Proteoglycans in Prostate Cancer Progression and Therapy Resistance
Abstract
1. Introduction
Search Strategy and Selection Criteria
2. Proteoglycans
Involvement of Proteoglycans in Cancer Progression
3. Role of (Major) Proteoglycans in Prostate Cancer Progression
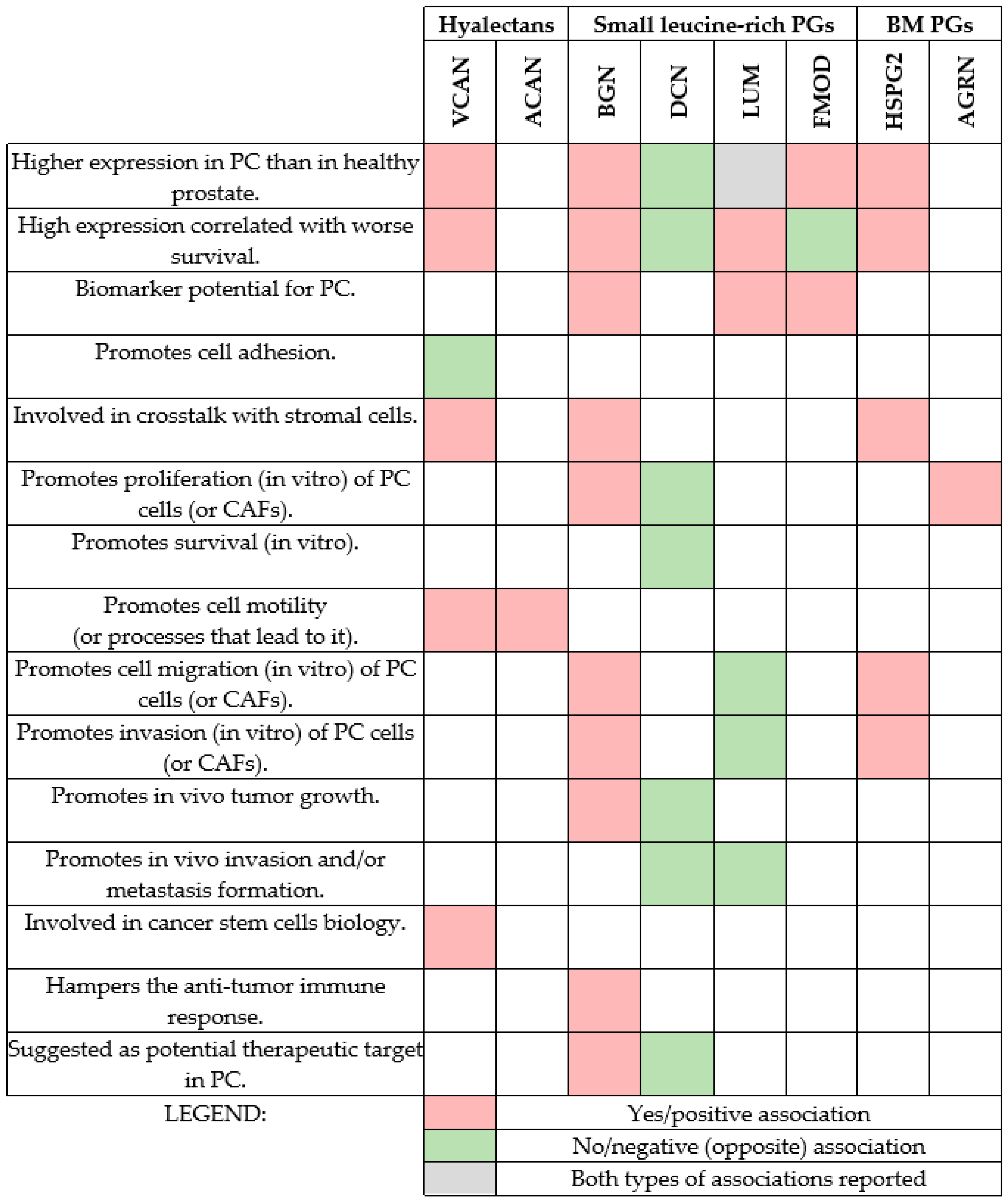
3.1. Hyalectans
3.2. Small Leucine-Rich Proteoglycans
3.3. Basement Membrane Proteoglycans
3.4. Cell Surface Proteoglycans
3.5. Intracellular Proteoglycans
3.6. Proteoglycans and Prostate Cancer Bone Metastasis Biology
4. Role of Proteoglycans in Prostate Cancer Therapy Resistance
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| TME | Tumor microenvironment |
| PGs | Proteoglycans |
| PC | Prostate cancer |
| GAG | Glycosaminoglycan |
| HS | Heparan sulfate |
| CS | Chondroitin sulfate |
| DS | Dermatan sulfate |
| KS | Keratan sulfate |
| HSPG2 | Heparan sulfate proteoglycan 2 |
| VCAN | Versican |
| ACAN | Aggrecan |
| BGN | Biglycan |
| DCN | Decorin |
| FMOD | Fibromodulin |
| LUM | Lumican |
| AGRN | Agrin |
| BM | Basement membrane |
| BGCAN | Betaglycan (also known as transforming growth factor beta receptor 3, TGFβRIII) |
| PCM | Pericellular matrix |
| CAFs | Cancer-associated-fibroblasts |
| PTEN | Phosphatase and tensin homolog |
| MDSCs | Myeloid-derived suppressor cells |
| MMP7 | Matrix metalloproteinase-7 |
| NEAT1 | Nuclear-enriched abundant transcript 1 |
| SDC1 | Syndecan 1 |
| EMT | Epithelial–mesenchymal transition |
| NEPC | Neuroendocrine prostate cancer |
| PSA | Prostate specific antigen |
| GPC1 | Glypican 1 |
| BSCs | Bone marrow-derived stromal cells |
| WNT | Wingless-related integration site |
| SRGN | Serglycin |
| AA/P | Abiraterone acetate/prednisone therapy |
| eccDNA | Extrachromosomal circular DNA |
References
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Naser, R.; Fakhoury, I.; El-Fouani, A.; Abi-Habib, R.; El-Sibai, M. Role of the Tumor Microenvironment in Cancer Hallmarks and Targeted Therapy (Review). Int. J. Oncol. 2023, 62, 23. [Google Scholar] [CrossRef]
- Chitty, J.L.; Cox, T.R. The Extracellular Matrix in Cancer: From Understanding to Targeting. Trends Cancer 2025, 11, 839–849. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; van Tienderen, G.S.; Schenke-Layland, K.; van der Laan, L.J.W.; Khalil, A.A.; Verstegen, M.M.A. The Extracellular Matrix as Hallmark of Cancer and Metastasis: From Biomechanics to Therapeutic Targets. Sci. Transl. Med. 2024, 16, eadg3840. [Google Scholar] [CrossRef]
- Vitale, D.; Kumar Katakam, S.; Greve, B.; Jang, B.; Oh, E.S.; Alaniz, L.; Götte, M. Proteoglycans and Glycosaminoglycans as Regulators of Cancer Stem Cell Function and Therapeutic Resistance. FEBS J. 2019, 286, 2870–2882. [Google Scholar] [CrossRef]
- Ahrens, T.D.; Bang-Christensen, S.R.; Jørgensen, A.M.; Løppke, C.; Spliid, C.B.; Sand, N.T.; Clausen, T.M.; Salanti, A.; Agerbæk, M.Ø. The Role of Proteoglycans in Cancer Metastasis and Circulating Tumor Cell Analysis. Front. Cell Dev. Biol. 2020, 8, 749. [Google Scholar] [CrossRef]
- Barkovskaya, A.; Buffone, A.; Žídek, M.; Weaver, V.M. Proteoglycans as Mediators of Cancer Tissue Mechanics. Front. Cell Dev. Biol. 2020, 8, 569377. [Google Scholar] [CrossRef] [PubMed]
- Dituri, F.; Gigante, G.; Scialpi, R.; Mancarella, S.; Fabregat, I.; Giannelli, G. Proteoglycans in Cancer: Friends or Enemies? A Special Focus on Hepatocellular Carcinoma. Cancers 2022, 14, 1902. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.J. Proteoglycans in Prostate Cancer. Nat. Rev. Urol. 2012, 9, 196–206. [Google Scholar] [CrossRef]
- Tzanakakis, G.; Giatagana, E.-M.; Kuskov, A.; Berdiaki, A.; Tsatsakis, A.; Neagu, M.; Nikitovic, D. Proteoglycans in the Pathogenesis of Hormone-Dependent Cancers: Mediators and Effectors. Cancers 2020, 12, 2401. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan Form and Function: A Comprehensive Nomenclature of Proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Monje, M. Cancer Hallmarks Intersect with Neuroscience in the Tumor Microenvironment. Cancer Cell 2023, 41, 573–580. [Google Scholar] [CrossRef]
- Wei, J.; Hu, M.; Huang, K.; Lin, S.; Du, H. Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Pro-gression. Int. J. Mol. Sci. 2020, 21, 5983. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Schaefer, L.; Iozzo, R.V. Global Impact of Proteoglycan Science on Human Diseases. iScience 2023, 26, 108095. [Google Scholar] [CrossRef]
- Karagiorgou, Z.; Fountas, P.N.; Manou, D.; Knutsen, E.; Theocharis, A.D. Proteoglycans Determine the Dynamic Landscape of EMT and Cancer Cell Stemness. Cancers 2022, 14, 5328. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, T.; You, Y.; Wu, B.; Wang, X.; Wu, J. Proteoglycans and Glycosaminoglycans in Stem Cell Homeostasis and Bone Tissue Regeneration. Front. Cell Dev. Biol. 2021, 9, 760532. [Google Scholar] [CrossRef]
- Gubbiotti, M.A.; Buraschi, S.; Kapoor, A.; Iozzo, R.V. Proteoglycan Signaling in Tumor Angiogenesis and Endothelial Cell Autophagy. Semin. Cancer Biol. 2020, 62, 1–8. [Google Scholar] [CrossRef]
- Douglass, S.; Goyal, A.; Iozzo, R.V. The Role of Perlecan and Endorepellin in the Control of Tumor Angiogenesis and Endothelial Cell Autophagy. Connect. Tissue Res. 2015, 56, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Goode, E.A.; Wang, N.; Munkley, J. Prostate Cancer Bone Metastases Biology and Clinical Management (Review). Oncol. Lett. 2023, 25, 163. [Google Scholar] [CrossRef]
- Ban, J.; Fock, V.; Aryee, D.N.T.; Kovar, H. Mechanisms, Diagnosis and Treatment of Bone Metastases. Cells 2021, 10, 2944. [Google Scholar] [CrossRef]
