Topical Spermidine Hyaluronate (Spd-HA) in Vulvovaginal Atrophy: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigational Device
2.2. Study Design and Participants
2.3. Clinical Endpoints
- -
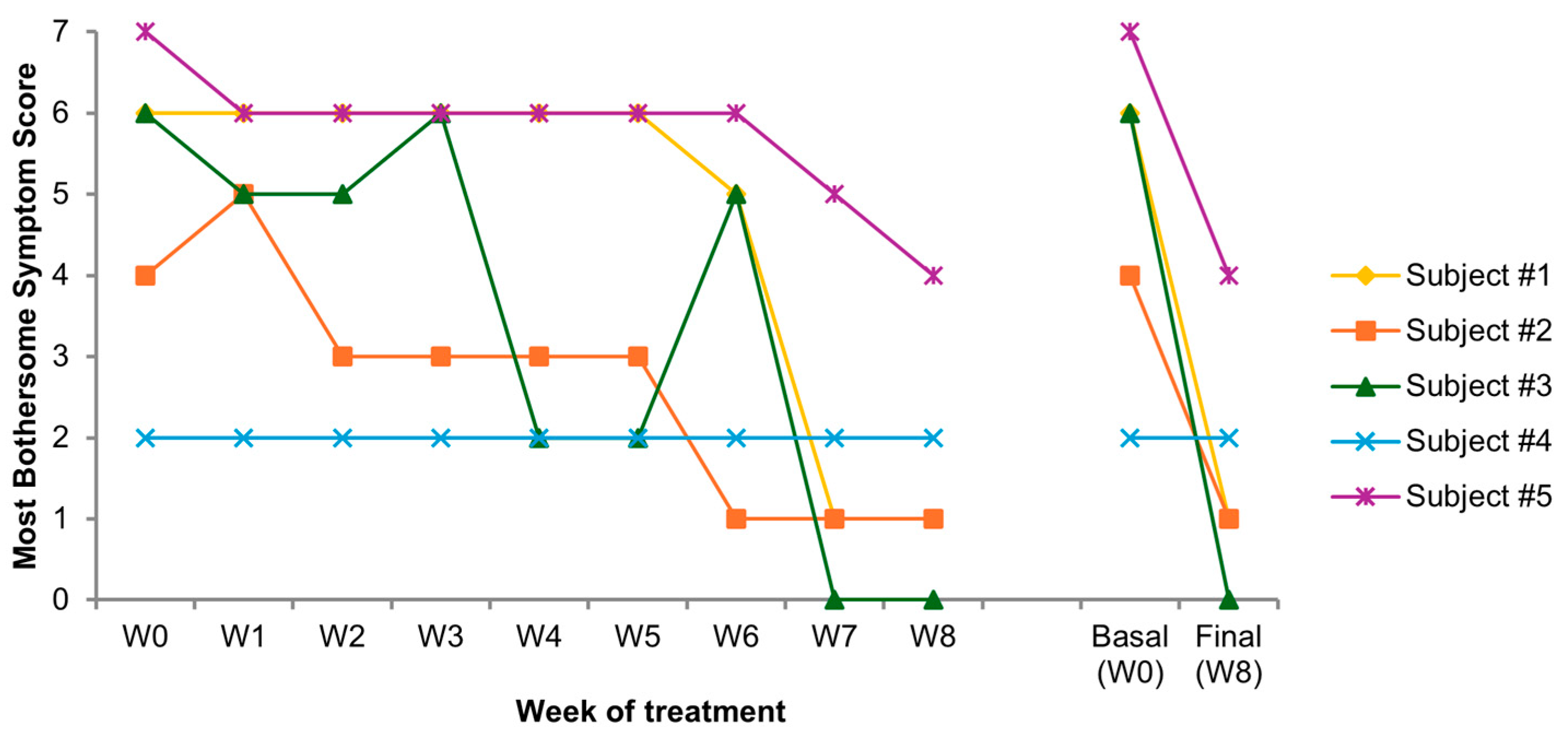
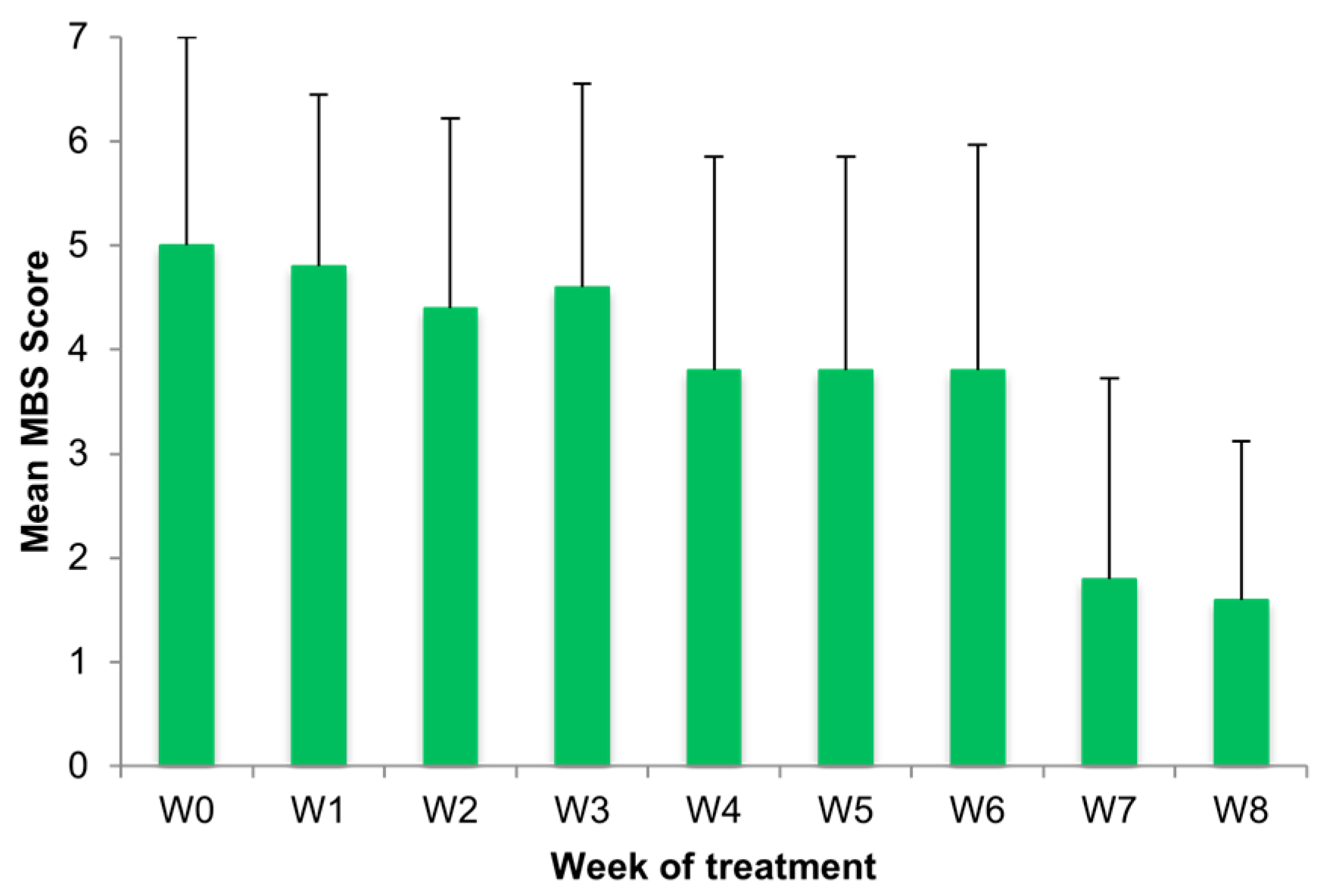
- Most bothersome symptom (MBS) score, a self-reported questionnaire, was used to measure VVA symptom severity by assessing four key symptoms: dryness, itching, burning, and dyspareunia. The intensity of each of the four symptoms was rated on a 4-point ordinal scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe). Dyspareunia was scored “severe” if sexual activity was not possible because of symptoms [36]. To assess the treatment’s overall