35) | 24.88 (21.61–31.24) | 24.97 (22.92–35.35) | |
| Length of time # (months) | ns * | |||
| Mean (SD) | 13.21 (22.01) | 16.97 (30.32) | 10.09 (11.06) | |
| Median (Range) | 7 (0.07–144) | 7 (0.36–144) | 8 (0.07–42) | |
| Length of hospitalization | ns * | |||
| Mean (SD) | 5.19 (3.06) | 4.92 (2.19) | 5.41 (3.66) | |
| Median (Range) | 4 (2–20) | 4 (3–11) | 4 (2–20) | |
| Total | 53 (100%) | 24 (100%) | 29 (100%) | |
| Type of Score | Total | Vivostat | Standard | Test * |
|---|---|---|---|---|
| Modified Cincinnati score | ||||
| Initial (preoperative) values | ||||
| Mean (SD) | 64.4 (21.66) | 69.5 (17.91) | 60.17 (23.81) | ns |
| Median (Range) | 65 (12–100) | 74 (34–100) | 62 (12–91) | |
| 12 months after surgery | ||||
| Mean (SD) | 93.3 (7.45) | 92.67 (7.91) | 93.82 (7.15) | ns |
| Median (Range) | 96 (70–100) | 94.5 (75–100) | 96 (70–100) | |
| Wilcoxon Signed-Rank Test | - | |||
| 12 months vs. initial | p < 0.01 | p < 0.01 | p < 0.01 | |
| IKDC score | ||||
| Initial (preoperative) values | ||||
| Mean (SD) | 57.4 (19.25) | 60.62 (17.16) | 54.74 (20.73) | ns |
| Median (Range) | 59.8 (14.9–93.1) | 61.5 (21.8–88.5) | 56.3 (14.9–93.1) | |
| 12 months after surgery | ||||
| Mean (SD) | 89.72 (9.44) | 90.33 (8.86) | 90.33 (8.86) | ns |
| Median (Range) | 89.7 (52.9–100) | 89.7 (67.8–100) | 89.7 (67.8–100) | |
| Wilcoxon Signed-Rank Test | - | |||
| 12 months vs. initial | p < 0.01 | p < 0.01 | p < 0.01 | |
| Tegner–Lysholm Score | ||||
| Initial (preoperative) values | ||||
| Mean (SD) | 71.83 (20.36) | 75.75 (17.06) | 68.59 (22.51) | ns |
| Median (Range) | 78 (16–100) | 80.5 (39–100) | 77 (16–94) | |
| 12 months after surgery | ||||
| Mean (SD) | 94.25 (12.7) | 95.75 (5.2) | 93 (16.54) | ns |
| Median (Range) | 95 (10–100) | 97 (81–100) | 95 (10–100) | |
| Wilcoxon Signed-Rank Test | - | |||
| 12 months vs. initial | p < 0.01 | p < 0.01 | p < 0.01 | |
| Activity score | ||||
| Initial (preoperative) values | ||||
| Mean (SD) | 6.45 (2.64) | 6.67 (2.7) | 6.28 (2.62) | ns |
| Median (Range) | 7 (0–10) | 7 (2–10) | 7 (0–10) | |
| 12 months after surgery | ||||
| Mean (SD) | 6.26 (2.1) | 6.5 (2.02) | 6.07 (2.17) | ns |
| Median (Range) | 6 (1–10) | 6 (3–10) | 6 (1–10) | |
| Wilcoxon Signed-Rank Test | - | |||
| 12 months vs. initial | ns | ns | ns | |
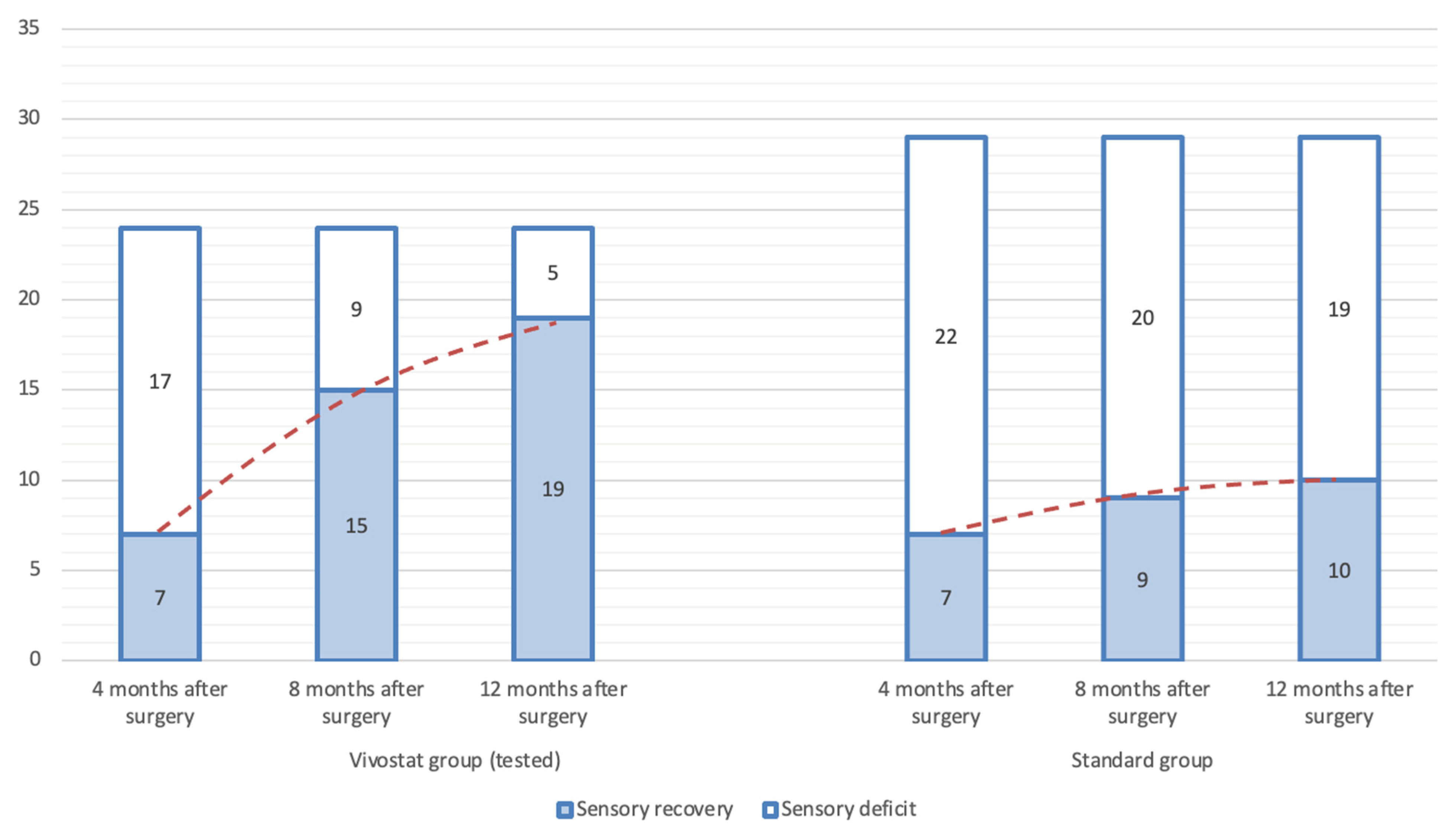