- Velasco, C.R.; Colliec-Jouault, S.; Redini, F.; Heymann, D.; Padrines, M. Proteoglycans on Bone Tumor Development. Drug Discov. Today 2010, 15, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Tredup, C.; Gubbiotti, M.A.; Iozzo, R.V. Proteoglycan Neofunctions: Regulation of Inflammation and Autophagy in Cancer Biology. FEBS J. 2017, 284, 10–26. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The Extracellular Matrix: Tools and Insights for the “Omics” Era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Iny Stein, T.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Islam, S.; Watanabe, H. Versican: A Dynamic Regulator of the Extracellular Matrix. J. Histochem. Cytochem. 2020, 68, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Zappia, J.; Joiret, M.; Sanchez, C.; Lambert, C.; Geris, L.; Muller, M.; Henrotin, Y. From Translation to Protein Degradation as Mechanisms for Regulating Biological Functions: A Review on the SLRP Family in Skeletal Tissues. Biomolecules 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, T.R.; Gressett, T.E.; Chastain, W.H.; Bix, G.J. Perlecan: A Review of Its Role in Neurologic and Musculoskeletal Disease. Front. Physiol. 2023, 14, 1189731. [Google Scholar] [CrossRef]
- Hayes, A.J.; Farrugia, B.L.; Biose, I.J.; Bix, G.J.; Melrose, J. Perlecan, a Multi-Functional, Cell-Instructive, Matrix-Stabilizing Proteoglycan with Roles in Tissue Development Has Relevance to Connective Tissue Repair and Regeneration. Front. Cell Dev. Biol. 2022, 10, 856261. [Google Scholar] [CrossRef]
- Melrose, J. Perlecan, a Modular Instructive Proteoglycan with Diverse Functional Properties. Int. J. Biochem. Cell Biol. 2020, 128, 105849. [Google Scholar] [CrossRef]
- Anakha, J.; Prasad, Y.R.; Pande, A.H. Endostatin in Disease Modulation: From Cancer to Beyond. Vasc. Pharmacol. 2025, 158, 107459. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Valdés, G.; Gómez-Hevia, F.; Lillo-Moya, J.; González-Fernández, T.; Abelli, J.; Cereceda-Cornejo, A.; Bragato, M.C.; Saso, L.; Rodrigo, R. Endostatin and Cancer Therapy: A Novel Potential Alternative to Anti-VEGF Monoclonal Antibodies. Biomedicines 2023, 11, 718. [Google Scholar] [CrossRef]
- Czarnowski, D. Syndecans in Cancer: A Review of Function, Expression, Prognostic Value, and Therapeutic Significance. Cancer Treat. Res. Commun. 2021, 27, 100312. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Spetz, M.R.; Ho, M. The Role of Glypicans in Cancer Progression and Therapy. J. Histochem. Cytochem. 2020, 68, 841–862. [Google Scholar] [CrossRef]
- Song, H.; Chou, J.; Zhao, P.; Chen, M.; Yang, J.; Hao, X. Exploring TGFBR3 in Disease Pathogenesis: Mechanisms, Clinical Implications, and Pharmacological Modulation. J. Pharm. Anal. 2025, 101372. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Yee, A.J. Roles of Versican in Cancer Biology—Tumorigenesis, Progression and Metastasis. Histol. Histopathol. 2013, 28, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, C.; Mayne, K.; Sykes, P.J.; Raymond, W.A.; McCaul, K.; Marshall, V.R.; Horsfall, D.J. Elevated Levels of Versican but Not Decorin Predict Disease Progression in Early-Stage Prostate Cancer. Clin. Cancer Res. 1998, 4, 963–971. [Google Scholar]
- Sakko, A.J.; Ricciardelli, C.; Mayne, K.; Suwiwat, S.; LeBaron, R.G.; Marshall, V.R.; Tilley, W.D.; Horsfall, D.J. Modulation of Prostate Cancer Cell Attachment to Matrix by Versican. Cancer Res. 2003, 63, 4786–4791. [Google Scholar]
- Ricciardelli, C.; Russell, D.L.; Ween, M.P.; Mayne, K.; Suwiwat, S.; Byers, S.; Marshall, V.R.; Tilley, W.D.; Horsfall, D.J. Formation of Hyaluronan- and Versican-Rich Pericellular Matrix by Prostate Cancer Cells Promotes Cell Motility. J. Biol. Chem. 2007, 282, 10814–10825. [Google Scholar] [CrossRef]
- Oktem, G.; Sercan, O.; Guven, U.; Uslu, R.; Uysal, A.; Goksel, G.; Ayla, S.; Bilir, A. Cancer Stem Cell Differentiation: TGFβ1 and Versican May Trigger Molecules for the Organization of Tumor Spheroids. Oncol. Rep. 2014, 32, 641–649. [Google Scholar] [CrossRef]
- Ayla, S.; Karakoc, E.; Byrne, Y.Y.; Parlayan, C.; Keskin, I.; Karahuseyinoglu, S.; Taskiran, A.; Oktem, G. Splicing Variants of Versican in CD133+/CD44+ Prostate Cancer Stem Cells. Pathol. Res. Pract. 2024, 260, 155440. [Google Scholar] [CrossRef]
- Nijenhuis, N.; Mizuno, D.; Spaan, J.A.E.; Schmidt, C.F. High-Resolution Microrheology in the Pericellular Matrix of Prostate Cancer Cells. J. R. Soc. Interface 2012, 9, 1733–1744. [Google Scholar] [CrossRef]
- Wang, J.; Levenson, A.S.; Satcher, R.L. Identification of a Unique Set of Genes Altered During Cell-Cell Contact in an in Vitro Model of Prostate Cancer Bone Metastasis. Int. J. Mol. Med. 2006, 17, 849–856. [Google Scholar] [CrossRef]
- Chen, N.; Ye, X.-C.; Chu, K.; Navone, N.M.; Sage, E.H.; Yu-Lee, L.-Y.; Logothetis, C.J.; Lin, S.-H. A Secreted Isoform of ErbB3 Promotes Osteonectin Expression in Bone and Enhances the Invasiveness of Prostate Cancer Cells. Cancer Res. 2007, 67, 6544–6548. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Gesteira, T.F.; Coulson-Thomas, Y.M.; Vicente, C.M.; Tersariol, I.L.S.; Nader, H.B.; Toma, L. Fibroblast and Prostate Tumor Cell Cross-Talk: Fibroblast Differentiation, TGF-β, and Extracellular Matrix Down-Regulation. Exp. Cell Res. 2010, 316, 3207–3226. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Wang, M.; Liu, M. Single-Cell and Bulk RNA Sequencing Reveal Cancer-Associated Fibroblast Heterogeneity and a Prognostic Signature in Prostate Cancer. Medicine 2023, 102, e34611. [Google Scholar] [CrossRef]
- Treacy, P.-J.; Martini, A.; Falagario, U.G.; Ratnani, P.; Wajswol, E.; Beksac, A.T.; Wiklund, P.; Nair, S.; Kyprianou, N.; Durand, M.; et al. Association between Expression of Connective Tissue Genes and Prostate Cancer Growth and Progression. Int. J. Mol. Sci. 2023, 24, 7520. [Google Scholar] [CrossRef]
- Gerke, J.S.; Orth, M.F.; Tolkach, Y.; Romero-Pérez, L.; Wehweck, F.S.; Stein, S.; Musa, J.; Knott, M.M.L.; Hölting, T.L.B.; Li, J.; et al. Integrative Clinical Transcriptome Analysis Reveals TMPRSS2-ERG Dependency of Prognostic Biomarkers in Prostate Adenocarcinoma. Int. J. Cancer 2020, 146, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, D.; Goddard, A.D.; Natraj, N.; Cherbavaz, D.B.; Clark-Langone, K.M.; Snable, J.; Watson, D.; Falzarano, S.M.; Magi-Galluzzi, C.; Klein, E.A.; et al. Analytical Validation of the Oncotype DX Prostate Cancer Assay—A Clinical RT-PCR Assay Optimized for Prostate Needle Biopsies. BMC Genom. 2013, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Suhovskih, A.V.; Mostovich, L.A.; Kunin, I.S.; Boboev, M.M.; Nepomnyashchikh, G.I.; Aidagulova, S.V.; Grigorieva, E.V. Proteoglycan Expression in Normal Human Prostate Tissue and Prostate Cancer. ISRN Oncol. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, F.; Kraft, J.; Schroeder, C.; Hube-Magg, C.; Kluth, M.; Lang, D.S.; Simon, R.; Sauter, G.; Izbicki, J.R.; Clauditz, T.S.; et al. Up-Regulation of Biglycan is Associated with Poor Prognosis and PTEN Deletion in Patients with Prostate Cancer. Neoplasia 2017, 19, 707–715. [Google Scholar] [CrossRef]