subjective efficacy, a composite symptom severity score was calculated weekly, as the sum of the individual severity scores for the four symptoms (range 0–12).
- -
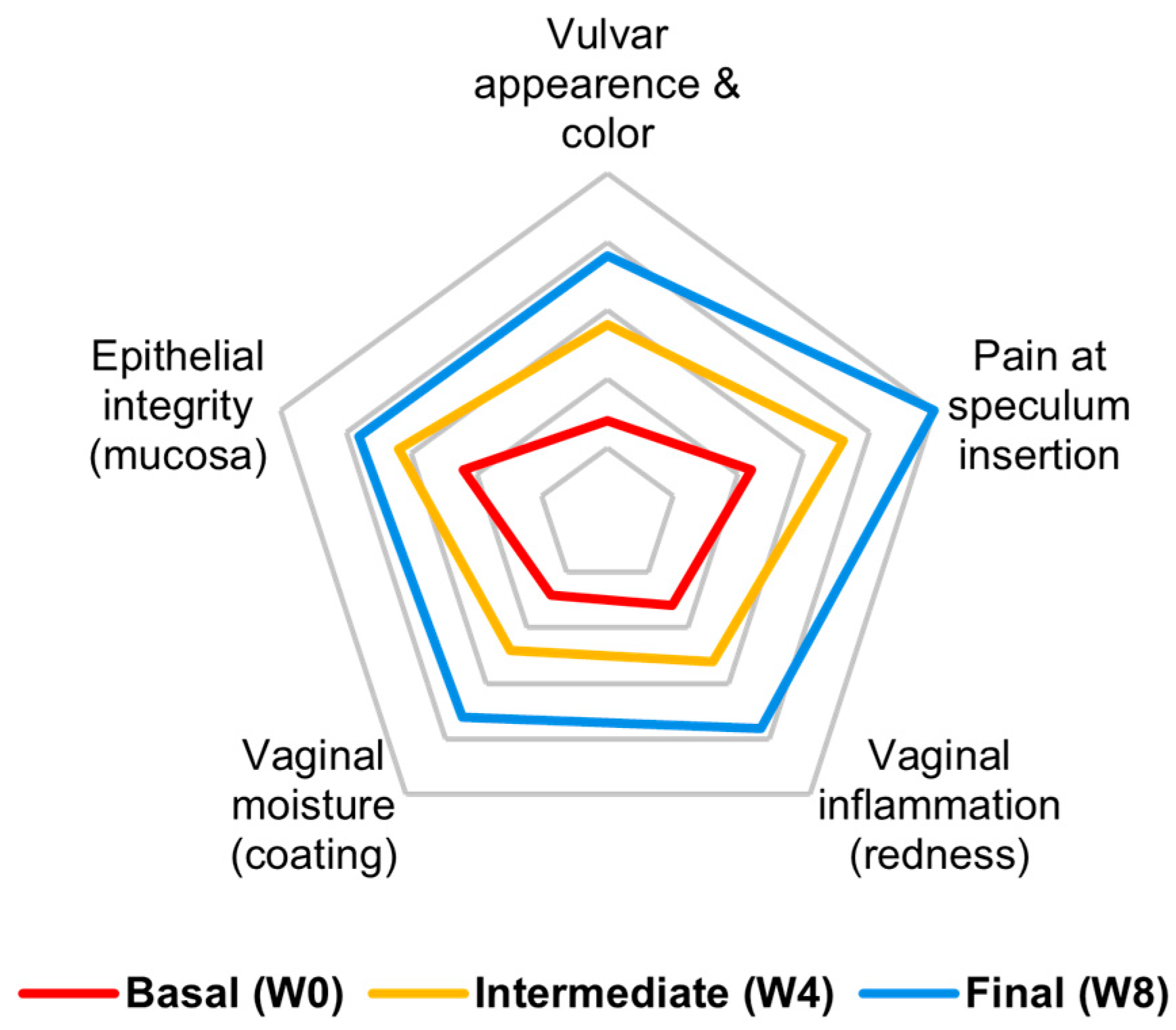
- Modified vulvovaginal health index (VHI) is a merged version of the vaginal health index [37] and vulvar health index [38], which evaluate five components: vulvar appearance/color, vaginal inflammation, pain at speculum insertion, vaginal moisture, and epithelial integrity (Appendix A). A score from 1 to 5 is given to each component (score 1 is the lowest health status). The sum of the five components represents the total VHI score. A score ≤ 15 defines the presence of VVA. VHI was assessed at each visit (V1, V2 and V3).