| Sensory Deficit | Total | Vivostat | Standard | Test * |
|---|---|---|---|---|
| 4 months after surgery | ||||
| Sensory 0 | 14 (26.42%) | 7 (29.17%) | 7 (24.14%) | ns |
| Sensory 1 | 39 (73.58%) | 17 (70.83%) | 22 (75.86%) | |
| 8 months after surgery | ||||
| Sensory 0 | 24 (45.28%) | 15 (62.5%) | 9 (31.03%) | p < 0.05 |
| Sensory 1 | 29 (54.72%) | 9 (37.5%) | 20 (68.97%) | |
| 12 months after surgery | ||||
| Sensory 0 | 29 (54.72%) | 19 (79.17%) | 10 (34.48%) | p < 0.01 |
| Sensory 1 | 24 (45.28%) | 5 (20.83%) | 19 (65.52%) | |
| Total | 53 (100%) | 24 (100%) | 29 (100%) | - |
| Sensitivity (Control Exams) | McNemar’s χ2 Test | ||
|---|---|---|---|
| Total | Vivostat | Standard | |
| 4 m vs. 8 m vs. 12 m | p # = 8.6 × 10−6 | p # = 8.8 × 10−5 | ns # |
| 4 m vs. 8 m | p * = 0.0044 | p * = 0.0133 | p * = 0.4795 |
| 4 m vs. 12 m | p * = 0.0003 | p * = 0.0015 | p * = 0.2482 |
| 8 m vs. 12 m | p * = 0.0736 | p * = 0.1336 | p * = 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanovic, D.; Kadija, M.; Gavrilović, D.; Sreckovic, S.; Bilanovic, M.; Matić, A.; Vukman, P. Sensory Deficit Following Anterior Cruciate Ligament Reconstruction with Bone–Patellar Tendon–Bone Autograft: Platelet-Rich Fibrin (PRF) Could Provide a Solution. Medicina 2025, 61, 2202. https://doi.org/10.3390/medicina61122202
Milovanovic D, Kadija M, Gavrilović D, Sreckovic S, Bilanovic M, Matić A, Vukman P. Sensory Deficit Following Anterior Cruciate Ligament Reconstruction with Bone–Patellar Tendon–Bone Autograft: Platelet-Rich Fibrin (PRF) Could Provide a Solution. Medicina. 2025; 61(12):2202. https://doi.org/10.3390/medicina61122202
Chicago/Turabian StyleMilovanovic, Darko, Marko Kadija, Dusica Gavrilović, Svetlana Sreckovic, Miljan Bilanovic, Aleksandar Matić, and Petar Vukman. 2025. "Sensory Deficit Following Anterior Cruciate Ligament Reconstruction with Bone–Patellar Tendon–Bone Autograft: Platelet-Rich Fibrin (PRF) Could Provide a Solution" Medicina 61, no. 12: 2202. https://doi.org/10.3390/medicina61122202
APA StyleMilovanovic, D., Kadija, M., Gavrilović, D., Sreckovic, S., Bilanovic, M., Matić, A., & Vukman, P. (2025). Sensory Deficit Following Anterior Cruciate Ligament Reconstruction with Bone–Patellar Tendon–Bone Autograft: Platelet-Rich Fibrin (PRF) Could Provide a Solution. Medicina, 61(12), 2202. https://doi.org/10.3390/medicina61122202