- Furumido, J.; Maishi, N.; Yanagawa-Matsuda, A.; Kikuchi, H.; Matsumoto, R.; Osawa, T.; Abe, T.; Matsuno, Y.; Shinohara, N.; Hida, Y.; et al. Stroma Biglycan Expression Can Be a Prognostic Factor in Prostate Cancers. Int. J. Urol. 2023, 30, 147–154. [Google Scholar] [CrossRef]
- Brina, D.; Ponzoni, A.; Troiani, M.; Calì, B.; Pasquini, E.; Attanasio, G.; Mosole, S.; Mirenda, M.; D’Ambrosio, M.; Colucci, M.; et al. The Akt/MTOR and MNK/EIF4E Pathways Rewire the Prostate Cancer Translatome to Secrete HGF, SPP1 and BGN and Recruit Suppressive Myeloid Cells. Nat. Cancer 2023, 4, 1102–1121. [Google Scholar] [CrossRef]
- Xu, W.; Niu, Q.; Zhao, K.; Zhao, H.; Zhang, L.; Li, W.; Yan, H.; Dong, Z. Association of High Tumor-Stroma Ratio with Prostate Cancer Progression: Insights from Clinical and Genomic Data. Int. J. Gen. Med. 2025, 18, 2599–2618. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, H.; Owens, R.T.; Wu, J.; Chen, Y.Q.; Berquin, I.M.; Perry, D.; O’Flaherty, J.T.; Edwards, I.J. Decorin Suppresses Prostate Tumor Growth through Inhibition of Epidermal Growth Factor and Androgen Receptor Pathways. Neoplasia 2009, 11, 1042–1053. [Google Scholar] [CrossRef]
- Rezaie, R.; Falakian, Z.; Mazloomzadeh, S.; Ayati, M.; Morakabati, A.; Dastjerdan, M.R.T.; Zare, M.; Moghimi, M.; Shahani, T.; Biglari, A. While Urine and Plasma Decorin Remain Unchanged in Prostate Cancer, Prostatic Tissue Decorin Has a Prognostic Value. Iran. Biomed. J. 2020, 24, 229–235. [Google Scholar] [CrossRef]
- Henke, A.; Grace, O.C.; Ashley, G.R.; Stewart, G.D.; Riddick, A.C.P.; Yeun, H.; O’Donnell, M.; Anderson, R.A.; Thomson, A.A. Stromal Expression of Decorin, Semaphorin6D, SPARC, Sprouty1 and Tsukushi in Developing Prostate and Decreased Levels of Decorin in Prostate Cancer. PLoS ONE 2012, 7, e42516. [Google Scholar] [CrossRef]
- Xu, W.; Neill, T.; Yang, Y.; Hu, Z.; Cleveland, E.; Wu, Y.; Hutten, R.; Xiao, X.; Stock, S.R.; Shevrin, D.; et al. The Systemic Delivery of an Oncolytic Adenovirus Expressing Decorin Inhibits Bone Metastasis in a Mouse Model of Human Prostate Cancer. Gene Ther. 2015, 22, 247–256. [Google Scholar] [CrossRef]
- Halin Bergström, S.; Semenas, J.; Nordstrand, A.; Thysell, E.; Wänman, J.; Crnalic, S.; Widmark, A.; Thellenberg-Karlsson, C.; Andersson, P.; Gidlund, S.; et al. Morphological Heterogeneities in Prostate Cancer Bone Metastases Are Related to Molecular Subtypes and Prognosis. Clin. Exp. Metastasis 2025, 42, 49. [Google Scholar] [CrossRef]
- Kim, J.-S.; Galvão, D.A.; Newton, R.U.; Gray, E.; Taaffe, D.R. Exercise-Induced Myokines and Their Effect on Prostate Cancer. Nat. Rev. Urol. 2021, 18, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Coulson-Thomas, V.J.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Andrade de Paula, C.A.; Carneiro, C.R.W.; Ortiz, V.; Toma, L.; Kao, W.W.Y.; Nader, H.B. Lumican Expression, Localization and Antitumor Activity in Prostate Cancer. Exp. Cell Res. 2013, 319, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Corn, P.G.; Zhang, M.; Nogueras-Gonzalez, G.M.; Xiao, L.; Zurita, A.J.; Subudhi, S.K.; Tu, S.-M.; Aparicio, A.M.; Coarfa, C.; Rajapakshe, K.; et al. A Phase II Study of Cabozantinib and Androgen Ablation in Patients with Hormone-Naïve Metastatic Prostate Cancer. Clin. Cancer Res. 2020, 26, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Tennstedt, P.; Wittig, A.; Huber, R.; Straub, O.; Schiess, R.; Steuber, T. A Novel Serum Biomarker Quintet Reveals Added Prognostic Value When Combined with Standard Clinical Parameters in Prostate Cancer Patients by Predicting Biochemical Recurrence and Adverse Pathology. PLoS ONE 2021, 16, e0259093. [Google Scholar] [CrossRef]
- Gabriele, C.; Aracri, F.; Prestagiacomo, L.E.; Rota, M.A.; Alba, S.; Tradigo, G.; Guzzi, P.H.; Cuda, G.; Damiano, R.; Veltri, P.; et al. Development of a Predictive Model to Distinguish Prostate Cancer from Benign Prostatic Hyperplasia by Integrating Serum Glycoproteomics and Clinical Variables. Clin. Proteom. 2023, 20, 52. [Google Scholar] [CrossRef]
- Reyes, N.; Benedetti, I.; Bettin, A.; Rebollo, J.; Geliebter, J. The Small Leucine Rich Proteoglycan Fibromodulin Is Overexpressed in Human Prostate Epithelial Cancer Cell Lines in Culture and Human Prostate Cancer Tissue. Cancer Biomark. 2016, 16, 191–202. [Google Scholar] [CrossRef]
- Bettin, A.; Reyes, I.; Reyes, N. Gene Expression Profiling of Prostate Cancer–Associated Genes Identifies Fibromodulin as Potential Novel Biomarker for Prostate Cancer. Int. J. Biol. Markers 2016, 31, 153–162. [Google Scholar] [CrossRef]
- Silva, T.; Gomes, C.P.; Voigt, D.D.; De Souza, R.B.; Medeiros, K.; Cosentino, N.L.; Fonseca, A.C.P.; Tilli, T.M.; Loayza, E.A.C.; Ramos, V.G.; et al. Fibromodulin Gene Variants (FMOD) as Potential Biomarkers for Prostate Cancer and Benign Prostatic Hyperplasia. Dis. Markers 2022, 2022, 5215247. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lai, C.; Hu, J.; Mulati, Y.; Xu, X.; Luo, J.; Kong, D.; Liu, C.; Xu, K. Integrative Analysis Regarding the Correlation Between Collagen-Related Genes and Prostate Cancer. BMC Cancer 2024, 24, 1038. [Google Scholar] [CrossRef]
- Samaržija, I. The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery. Biomedicines 2024, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Samaržija, I.; Konjevoda, P. Extracellular Matrix- and Integrin Adhesion Complexes-Related Genes in the Prognosis of Prostate Cancer Patients’ Progression-Free Survival. Biomedicines 2023, 11, 2006. [Google Scholar] [CrossRef]
- Grindel, B.J.; Martinez, J.R.; Pennington, C.L.; Muldoon, M.; Stave, J.; Chung, L.W.; Farach-Carson, M.C. Matrilysin/Matrix Metalloproteinase-7(MMP7) Cleavage of Perlecan/HSPG2 Creates a Molecular Switch to Alter Prostate Cancer Cell Behavior. Matrix Biol. 2014, 36, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Grindel, B.; Li, Q.; Arnold, R.; Petros, J.; Zayzafoon, M.; Muldoon, M.; Stave, J.; Chung, L.W.K.; Farach-Carson, M.C. Perlecan/HSPG2 and Matrilysin/MMP-7 as Indices of Tissue Invasion: Tissue Localization and Circulating Perlecan Fragments in a Cohort of 288 Radical Prostatectomy Patients. Oncotarget 2016, 7, 10433–10447. [Google Scholar] [CrossRef]