- -
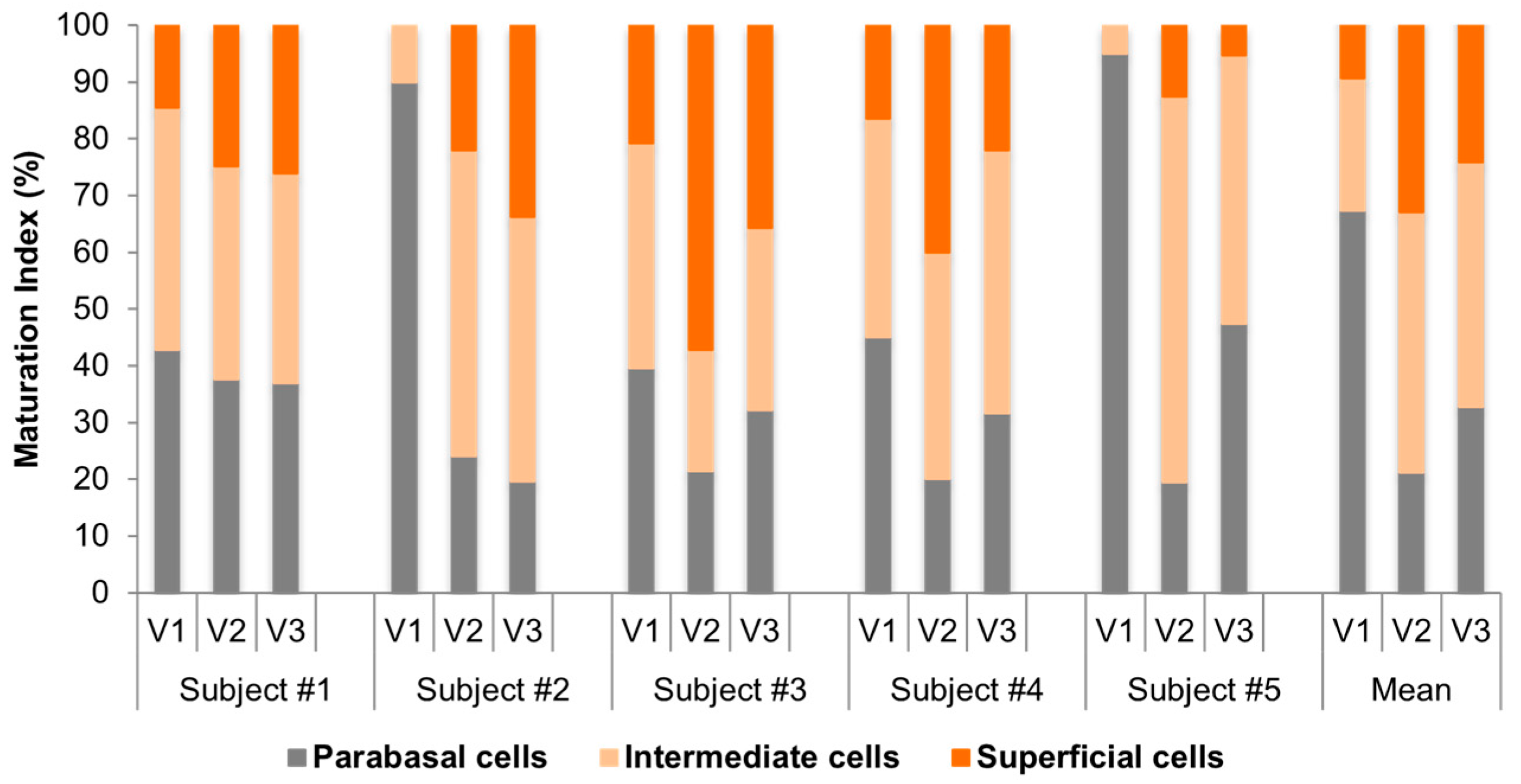
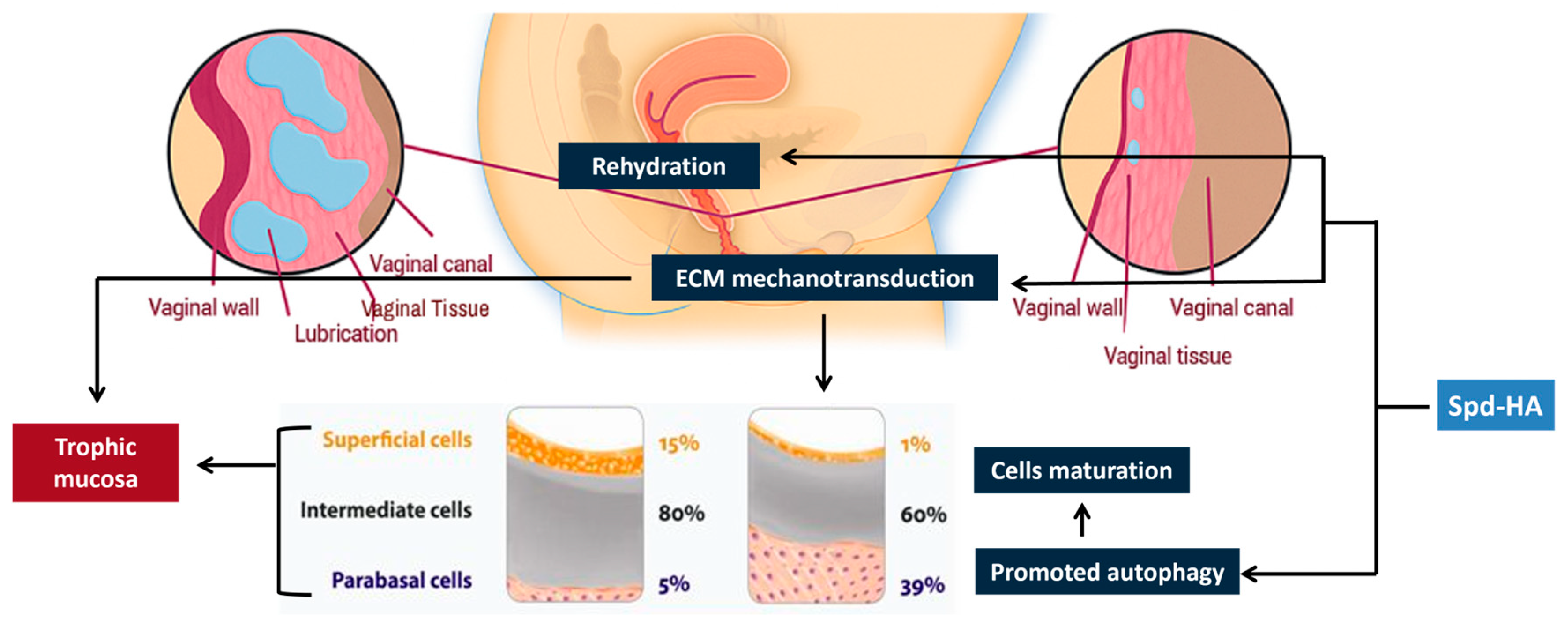
- Maturation index (MI) is obtained by cytological examination and is the percentage of parabasal, intermediate, and superficial squamous cells/100 cells. Predominance of superficial cells indicates a normal trophic condition, whilst, vice versa, predominance of parabasal cells indicates a poor trophism, often associated with hypoestrogenism [39].
- -
- Maturation value (MV) is calculated with the following formula: MV = % surface cells + (0.5 × % intermediate cells). A threshold of 40 defines vaginal atrophy [40].
- -
- Potential adverse effects (PAEs) were recorded weekly.
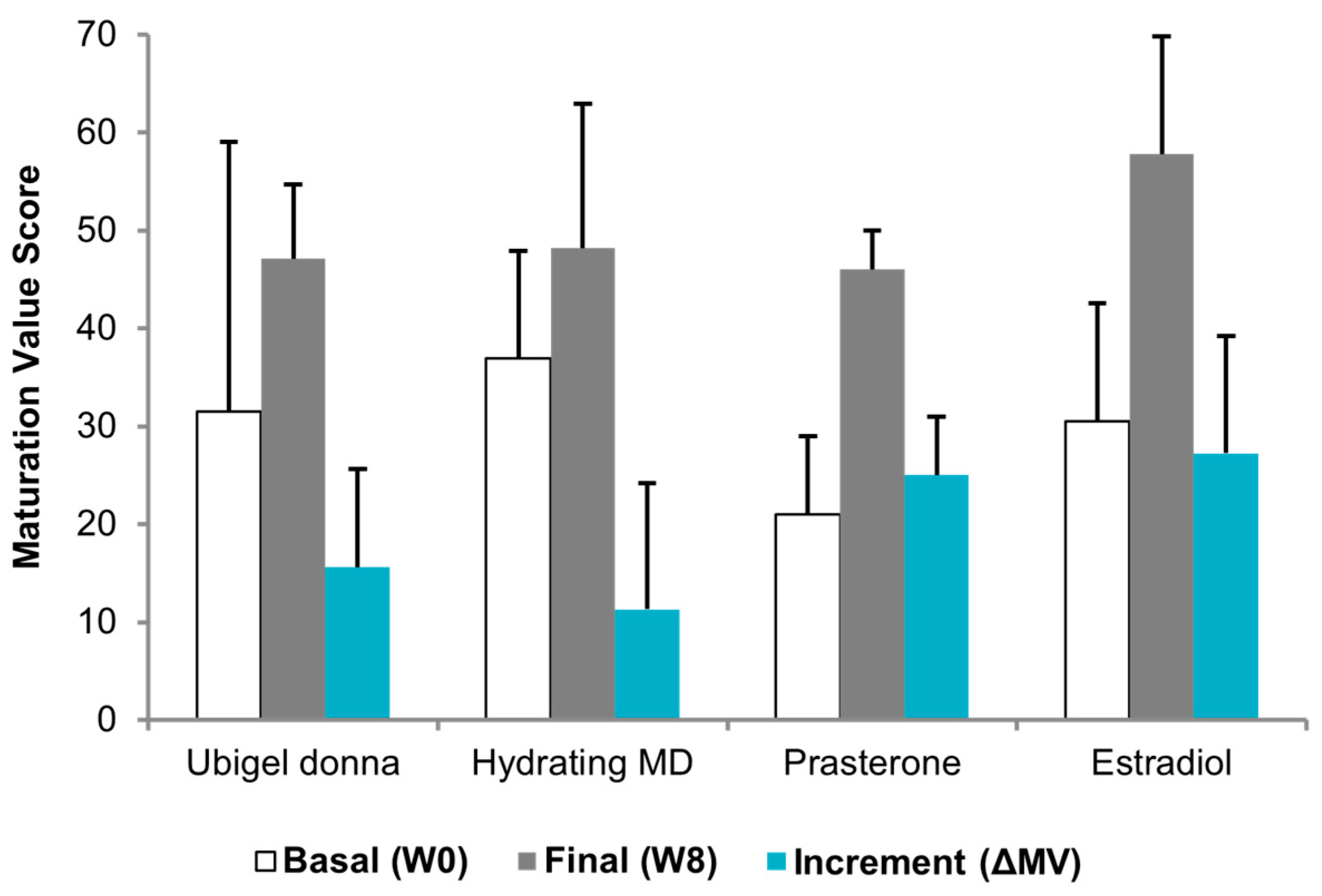
2.4. Comparison with Other Treatments
2.5. Ethical Standard
3. Results
3.1. Individual Case Reports
3.2. Tolerability and Remarks
3.3. Individual Subjective Patterns
3.4. Cumulative Subjective Outcome
3.5. Cumulative Objective Outcome
3.6. Individual Cytologic Data
3.7. Comparative Cytologic Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| DHEA | Dehydroepiandrosterone |
| ECM | Extracellular Matrix |
| E2 | Estradiol |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GSM | Genitourinary Syndrome of Menopause |
| HMW-HA | High Molecular Weight Hyaluronic Acid |
| HRT | Hormone Replacement Therapy |
| IRB | Institutional Review Board |
| ISO | International Organization for Standardization |
| MBS | Most Bothersome Symptom |
| MI | Maturation Index |
| MV | Maturation Value |
| SERM | Selective Estrogen Receptor Modulator |
| SMC | Supramolecular Complexes |
| SUI | Stress Urinary Incontinence |
| V1, V2, V3 | Visit 1, Visit 2, Visit 3 |
| VHI | Vaginal Health Index |
| VVA | Vulvovaginal Atrophy |
Appendix A
Modified Vulvovaginal Health Index
| VHI SCORE | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Vulvar appearance and color | Very pale, reduced size | Pale, smaller labia | Intermediate | Mostly rose-colored | Rosy and healthy |
| Pain at speculum insertion | Very strong | Strong | Moderate | Minimal | None |
| Vaginal inflammation (redness) | Vivid red with rashes | Very red and inflamed | Intermediate, minor spots | Fair to good | Excellent, redness absent |
| Vaginal moisture (coating) | None | Poor | Fair | Good | Excellent |
| Epithelial integrity (mucosa) | Petechiae, ulcerate mucosa | Bleeds with light contact | Not friable, thin epithelium | Almost fully trophic | Normal |
References