- Grindel, B.J.; Martinez, J.R.; Tellman, T.V.; Harrington, D.A.; Zafar, H.; Nakhleh, L.; Chung, L.W.; Farach-Carson, M.C. Matrilysin/MMP-7 Cleavage of Perlecan/HSPG2 Complexed with Semaphorin 3A Supports FAK-Mediated Stromal Invasion by Prostate Cancer Cells. Sci. Rep. 2018, 8, 7262. [Google Scholar] [CrossRef]
- Tellman, T.V.; Cruz, L.A.; Grindel, B.J.; Farach-Carson, M.C. Cleavage of the Perlecan-Semaphorin 3a-Plexin A1-Neuropilin-1 (Pspn) Complex by Matrix Metalloproteinase 7/Matrilysin Triggers Prostate Cancer Cell Dyscohesion and Migration. Int. J. Mol. Sci. 2021, 22, 3218. [Google Scholar] [CrossRef]
- Cruz, L.A.; Tellman, T.V.; Farach-Carson, M.C. Flipping the Molecular Switch: Influence of Perlecan and Its Modifiers in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 133–146. [Google Scholar]
- Ojalill, M.; Virtanen, N.; Rappu, P.; Siljamäki, E.; Taimen, P.; Heino, J. Interaction between Prostate Cancer Cells and Prostate Fibroblasts Promotes Accumulation and Proteolytic Processing of Basement Membrane Proteins. Prostate 2020, 80, 715–726. [Google Scholar] [CrossRef]
- Samaržija, I.; Lukiyanchuk, V.; Lončarić, M.; Rac-Justament, A.; Stojanović, N.; Gorodetska, I.; Kahya, U.; Humphries, J.D.; Fatima, M.; Humphries, M.J.; et al. The Extracellular Matrix Component Perlecan/HSPG2 Regulates Radioresistance in Prostate Cancer Cells. Front. Cell Dev. Biol. 2024, 12, 1452463. [Google Scholar] [CrossRef]
- Lima, T.; Barros, A.S.; Trindade, F.; Ferreira, R.; Leite-Moreira, A.; Barros-Silva, D.; Jerónimo, C.; Araújo, L.; Henrique, R.; Vitorino, R.; et al. Application of Proteogenomics to Urine Analysis towards the Identification of Novel Biomarkers of Prostate Cancer: An Exploratory Study. Cancers 2022, 14, 2001. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Song, W.; Xu, H.; Huang, R.; Wang, Y.; Zhao, W.; Xiao, Z.; Yang, X. Oncogenic Properties of NEAT1 in Prostate Cancer Cells Depend on the CDC5L–AGRN Transcriptional Regulation Circuit. Cancer Res. 2018, 78, 4138–4149. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.-L.; Pang, Z.-Q.; Gao, K.; Shen, L.-K.; Xu, W.-H.; Ren, M.-H. Endostatin 33 Peptide Is a Deintegrin α6β1 Agent That Exerts Antitumor Activity by Inhibiting the PI3K-Akt Signaling Pathway in Prostate Cancer. J. Clin. Med. 2023, 12, 1861. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, M.; Wang, H.; Naik, G.; Mobley, J.A.; Sonpavde, G.; Garvey, W.T.; Darley-Usmar, V.M.; Ponnazhagan, S. Endostatin Inhibits Androgen-Independent Prostate Cancer Growth by Suppressing Nuclear Receptor-Mediated Oxidative Stress. FASEB J. 2017, 31, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Isayeva, T.; Larson, M.R.; Sawant, A.; Cha, H.-R.; Chanda, D.; Chesnokov, I.N.; Ponnazhagan, S. Endostatin: A Novel Inhibitor of Androgen Receptor Function in Prostate Cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Luo, R.; Wu, J.; Xie, F.; Li, H.; Xiao, X.; Fu, L.; Zhu, X.; Liu, R.; Zhu, Y.; et al. E10A, an Adenovirus Carrying Human Endostatin Gene, in Combination with Docetaxel Treatment Inhibits Prostate Cancer Growth and Metastases. J. Cell. Mol. Med. 2010, 14, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Isayeva, T.; Moore, L.D.; Chanda, D.; Chen, D.; Ponnazhagan, S. Tumoristatic Effects of Endostatin in Prostate Cancer is Dependent on Androgen Receptor Status. Prostate 2009, 69, 1055–1066. [Google Scholar] [CrossRef]
- Ojalill, M.; Rappu, P.; Siljamäki, E.; Taimen, P.; Boström, P.; Heino, J. The Composition of Prostate Core Matrisome In Vivo and In Vitro Unveiled by Mass Spectrometric Analysis. Prostate 2018, 78, 583–594. [Google Scholar] [CrossRef]
- Shimada, K.; Anai, S.; Fujii, T.; Tanaka, N.; Fujimoto, K.; Konishi, N. Syndecan-1 (CD138) Contributes to Prostate Cancer Progression by Stabilizing Tumour-Initiating Cells. J. Pathol. 2013, 231, 495–504. [Google Scholar] [CrossRef]
- Fujii, T.; Shimada, K.; Tatsumi, Y.; Tanaka, N.; Fujimoto, K.; Konishi, N. Syndecan-1 up-Regulates microRNA-331-3p and Mediates Epithelial-to-Mesenchymal Transition in Prostate Cancer. Mol. Carcinog. 2016, 55, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, B.; Alghezi, D.A.; Cattermole, C.; Beresford, M.; Bowen, R.; Mitchard, J.; Chalmers, A.D. A Subset of High Gleason Grade Prostate Carcinomas Contain a Large Burden of Prostate Cancer Syndecan-1 Positive Stromal Cells. Prostate 2017, 77, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Reis, H.; vom Dorp, F.; Tschirdewahn, S.; Niedworok, C.; Nyirady, P.; Schmid, K.W.; Rübben, H.; Kovalszky, I. Soluble Syndecan-1 (SDC1) Serum Level as an Independent Pre-Operative Predictor of Cancer-Specific Survival in Prostate Cancer. Prostate 2016, 76, 977–985. [Google Scholar] [CrossRef]
- Logan, J.M.; Martini, C.; Sorvina, A.; Johnson, I.R.D.; Brooks, R.D.; Caruso, M.C.; Huzzell, C.; Moore, C.R.; Karageorgos, L.; Butler, L.M.; et al. Reinterpretation of Prostate Cancer Pathology by Appl1, Sortilin and Syndecan-1 Biomarkers. Sci. Data 2024, 11, 852. [Google Scholar] [CrossRef]
- Martini, C.; Logan, J.M.; Sorvina, A.; Prabhakaran, S.; Ung, B.S.Y.; Johnson, I.R.D.; Hickey, S.M.; Brooks, R.D.; Caruso, M.C.; Klebe, S.; et al. Distinct Patterns of Biomarker Expression for Atypical Intraductal Proliferations in Prostate Cancer. Virchows Arch. 2024, 485, 723–728. [Google Scholar] [CrossRef]
- Lazniewska, J.; Li, K.L.; Johnson, I.R.D.; Sorvina, A.; Logan, J.M.; Martini, C.; Moore, C.; Ung, B.S.Y.; Karageorgos, L.; Hickey, S.M.; et al. Dynamic Interplay between Sortilin and Syndecan-1 Contributes to Prostate Cancer Progression. Sci. Rep. 2023, 13, 13489. [Google Scholar] [CrossRef]
- Sorvina, A.; Martini, C.; Prabhakaran, S.; Logan, J.M.; Ung, B.S.-Y.; Moore, C.; Johnson, I.R.D.; Lazniewska, J.; Tewari, P.; Malone, V.; et al. Appl1, Sortilin and Syndecan-1 Immunohistochemistry on Intraductal Carcinoma of the Prostate Provides Evidence of Retrograde Spread. Pathology 2023, 55, 792–799. [Google Scholar] [CrossRef]
- Logan, J.M.; Hopkins, A.M.; Martini, C.; Sorvina, A.; Tewari, P.; Prabhakaran, S.; Huzzell, C.; Johnson, I.R.D.; Hickey, S.M.; Ung, B.S.-Y.; et al. Prediction of Prostate Cancer Biochemical and Clinical Recurrence Is Improved by IHC-Assisted Grading Using Appl1, Sortilin and Syndecan-1. Cancers 2023, 15, 3215. [Google Scholar] [CrossRef]
- Martini, C.; Logan, J.M.; Sorvina, A.; Gordon, C.; Beck, A.R.; Ung, B.S.-Y.; Caruso, M.C.; Moore, C.; Hocking, A.; Johnson, I.R.D.; et al. Aberrant Protein Expression of Appl1, Sortilin and Syndecan-1 during the Biological Progression of Prostate Cancer. Pathology 2023, 55, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Heatlie, J.K.; Lazniewska, J.; Moore, C.R.; Johnson, I.R.D.; Nturubika, B.D.; Williams, R.; Ward, M.P.; O’Leary, J.J.; Butler, L.M.; Brooks, D.A. Extracellular Vesicles and Tunnelling Nanotubes as Mediators of Prostate Cancer Intercellular Communication. Biomolecules 2025, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Sulsenti, R.; Scialpi, G.B.; Frossi, B.; Botti, L.; Ferri, R.; Tripodi, I.; Piva, A.; Sangaletti, S.; Pernici, D.; Cancila, V.; et al. Intracellular Osteopontin Promotes the Release of TNFα by Mast Cells to Restrain Neuroendocrine Prostate Cancer. Cancer Immunol. Res. 2024, 12, 1147–1169. [Google Scholar] [CrossRef]
- Contreras, H.R.; Ledezma, R.A.; Vergara, J.; Cifuentes, F.; Barra, C.; Cabello, P.; Gallegos, I.; Morales, B.; Huidobro, C.; Castellón, E.A. The Expression of Syndecan-1 and -2 Is Associated with Gleason Score and Epithelial-Mesenchymal Transition Markers, E-Cadherin and β-Catenin, in Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Ledezma, R.; Cifuentes, F.; Gallegos, I.; Fullá, J.; Ossandon, E.; Castellon, E.A.; Contreras, H.R. Altered Expression Patterns of Syndecan-1 and -2 Predict Biochemical Recurrence in Prostate Cancer. Asian J. Androl. 2011, 13, 476–480. [Google Scholar] [CrossRef]
- Santos, N.J.; Barquilha, C.N.; Barbosa, I.C.; Macedo, R.T.; Lima, F.O.; Justulin, L.A.; Barbosa, G.O.; Carvalho, H.F.; Felisbino, S.L. Syndecan Family Gene and Protein Expression and Their Prognostic Values for Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 8669. [Google Scholar] [CrossRef]
- Levin, R.A.; Lund, M.E.; Truong, Q.; Wu, A.; Shore, N.D.; Saltzstein, D.R.; Concepcion, R.S.; Paivanas, T.A.; van Breda, A.; Beebe-Dimmer, J.; et al. Development of a Reliable Assay to Measure Glypican-1 in Plasma and Serum Reveals Circulating Glypican-1 as a Novel Prostate Cancer Biomarker. Oncotarget 2018, 9, 22359–22367. [Google Scholar] [CrossRef]
- Campbell, D.H.; Lund, M.E.; Nocon, A.L.; Cozzi, P.J.; Frydenberg, M.; De Souza, P.; Schiller, B.; Beebe-Dimmer, J.L.; Ruterbusch, J.J.; Walsh, B.J. Detection of Glypican-1 (GPC-1) Expression in Urine Cell Sediments in Prostate Cancer. PLoS ONE 2018, 13, e0196017. [Google Scholar] [CrossRef]
- Shore, N.D.; Pieczonka, C.M.; Henderson, R.J.; Bailen, J.L.; Saltzstein, D.R.; Concepcion, R.S.; Beebe-Dimmer, J.L.; Ruterbusch, J.J.; Levin, R.A.; Wissmueller, S.; et al. Development and Evaluation of the MiCheck Test for Aggressive Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 683.e11–683.e18. [Google Scholar] [CrossRef]
- Quach, N.D.; Kaur, S.P.; Eggert, M.W.; Ingram, L.; Ghosh, D.; Sheth, S.; Nagy, T.; Dawson, M.R.; Arnold, R.D.; Cummings, B.S. Paradoxical Role of Glypican-1 in Prostate Cancer Cell and Tumor Growth. Sci. Rep. 2019, 9, 11478. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.P.; Verma, A.; Lee, H.K.; Barnett, L.M.; Somanath, P.R.; Cummings, B.S. Inhibition of Glypican-1 Expression Induces an Activated Fibroblast Phenotype in a Human Bone Marrow-Derived Stromal Cell-Line. Sci. Rep. 2021, 11, 9262. [Google Scholar] [CrossRef]
- Sabanathan, D.; Campbell, D.H.; Velonas, V.M.; Wissmueller, S.; Mazure, H.; Trifunovic, M.; Poursoltan, P.; Shon, K.H.; Mackay, T.R.; Lund, M.E.; et al. Safety and Tolerability of Miltuximab®-a First in Human Study in Patients with Advanced Solid Cancers. Asia Ocean. J. Nucl. Med. Biol. 2021, 9, 86–100. [Google Scholar] [CrossRef]
- Lund, M.E.; Howard, C.B.; Thurecht, K.J.; Campbell, D.H.; Mahler, S.M.; Walsh, B.J. A Bispecific T Cell Engager Targeting Glypican-1 Redirects T Cell Cytolytic Activity to Kill Prostate Cancer Cells. BMC Cancer 2020, 20, 1214. [Google Scholar] [CrossRef]
- Yeh, M.-C.; Tse, B.W.C.; Fletcher, N.L.; Houston, Z.H.; Lund, M.; Volpert, M.; Stewart, C.; Sokolowski, K.A.; Jeet, V.; Thurecht, K.J.; et al. Targeted Beta Therapy of Prostate Cancer with 177Lu-Labelled Miltuximab® Antibody Against Glypican-1 (GPC-1). EJNMMI Res. 2020, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Truong, Q.; Justiniano, I.O.; Nocon, A.L.; Soon, J.T.; Wissmueller, S.; Campbell, D.H.; Walsh, B.J. Glypican-1 as a Biomarker for Prostate Cancer: Isolation and Characterization. J. Cancer 2016, 7, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liao, J.; Li, J.; Wang, S. GPC2 Promotes Prostate Cancer Progression via MDK-Mediated Activation of PI3K/AKT Signaling Pathway. Funct. Integr. Genom. 2024, 24, 127. [Google Scholar] [CrossRef]
- Lee, W.; Chung, J.-Y.; Baidoo, K.E.; Nambiar, D.; Basuli, F.; Coleman, I.; Bakht, M.; Li, C.; Shin, J.; Jeong, S.U.; et al. Glypican-3 as a Radiotheranostic Target for Neuroendocrine Prostate Cancer. Eur. Urol. 2025, 88, 301–303. [Google Scholar] [CrossRef]