- Simon, J.A.; Nappi, R.E.; Chedraui, P.; Clark, A.L.; Gompel, A.; Nasreen, S.Z.A.; Palacios, S.; Wolfman, W. Genitourinary Syndrome of Menopause (GSM): Recommendations from the Fifth International Consultation on Sexual Medicine (ICSM 2024). Sex. Med. Rev. 2025, 13, qeaf055. [Google Scholar] [CrossRef]
- Murina, F.; Torraca, M.; Graziottin, A.; Nappi, R.E.; Villa, P.; Cetin, I. Validation of a Clinical Tool for Vestibular Trophism in Postmenopausal Women. Climacteric 2023, 26, 149–153. [Google Scholar] [CrossRef]
- Nappi, R.E.; Martini, E.; Cucinella, L.; Martella, S.; Tiranini, L.; Inzoli, A.; Brambilla, E.; Bosoni, D.; Cassani, C.; Gardella, B. Addressing Vulvovaginal Atrophy (VVA)/Genitourinary Syndrome of Menopause (GSM) for Healthy Aging in Women. Front. Endocrinol. 2019, 10, 561. [Google Scholar] [CrossRef]
- Portman, D.J.; Gass, M.L.S. Vulvovaginal Atrophy Terminology Consensus Conference Panel Genitourinary Syndrome of Menopause: New Terminology for Vulvovaginal Atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014, 21, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- The NAMS 2020 GSM Position Statement Editorial Panel. The 2020 Genitourinary Syndrome of Menopause Position Statement of The North American Menopause Society. Menopause 2020, 27, 976–992. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.M.C.; Crandall, C.J.M.; Davis, L.D.; El Khoudary, S.R.P.; Hodis, H.N.; Lobo, R.A.; Maki, P.M.; Manson, J.E.M.; Pinkerton, J.V.M.; Santoro, N.F.; et al. The 2022 Hormone Therapy Position Statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [Google Scholar] [CrossRef]
- Filippini, M.; Porcari, I.; Ruffolo, A.F.; Casiraghi, A.; Farinelli, M.; Uccella, S.; Franchi, M.; Candiani, M.; Salvatore, S. CO2-Laser Therapy and Genitourinary Syndrome of Menopause: A Systematic Review and Meta-Analysis. J. Sex. Med. 2022, 19, 452–470. [Google Scholar] [CrossRef]
- Kennedy, S.K.F.; Mekhaeil, S.; Zhang, E.; Jolfaei, N.A.; Wong, H.C.Y.; Chan, A.W.; Lee, S.F.; Haywood, D.; Kirk, D.; Abdou, A.M.; et al. Sexual Health after Breast Cancer: A Clinical Practice Review. Ann. Palliat. Med. 2024, 13, 1281–1290. [Google Scholar] [CrossRef]
- New Drug: Prasterone for Vulvar and Vaginal Atrophy in Postmenopausal Women. Aust. Prescr. 2024, 47, 164. [CrossRef]