- Butler, W.; Xu, L.; Zhou, Y.; Cheng, Q.; Hauck, J.S.; He, Y.; Marek, R.; Hartman, Z.; Cheng, L.; Yang, Q.; et al. Oncofetal Protein Glypican-3 Is a Biomarker and Critical Regulator of Function for Neuroendocrine Cells in Prostate Cancer. J. Pathol. 2023, 260, 43–55. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Wang, L.; Qiao, B.; Du, E.; Li, L.; Xu, Y.; Zhang, Z. Prognostic Significance of GPC5 Expression in Patients with Prostate Cancer. Tumor Biol. 2016, 37, 6413–6418. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, K.; He, M.; Fan, G.; Lu, H. Overexpression of Glypican 5 (GPC5) Inhibits Prostate Cancer Cell Proliferation and Invasion via Suppressing Sp1-Mediated EMT and Activation of Wnt/β-Catenin Signaling. Oncol. Res. 2018, 26, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Turley, R.S.; Finger, E.C.; Hempel, N.; How, T.; Fields, T.A.; Blobe, G.C. The Type III Transforming Growth Factor-β Receptor as a Novel Tumor Suppressor Gene in Prostate Cancer. Cancer Res. 2007, 67, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Wang, L.; López-Casillas, F.; Mendoza, V.; Yeh, I.-T.; Sun, L. Systemic Administration of a Soluble Betaglycan Suppresses Tumor Growth, Angiogenesis, and Matrix Metalloproteinase-9 Expression in a Human Xenograft Model of Prostate Cancer. Prostate 2005, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yu-Lee, L.-Y.; Yu, G.; Lee, Y.-C.; Lin, S.-C.; Pan, J.; Pan, T.; Yu, K.-J.; Liu, B.; Creighton, C.J.; Rodriguez-Canales, J.; et al. Osteoblast-Secreted Factors Mediate Dormancy of Metastatic Prostate Cancer in the Bone via Activation of the TGFβRIII–p38MAPK–pS249/T252RB Pathway. Cancer Res. 2018, 78, 2911–2924. [Google Scholar] [CrossRef]
- Cook, L.M.; Frieling, J.S.; Nerlakanti, N.; McGuire, J.J.; Stewart, P.A.; Burger, K.L.; Cleveland, J.L.; Lynch, C.C. Betaglycan Drives the Mesenchymal Stromal Cell Osteogenic Program and Prostate Cancer-Induced Osteogenesis. Oncogene 2019, 38, 6959–6969. [Google Scholar] [CrossRef]
- Korpetinou, A.; Papachristou, D.J.; Lampropoulou, A.; Bouris, P.; Labropoulou, V.T.; Noulas, A.; Karamanos, N.K.; Theocharis, A.D. Increased Expression of Serglycin in Specific Carcinomas and Aggressive Cancer Cell Lines. BioMed Res. Int. 2015, 2015, 690721. [Google Scholar] [CrossRef]
- Ge, S.; Cen, J.; Liu, X.; Hong, Y.; Tang, Y.; Yu, Y.; Li, H.; Xie, T.; Wang, C.; Cai, M.; et al. TGFβ-Activated Asporin Interacts with STMN1 to Promote Prostate Cancer Docetaxel Chemoresistance and Metastasis by Upregulating the Wnt/β-Catenin Signaling Pathway. Drug Resist. Updat. 2025, 81, 101227. [Google Scholar] [CrossRef]
- Hughes, R.M.; Simons, B.W.; Khan, H.; Miller, R.; Kugler, V.; Torquato, S.; Theodros, D.; Haffner, M.C.; Lotan, T.; Huang, J.; et al. Asporin Restricts Mesenchymal Stromal Cell Differentiation, Alters the Tumor Microenvironment, and Drives Metastatic Progression. Cancer Res. 2019, 79, 3636–3650. [Google Scholar] [CrossRef]
- Rochette, A.; Boufaied, N.; Scarlata, E.; Hamel, L.; Brimo, F.; Whitaker, H.C.; Ramos-Montoya, A.; Neal, D.E.; Dragomir, A.; Aprikian, A.; et al. Asporin Is a Stromally Expressed Marker Associated with Prostate Cancer Progression. Br. J. Cancer 2017, 116, 775–784. [Google Scholar] [CrossRef]
- Pan, K.; Lee, W.; Chou, C.; Yang, Y.; Chang, Y.; Chien, M.; Hsiao, M.; Hua, K. Direct Interaction of β-Catenin with Nuclear ESM1 Supports Stemness of Metastatic Prostate Cancer. EMBO J. 2021, 40, e105450. [Google Scholar] [CrossRef] [PubMed]
- Dadali, M.; Tad, M.; Bagbanci, M.S. Expression of Endocan in Tissue Samples from Prostate Adenocarcinoma and Prostate Hyperplasia: A Comparative Retrospective Study. Urol. J. 2021, 18, 530–536. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Chen, C.-M.; Hsu, W.-H.; Hsieh, Y.-H.; Liu, C.-J. Overexpression of Endothelial Cell-Specific Molecule 1 Correlates with Gleason Score and Expression of Androgen Receptor in Prostate Carcinoma. Int. J. Med Sci. 2017, 14, 1263–1267. [Google Scholar] [CrossRef]
- Rebollo, J.; Geliebter, J.; Reyes, N. ESM-1 SiRNA Knockdown Decreased Migration and Expression of CXCL3 in Prostate Cancer Cells. Int. J. Biomed. Sci. 2017, 13, 35–42. [Google Scholar] [CrossRef]
- Chen, C.M.; Lin, C.L.; Chiou, H.L.; Hsieh, S.C.; Lin, C.L.; Cheng, C.W.; Hung, C.H.; Tsai, J.P.; Hsieh, Y.H. Loss of Endothelial Cell-Specific Molecule 1 Promotes the Tumorigenicity and Metastasis of Prostate Cancer Cells Through Regulation of the TIMP-1/MMP-9 Expression. Oncotarget 2017, 8, 13886–13897. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Onuk, Ö.; Hazar, I.; Aydin, M.; Çilesiz, N.C.; Eroglu, A.; Nuhoglu, B. Prognostic Value of Endocan in Prostate Cancer: Clinicopathologic Association between Serum Endocan Levels and Biochemical Recurrence after Radical Prostatectomy. Tumori J. 2017, 103, 204–208. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, G.F.A.; Listik, E.; Justo, G.Z.; Vicente, C.M.; Toma, L. The Glypican Proteoglycans Show Intrinsic Interactions with Wnt-3a in Human Prostate Cancer Cells That Are Not Always Associated with Cascade Activation. BMC Mol. Cell Biol. 2021, 22, 26. [Google Scholar] [CrossRef]
- Kameda, T.; Sugihara, T.; Obinata, D.; Oshima, M.; Yamada, Y.; Kimura, N.; Takayama, K.; Inoue, S.; Takahashi, S.; Fujimura, T. Androgen Receptor and Osteoglycin Gene Expression Predicting Prognosis of Metastatic Prostate Cancer. Sci. Rep. 2024, 14, 30654. [Google Scholar] [CrossRef]
- Datta, M.W.; Hernandez, A.M.; Schlicht, M.J.; Kahler, A.J.; DeGueme, A.M.; Dhir, R.; Shah, R.B.; Farach-Carson, C.; Barrett, A.; Datta, S. Perlecan, a Candidate Gene for the CAPB Locus, Regulates Prostate Cancer Cell Growth via the Sonic Hedgehog Pathway. Mol. Cancer 2006, 5, 9. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Q.; Jiang, W.; Li, H.; Li, X.; Zi, T.; Qin, X.; Zhao, Y.; Wu, D.; Wu, G. Extrachromosomal Circular DNA-Related SPOCK1 Contributes to Development and Enzalutamide Resistance of Prostate Cancer by Regulating Epithelial Mesenchymal Transition. Heliyon 2024, 10, e37075. [Google Scholar] [CrossRef]
- Chen, M.-L.; Ho, C.-J.; Yeh, C.-M.; Chen, S.-L.; Sung, W.-W.; Wang, S.-C.; Chen, C.-J. High SPOCK1 Expression Is Associated with Advanced Stage, T Value, and Gleason Grade in Prostate Cancer. Medicina 2019, 55, 343. [Google Scholar] [CrossRef]
- Chien, M.-H.; Lin, Y.-W.; Wen, Y.-C.; Yang, Y.-C.; Hsiao, M.; Chang, J.-L.; Huang, H.-C.; Lee, W.-J. Targeting the SPOCK1-Snail/Slug Axis-Mediated Epithelial-to-Mesenchymal Transition by Apigenin Contributes to Repression of Prostate Cancer Metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 246. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.T.; Xu, H.; Chen, Y.B.; Gu, M.; Cai, Z.K.; Wang, Z. SPOCK1 Promotes Tumor Growth and Metastasis in Human Prostate Cancer. Drug Des. Dev. Ther. 2016, 10, 2311–2321. [Google Scholar] [CrossRef]
- Yang, C.; Fischer-Kešo, R.; Schlechter, T.; Ströbel, P.; Marx, A.; Hofmann, I. Plakophilin 1-Deficient Cells Upregulate SPOCK1: Implications for Prostate Cancer Progression. Tumor Biol. 2015, 36, 9567–9577. [Google Scholar] [CrossRef]
- Liu, G.; Ren, F.; Song, Y. Upregulation of SPOCK2 Inhibits the Invasion and Migration of Prostate Cancer Cells by Regulating the MT1-MMP/MMP2 Pathway. PeerJ 2019, 7, e7163. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lai, C.; Xu, X.; Shi, J.; Hu, J.; Guo, K.; Mulati, Y.; Xiao, Y.; Kong, D.; Liu, C.; et al. Mechanism of Prognostic Marker SPOCK3 Affecting Malignant Progression of Prostate Cancer and Construction of Prognostic Model. BMC Cancer 2023, 23, 741. [Google Scholar] [CrossRef] [PubMed]
- Saraji, A.; Duan, K.; Watermann, C.; Hempel, K.; Roesch, M.C.; Krupar, R.; Stegmann-Frehse, J.; Jonigk, D.; Kuehnel, M.P.; Klapper, W.; et al. The Gene Expression Landscape of Prostate Cancer BM Reveals Close Interaction with the Bone Microenvironment. Int. J. Mol. Sci. 2022, 23, 13029. [Google Scholar] [CrossRef]
- Samaržija, I. Site-Specific and Common Prostate Cancer Metastasis Genes as Suggested by Meta-Analysis of Gene Expression Data. Life 2021, 11, 636. [Google Scholar] [CrossRef]
- Raychaudhuri, R.; Lin, D.W.; Montgomery, R.B. Prostate Cancer: A Review. JAMA 2025, 333, 1433–1446. [Google Scholar] [CrossRef]
- Puente, J.; Grande, E.; Medina, A.; Maroto, P.; Lainez, N.; Arranz, J.A. Docetaxel in Prostate Cancer: A Familiar Face as the New Standard in a Hormone-Sensitive Setting. Ther. Adv. Med Oncol. 2017, 9, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Sevcenco, S.; Módos, O.; Keresztes, D.; Nyirády, P.; Kubik, A.; Romics, M.; Kovalszky, I.; Reis, H.; Hadaschik, B.; et al. Circulating Syndecan-1 Is Associated with Chemotherapy-Resistance in Castration-Resistant Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 312.e9–312.e15. [Google Scholar] [CrossRef]
- Arichi, N.; Mitsui, Y.; Hiraki, M.; Nakamura, S.; Hiraoka, T.; Sumura, M.; Hirata, H.; Tanaka, Y.; Dahiya, R.; Yasumoto, H.; et al. Versican Is a Potential Therapeutic Target in Docetaxel-Resistant Prostate Cancer. Oncoscience 2015, 2, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Sicotte, H.; Kalari, K.R.; Qin, S.; Dehm, S.M.; Bhargava, V.; Gormley, M.; Tan, W.; Sinnwell, J.P.; Hillman, D.W.; Li, Y.; et al. Molecular Profile Changes in Patients with Castrate-Resistant Prostate Cancer Pre- and Post-Abiraterone/Prednisone Treatment. Mol. Cancer Res. 2022, 20, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Kyprianou, N. Mechanisms Navigating the TGF-β Pathway in Prostate Cancer. Asian J. Urol. 2015, 2, 11–18. [Google Scholar] [CrossRef]
- Pan, C.; He, Y.; Wang, H.; Yu, Y.; Li, L.; Huang, L.; Lyu, M.; Ge, W.; Yang, B.; Sun, Y.; et al. Identifying Patients with Rapid Progression from Hormone-Sensitive to Castration-Resistant Prostate Cancer: A Retrospective Study. Mol. Cell. Proteom. 2023, 22, 100613. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A. Proteoglycans in Biomedicine: Resurgence of an Underexploited Class of ECM Molecules. Front. Pharmacol. 2020, 10, 1661. [Google Scholar] [CrossRef] [PubMed]


| Proteoglycan | Reported Findings | Year | Reference |
|---|---|---|---|
| Aggrecan (ACAN) | Stimulates motility-related processes in an experiment of microrheology on cultured PC3 cells. | 2012 | [50] |
| Agrin (AGRN) | CDC5L-AGRN signaling mediates the PC-promoting function of the long noncoding RNA NEAT1. | 2018 | [87] |
| Asporin (ASPN) | Promotes PC metastasis through the Wnt/β-catenin signaling pathway; enhanced stemness and epithelial–mesenchymal transition (EMT) are involved. | 2025 | [128] |
| Promotes PC metastatic progression; restriction of mesenchymal stromal cell differentiation and alteration of the tumor microenvironment are involved. | 2019 | [129] | |
| In a cohort of 326 PC patients, increased expression of ASPN was correlated with decreased time to biochemical recurrence. | 2017 | [130] | |
| Betaglycan (BGCAN) | Drives PC-induced osteogenesis. | 2019 | [126] |
| Part of the signaling axis that mediates dormancy of metastatic PC in the bone. | 2018 | [125] | |
| Inhibits PC growth and angiogenesis. | 2012, 2007, 2005 | [13,123,124] | |
| Biglycan (BGN) | Downregulation of BGN in cancer-associated fibroblasts suppresses cell proliferation, migration, and invasion in vitro, and in vivo xenograft assays. | 2025 | [62] |
| Among the key secretome factors that regulate recruitment of myeloid-derived suppressor cells in PC. | 2023 | [61] | |
| Upregulation associated with poor prognosis and PTEN deletion in PC patients. | 2023, 2017 | [59,60] | |
| Potentially promotes PC bone metastasis. | 2006 | [51] | |
| Decorin (DCN) | High DCN expression in the PC bone microenvironment indicates better prognosis after androgen deprivation therapy. | 2025 | [67] |
| Lower expression in PC than benign prostatic hyperplasia tissue has a potential prognostic value. | 2020 | [64] | |
| Inhibits bone metastasis. | 2015 | [66] | |
| Reduced expression in PC stroma compared to non-malignant prostate stroma. | 2012 | [65] | |
| Suppresses PC growth. | 2009 | [63] | |
| Endocan (ESM1) | Interaction of β-catenin with nuclear ESM1 promotes stemness of metastatic PC. | 2021 | [131] |
| Tissue endocan expression level is higher in PC patients compared to those with benign prostate hyperplasia. | 2021 | [132] | |
| Overexpression of ESM1 in PC correlates with Gleason score and androgen receptor expression. | 2017 | [133] | |
| ESM1 downregulation decreases migration in PC cells. | 2017 | [134] | |
| Loss of ESM1 expression promotes PC tumorigenicity and metastasis. | 2017 | [135] | |
| Associated with tumor recurrence in PC. | 2017 | [136] | |
| Fibromodulin (FMOD) | Potential biomarker in PC. | 2024, 2023 | [76,77,78] |
| Glypican 1 (GPC1) | PC target. | 2021, 2020, 2016 | [114,115,116,117] |
| Expressed in PC cell lines; interacts with WNT3A. | 2021 | [137] | |
| Influences the biology of human bone marrow-derived stromal cells and PC cell aggressiveness. | 2021 | [113] | |
| Part of a MiCheck test for aggressive PC. | 2020 | [111] | |
| The role of GPC-1 in PC is cell type-specific; discrepancy between the in vitro and in vivo data possibly mediated by stromal cells in the tumor microenvironment. | 2019 | [112] | |