- Bouchard, C.; Labrie, F.; Derogatis, L.; Girard, G.; Ayotte, N.; Gallagher, J.; Cusan, L.; Archer, D.F.; Portman, D.; Lavoie, L.; et al. Effect of Intravaginal Dehydroepiandrosterone (DHEA) on the Female Sexual Function in Postmenopausal Women: ERC-230 Open-Label Study. Horm. Mol. Biol. Clin. Investig. 2016, 25, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.L.; Sloan, J.A.; Shuster, L.T.; Gill, P.; Griffin, P.; Flynn, K.; Terstriep, S.A.; Rana, F.N.; Dockter, T.; Atherton, P.J.; et al. Evaluating the Efficacy of Vaginal Dehydroepiandosterone for Vaginal Symptoms in Postmenopausal Cancer Survivors: NCCTG N10C1 (Alliance). Support. Care Cancer 2018, 26, 643–650. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, R.; Taithongchai, A.; Da Silva, A.S.; Robinson, D.; Cardozo, L. Treating and Managing Urinary Incontinence: Evolving and Potential Multicomponent Medical and Lifestyle Interventions. Res. Rep. Urol. 2023, 15, 193–203. [Google Scholar] [CrossRef]
- Nappi, R.E.; Kotek, M.; Breštánský, A.; Giordan, N.; Beriotto, I.; Tramentozzi, E. Treatment of Vulvo-Vaginal Atrophy with Hyaluronate-Based Gel: A Randomized Controlled Study. Minerva Obstet. Gynecol. 2022, 74, 480–488. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Caruso, S.; Di Lorenzo, G.; Romano, F.; Mirandola, M.; Nappi, R.E. Efficacy and Safety of a New Vaginal Gel for the Treatment of Symptoms Associated with Vulvovaginal Atrophy in Postmenopausal Women: A Double-Blind Randomized Placebo-Controlled Study. Maturitas 2021, 147, 34–40. [Google Scholar] [CrossRef]
- Gustavino, C.; Sala, P.; Cusini, N.; Gravina, B.; Ronzini, C.; Marcolin, D.; Vellone, V.G.; Paudice, M.; Nappi, R.; Costantini, S.; et al. Efficacy and Safety of Prolonged-Release Hyaluronic Acid Derivative Vaginal Application in the Postpartum Period: A Prospective Randomised Clinical Trial. Ann. Med. 2021, 53, 1589–1597. [Google Scholar] [CrossRef]
- Misasi, G.; Russo, E.; Guevara, M.M.M.; Tomatis, V.; Fidecicchi, T.; Luisi, S.; Giannini, A.; Mannella, P.; Caretto, M.; Pomara, G.; et al. Effects of Vaginal DHEA on Stress Urinary Incontinence in Postmenopausal Women with Vulvovaginal Atrophy. Maturitas 2025, 196, 108232. [Google Scholar] [CrossRef] [PubMed]
- Buzzaccarini, G.; Marin, L.; Noventa, M.; Vitagliano, A.; Riva, A.; Dessole, F.; Capobianco, G.; Bordin, L.; Andrisani, A.; Ambrosini, G. Hyaluronic Acid in Vulvar and Vaginal Administration: Evidence from a Literature Systematic Review. Climacteric 2021, 24, 560–571. [Google Scholar] [CrossRef]
- Morali, G.; Polatti, F.; Metelitsa, E.; Mascarucci, P.; Magnani, P.; Brunenghi Marrè, G. Open, Non-Controlled Clinical Studies to Assess the Efficacy and Safety of a Medical Device in Form of Gel Topically and Intravaginally Used in Postmenopausal Women with Genital Atrophy. Arzneimittelforschung 2011, 56, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Tersigni, C.; Di Simone, N.; Tempestilli, E.; Cianfrini, F.; Russo, R.; Moruzzi, M.C.; Amar, I.D.; Fiorelli, A.; Scambia, G.; Villa, P. Non-Hormonal Treatment of Vulvo-Vaginal Atrophy-Related Symptoms in Post-Menopausal Women. J. Obstet. Gynaecol. 2015, 35, 835–838. [Google Scholar] [CrossRef]
- Albalawi, N.S.; Almohammadi, M.A.; Albalawi, A.R. Comparison of the Efficacy of Vaginal Hyaluronic Acid to Estrogen for the Treatment of Vaginal Atrophy in Postmenopausal Women: A Systematic Review. Cureus 2023, 15, e44191. [Google Scholar] [CrossRef]
- Sánchez-Prieto, M.; Pingarrón, C.; Bergamaschi, L.; Bermúdez, J.C.; Subiris González, J.; Sánchez Sánchez, R.; Poyo Torcal, S.; Gómez, M.; Ruiz Pérez, M.L.; Castillo Martínez, M.; et al. Prospective, Multicenter, Uncontrolled Study on the Effectiveness and Safety of a Hyaluronic Acid Water-Based Vaginal Lubricant in Alleviating Vaginal Dryness and Dyspareunia. Gynecol. Endocrinol. 2024, 40, 2317268. [Google Scholar] [CrossRef]
- Salvatore, S.; Nappi, R.E.; Zerbinati, N.; Calligaro, A.; Ferrero, S.; Origoni, M.; Candiani, M.; Leone Roberti Maggiore, U. A 12-Week Treatment with Fractional CO2 Laser for Vulvovaginal Atrophy: A Pilot Study. Climacteric 2014, 17, 363–369. [Google Scholar] [CrossRef]
- Cagnacci, A.; Barattini, D.F.; Casolati, E.; Pecoroni, A.; Mangrella, M.; Patrascu, L.C. Polycarbophil Vaginal Moisturizing Gel versus Hyaluronic Acid Gel in Women Affected by Vaginal Dryness in Late Menopausal Transition: A Prospective Randomized Trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 239–245. [Google Scholar] [CrossRef]
- Murina, F.; Ghisalberti, C. Clinical Significance of Topical Spermidine Hyaluronate in Vestibulodynia: An Early Appraisal. Open J. Obstet. Gynecol. 2023, 13, 1974–1984. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of Cellular Function by Polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Minois, N. Molecular Basis of the “anti-Aging” Effect of Spermidine and Other Natural Polyamines—A Mini-Review. Gerontology 2014, 60, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenhoek, A.V. Classic Pages in Obstetrics and Gynecology. Observationes…de Natis è Semine Genitali Animalculis. Antoni Van Leeuwenhoek. Philosophical Transactions of the Royal Society (London), Vol. 12, pp. 1040–1043, 1678–1679. Am. J. Obstet. Gynecol. 1978, 131, 469–470. Available online: https://pubmed.ncbi.nlm.nih.gov/352154/ (accessed on 23 November 2025).