| PC biomarker. | 2018, 2018 | [109,110] | |
| Glypican 2 (GPC2) | Promotes PC proliferation, migration, and invasion. | 2024 | [118] |
| Glypican 3 (GPC3) | Potential target for neuroendocrine PC. | 2025, 2023 | [119,120] |
| Glypican 5 (GPC5) | Inhibits PC cell proliferation and invasion; suppression of EMT and WNT/β-catenin signaling are involved. | 2018 | [122] |
| Potential diagnostic and prognostic PC biomarker (lower expression in PC tissue, especially in high-risk PC). | 2016 | [121] | |
| Lumican (LUM) | Part of a serum biomarker signature to (a) distinguish PC from benign prostatic hyperplasia and (b) predict biochemical recurrence and adverse pathology. | 2023, 2021, 2020 | [70,71,72] |
| LUM in the reactive stroma has a suppressive role on the PC progression. | 2013 | [53,69] | |
| Osteoglycin (OGN) | PC patients with high OGN expression show better survival. | 2024 | [138] |
| Perlecan (HSPG2) | High expression correlates with worse survival of The Cancer Genome Atlas prostate adenocarcinoma patients. | 2024 | [85] |
| HSPG2 cleavage triggers PC cell dyscohesion, migration, and tissue invasion. | 2021, 2018, 2016, 2014 | [79,80,81,82] | |
| HSPG2 expression in PC tissues correlates with a high Gleason score and rapid cell proliferation; inhibition of HSPG2 expression in PC cell lines decreases cell growth and Sonic Hedgehog signaling. | 2006 | [139] | |
| Serglycin (SRGN) | Detected in both the neoplastic and the normal prostatic epithelia. | 2015 | [127] |
| Syndecan 1 (SDC1) | Found in PC extracellular vesicles. | 2025 | [104] |
| Potential PC biomarker (part of the Appl1, Sortilin, and SDC1 biomarker panel). | 2024, 2023 | [98,99,100,101,102,103] | |
| Part of the signaling axis that promotes the release of TNFα by mast cells to suppress neuroendocrine PC. | 2024 | [105] | |
| SDC1 expression identifies a previously unreported cell type that is frequent in a subset of poor prognosis high Gleason grade tumors. | 2017 | [96] | |
| Soluble SDC1 serum level is an independent pre-operative predictor of cancer-specific survival in PC. | 2016 | [97] | |
| Mediates EMT in PC. | 2016 | [95] | |
| Contributes to PC progression by stabilizing tumor-initiating cells. | 2013 | [94] | |
| Syndecan 2 (SDC2) | SDC2 is expressed preferentially in basal cells in non-affected prostate; in PC the expression pattern shifts to granular-cytoplasmic localization, and PC patients with altered expression have worse PSA recurrence-free survival. | 2011 | [107] |
| The expression of SDC2 is associated with Gleason score and EMT markers in PC. | 2010 | [106] | |
| Syndecan 3 (SDC3) | SDC3 expression is associated with more aggressive PC tumors and a worse prognosis. | 2021 | [108] |
| Syndecan 4 (SDC4) | SDC4 expression is associated with a better prognosis in PC patients. | 2021 | [108] |
| Testican 1 (SPOCK1) | Extrachromosomal circular DNA-related SPOCK1 contributes to the development of PC; regulation of epithelial–mesenchymal transition (EMT) is involved. | 2024 | [140] |
| High SPOCK1 expression is associated with advanced PC. | 2019 | [141] | |
| SPOCK1-snail/slug axis is involved in EMT; its targeting contributes to inhibition of PC metastasis. | 2019 | [142] | |
| Promotes tumor growth and metastasis in human PC. | 2016 | [143] | |
| Upregulation of SPOCK1 mRNA and protein in PC samples. | 2015 | [144] | |
| Testican 2 (SPOCK2) | Upregulation of SPOCK2 inhibits the invasion and migration of PC cells; MT1-MMP/MMP2 pathway is involved. | 2019 | [145] |
| Testican 3 (SPOCK3) | SPOCK3 expression is associated with immune cell infiltration; PC patients with higher SPOCK3 expression show better disease-free survival. | 2023 | [146] |
| Lower expression in bone metastasis than in primary PC. | 2022 | [147] | |
| Among the genes with the most downregulated expression in PC lymph node and liver metastases compared to primary tumors. | 2021 | [148] | |
| Versican (VCAN | Promotes PC cell motility and invasion. | 2012, 2007 | [13,47] |
| Proteoglycan | Reported Findings | Year | Ref. |
|---|---|---|---|
| Asporin (ASPN) | Promotes PC docetaxel chemoresistance through the Wnt/β-catenin signaling pathway. | 2025 | [128] |
| Betaglycan (BGCAN) | Among other characteristics, patients with castration-resistant PC non-responding to abiraterone/prednisone treatment had low expression of BGCAN. | 2022 | [153] |
| Biglycan (BGN) | One of the two proteins associated with a fast progression from hormone-sensitive to castration-resistant PC. | 2023 | [155] |
| Perlecan (HSPG2) | Regulates radioresistance in PC DU145 cells. | 2024 | [85] |
| Syndecan 1 (SDC1) | Circulating SDC1 is associated with chemotherapy-resistance in castration-resistant PC. | 2018 | [151] |
| Testican 1 (SPOCK1) | Extrachromosomal circular DNA-related gene SPOCK1 contributes to PC enzalutamide resistance; regulation of EMT is involved. | 2024 | [140] |
| Versican (VCAN) | Potential therapeutic target in docetaxel-resistant PC. | 2015 | [152] |
| Strategy | Example | Comment |
|---|---|---|
| Antibodies against PGs | Antibodies against different glypicans | Targeting cell surface PGs. |
| Interference with enzymes that process PGs | Heparanase inhibitors | Block the activity of enzymes that modify, e.g., heparan sulfate chains. |
| Modification of PGs | Enzymatic glycosaminoglycan (GAG) editing | Methods include synthesis of new GAG chains, degradation of existing GAGs or production of GAGs with specific characteristics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaržija, I. Proteoglycans in Prostate Cancer Progression and Therapy Resistance. Medicina 2025, 61, 2112. https://doi.org/10.3390/medicina61122112
Samaržija I. Proteoglycans in Prostate Cancer Progression and Therapy Resistance. Medicina. 2025; 61(12):2112. https://doi.org/10.3390/medicina61122112
Chicago/Turabian StyleSamaržija, Ivana. 2025. "Proteoglycans in Prostate Cancer Progression and Therapy Resistance" Medicina 61, no. 12: 2112. https://doi.org/10.3390/medicina61122112
APA StyleSamaržija, I. (2025). Proteoglycans in Prostate Cancer Progression and Therapy Resistance. Medicina, 61(12), 2112. https://doi.org/10.3390/medicina61122112