- Lefèvre, P.L.C.; Palin, M.-F.; Murphy, B.D. Polyamines on the Reproductive Landscape. Endocr. Rev. 2011, 32, 694–712. [Google Scholar] [CrossRef]
- Watanabe, S.; Kusama-Eguchi, K.; Kobayashi, H.; Igarashi, K. Estimation of Polyamine Binding to Macromolecules and ATP in Bovine Lymphocytes and Rat Liver. J. Biol. Chem. 1991, 266, 20803–20809. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in Health and Disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed]
- Niechcial, A.; Schwarzfischer, M.; Wawrzyniak, M.; Atrott, K.; Laimbacher, A.; Morsy, Y.; Katkeviciute, E.; Häfliger, J.; Westermann, P.; Akdis, C.A.; et al. Spermidine Ameliorates Colitis via Induction of Anti-Inflammatory Macrophages and Prevention of Intestinal Dysbiosis. J. Crohn’s Colitis 2023, 17, 1489–1503. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Silvestri, Y.; Minguzzi, M.; Santi, S.; Cattini, L.; Filardo, G.; Flamigni, F.; Borzì, R.M. Spermidine Rescues the Deregulated Autophagic Response to Oxidative Stress of Osteoarthritic Chondrocytes. Free Radic. Biol. Med. 2020, 153, 159–172. [Google Scholar] [CrossRef]
- Ghisalberti, C.A.; Morisetti, A.; Bestetti, A.; Cairo, G. Potent Trophic Activity of Spermidine Supramolecular Complexes in In Vitro Models. World J. Biol. Chem. 2013, 4, 71–78. [Google Scholar] [CrossRef]
- Murina, F.; Graziottin, A.; Toni, N.; Schettino, M.T.; Bello, L.; Marchi, A.; Del Bravo, B.; Gambini, D.; Tiranini, L.; Nappi, R.E. Clinical Evidence Regarding Spermidine–Hyaluronate Gel as a Novel Therapeutic Strategy in Vestibulodynia Management. Pharmaceutics 2024, 16, 1448. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, W.J.; Perren, S.M. Standards, guidelines and principles for clinical trials: Medical products. Swiss Surg. 1995, 81–83. Available online: https://pubmed.ncbi.nlm.nih.gov/8581810/ (accessed on 23 November 2025).
- Ettinger, B.; Hait, H.; Reape, K.Z.; Shu, H. Measuring Symptom Relief in Studies of Vaginal and Vulvar Atrophy: The Most Bothersome Symptom Approach. Menopause 2008, 15, 885–889. [Google Scholar] [CrossRef]
- Bachmann, G. Urogenital Ageing: An Old Problem Newly Recognized. Maturitas 1995, 22, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Nappi, R.E.; Bruyniks, N.; Particco, M.; Panay, N. The European Vulvovaginal Epidemiological Survey (EVES): Prevalence, Symptoms and Impact of Vulvovaginal Atrophy of Menopause. Climacteric 2018, 21, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.; Austin, R.M.; Dillon, S.; Chang, C.-C.H.; Ness, R.B. Vaginal Maturation Index Self-Sample Collection in Mid-Life Women: Acceptability and Correlation with Physician-Collected Samples. Menopause 2008, 15, 726–729. [Google Scholar] [CrossRef]
- Meisels, A. The Maturation Value. Acta Cytol. 1967, 11, 249. [Google Scholar]
- Ekin, M.; Yaşar, L.; Savan, K.; Temur, M.; Uhri, M.; Gencer, I.; Kıvanç, E. The Comparison of Hyaluronic Acid Vaginal Tablets with Estradiol Vaginal Tablets in the Treatment of Atrophic Vaginitis: A Randomized Controlled Trial. Arch. Gynecol. Obstet. 2011, 283, 539–543. [Google Scholar] [CrossRef]
- Labrie, F.; Archer, D.; Bouchard, C.; Fortier, M.; Cusan, L.; Gomez, J.-L.; Girard, G.; Baron, M.; Ayotte, N.; Moreau, M.; et al. Intravaginal Dehydroepiandrosterone (Prasterone), a Physiological and Highly Efficient Treatment of Vaginal Atrophy. Menopause 2009, 16, 907–922. [Google Scholar] [CrossRef]
- Simon, J.A.; Laliberté, F.; Duh, M.S.; Pilon, D.; Kahler, K.H.; Nyirady, J.; Davis, P.J.; Lefebvre, P. Venous Thromboembolism and Cardiovascular Disease Complications in Menopausal Women Using Transdermal versus Oral Estrogen Therapy. Menopause 2016, 23, 600–610. [Google Scholar] [CrossRef]
- Van Der Laak, J.A.W.M.; De Bie, L.M.T.; De Leeuw, H.; De Wilde, P.C.M.; Hanselaar, A.G.J.M. The Effect of Replens(R) on Vaginal Cytology in the Treatment of Postmenopausal Atrophy: Cytomorphology versus Computerised Cytometry. J. Clin. Pathol. 2002, 55, 446–451. [Google Scholar] [CrossRef]
- Alvisi, S.; Gava, G.; Orsili, I.; Giacomelli, G.; Baldassarre, M.; Seracchioli, R.; Meriggiola, M.C. Vaginal Health in Menopausal Women. Medicina 2019, 55, 615. [Google Scholar] [CrossRef]
- Meriggiola, M.C.; Villa, P.; Maffei, S.; Becorpi, A.; Di Paolantonio, T.; Nicolucci, A.; Salvatore, S.; Nappi, R.E. Vulvovaginal Atrophy in Women with and without a History of Breast Cancer: Baseline Data from the Patient Satisfaction Study (PEONY) in Italy. Maturitas 2024, 183, 107950. [Google Scholar] [CrossRef]
- Davis, S.R. Androgen Therapy in Women, beyond Libido. Climacteric 2013, 16, 18–24. [Google Scholar] [CrossRef]
- Davis, S.R.; Baber, R.; Panay, N.; Bitzer, J.; Perez, S.C.; Islam, R.M.; Kaunitz, A.M.; Kingsberg, S.A.; Lambrinoudaki, I.; Liu, J.; et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. J. Sex. Med. 2019, 16, 1331–1337. [Google Scholar] [CrossRef]
- Zaidel-Bar, R.; Cohen, M.; Addadi, L.; Geiger, B. Hierarchical Assembly of Cell–Matrix Adhesion Complexes. Biochem. Soc. Trans. 2004, 32, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, Y.; Niu, C.; Long, S.; Jiang, Y.; Wang, Z.; Wang, X.; Sun, Q.; Ling, W.; An, X.; et al. Exploration of the Antioxidant Effect of Spermidine on the Ovary and Screening and Identification of Differentially Expressed Proteins. Int. J. Mol. Sci. 2023, 24, 5793. [Google Scholar] [CrossRef] [PubMed]
- Gallant, N.D.; Michael, K.E.; García, A.J. Cell Adhesion Strengthening: Contributions of Adhesive Area, Integrin Binding, and Focal Adhesion Assembly. Mol. Biol. Cell 2005, 16, 4329–4340. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, X.; Rao, J.N.; Zou, T.; Xiao, L.; Yu, T.; Timmons, J.A.; Turner, D.J.; Wang, J.-Y. Polyamines Regulate E-Cadherin Transcription through c-Myc Modulating Intestinal Epithelial Barrier Function. Am. J. Physiol.-Cell Physiol. 2009, 296, C801–C810. [Google Scholar] [CrossRef]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y. Cell Movement Is Guided by the Rigidity of the Substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Mechulam, A.; Chernov, K.G.; Mucher, E.; Hamon, L.; Curmi, P.A.; Pastré, D. Polyamine Sharing between Tubulin Dimers Favours Microtubule Nucleation and Elongation via Facilitated Diffusion. PLoS Comput. Biol. 2009, 5, e1000255. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of Autophagy by Spermidine Promotes Longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Ghisalberti, C.A.; Tezze, C. Assessing the Noninferiority of the Spermidine Hyaluronate Complex Relative to 17β-Estradiol Treatment in the Ovariectomized Murine Model of Vulvovaginal Atrophy. J. Menopausal Med. 2025, 31, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Gorodeski, G.I. Aging and Estrogen Effects on Transcervical-Transvaginal Epithelial Permeability. J. Clin. Endocrinol. Metab. 2005, 90, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.T.; Lee, K.B.; Kim, S.Y.; Choi, H.R.; Park, S.C. Autophagy Impairment Induces Premature Senescence in Primary Human Fibroblasts. PLoS ONE 2011, 6, e23367. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in Polyamines with Aging and Their Ingestion from Food and Drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef] [PubMed]
| Subject N. | Age | Years from Menopause | N. of Pregnancies | Medical History |
|---|---|---|---|---|
| #1 | 63 | 11 | 1 | Factor VII deficiency; medications: alendronic acid and colecalciferol, Ca and Vitamin D; no history of HRT. |
| #2 | 63 | 13 | 1 | Insomnia treated with melatonin; mild smoker; under HRT for 10 years, abandoned. |
| #3 | 71 | 18 | 3 | Celiac; SUI; recurrent infective cystitis; hysterectomized; no history of HRT. |
| #4 | 71 | 21 | 2 | Recurrent infective cystitis; no history of HRT. |
| #5 | 66 | 11 | 1 | Hypothyroidism treated with thyroxine; uterine fibromatosis; no history of HRT. |
| Subject #1 | Subject #2 | Subject #3 | Subject #4 | Subject #5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V1 | V2 | V3 | V1 | V2 | V3 | V1 | V2 | V3 | V1 | V2 | V3 | |
| Vulvar appearance and color | 2 | 3 | 4 | 1 | 3 | 4 | 1 | 3 | 4 | 2 | 3 | 4 | 1 | 2 | 3 |
| Pain at speculum insertion | 2 | 3 | 5 | 1 | 2 | 5 | 3 | 5 | 5 | 4 | 5 | 5 | 1 | 3 | 5 |
| Vaginal inflammation (redness) | 1 | 2 | 3 | 1 | 1 | 3 | 3 | 4 | 5 | 2 | 3 | 4 | 1 | 3 | 4 |
| Vaginal moisture (coating) | 2 | 3 | 4 | 2 | 2 | 4 | 1 | 3 | 4 | 1 | 2 | 4 | 1 | 2 | 2 |
| Epithelial integrity (mucosa) | 3 | 3 | 4 | 2 | 3 | 3 | 3 | 4 | 4 | 2 | 3 | 4 | 1 | 3 | 4 |
| Mean | 2.0 | 2.8 | 4.0 | 1.4 | 2.2 | 3.8 | 2.2 | 3.8 | 4.4 | 2.2 | 3.2 | 4.2 | 1.0 | 2.6 | 3.6 |
| SD | 0.7 | 0.4 | 0.7 | 0.5 | 0.8 | 0.8 | 1.1 | 0.8 | 0.5 | 1.1 | 1.1 | 0.4 | 0.0 | 0.5 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcari, I.; Carena Maini, M.; Ghisalberti, C.A.; Tezze, C.; Trimarchi, E.; Graziottin, A.; Bosco, M.; Casprini, C.; Garzon, S.; Uccella, S. Topical Spermidine Hyaluronate (Spd-HA) in Vulvovaginal Atrophy: A Preliminary Study. Medicina 2025, 61, 2104. https://doi.org/10.3390/medicina61122104
Porcari I, Carena Maini M, Ghisalberti CA, Tezze C, Trimarchi E, Graziottin A, Bosco M, Casprini C, Garzon S, Uccella S. Topical Spermidine Hyaluronate (Spd-HA) in Vulvovaginal Atrophy: A Preliminary Study. Medicina. 2025; 61(12):2104. https://doi.org/10.3390/medicina61122104
Chicago/Turabian StylePorcari, Irene, Michela Carena Maini, Carlo Angelo Ghisalberti, Caterina Tezze, Erica Trimarchi, Alessandra Graziottin, Mariachiara Bosco, Chiara Casprini, Simone Garzon, and Stefano Uccella. 2025. "Topical Spermidine Hyaluronate (Spd-HA) in Vulvovaginal Atrophy: A Preliminary Study" Medicina 61, no. 12: 2104. https://doi.org/10.3390/medicina61122104
APA StylePorcari, I., Carena Maini, M., Ghisalberti, C. A., Tezze, C., Trimarchi, E., Graziottin, A., Bosco, M., Casprini, C., Garzon, S., & Uccella, S. (2025). Topical Spermidine Hyaluronate (Spd-HA) in Vulvovaginal Atrophy: A Preliminary Study. Medicina, 61(12), 2104. https://doi.org/10.3390/medicina61122104






