Proliferative Effect of Proanthocyanidins on HGF-1 and HPDLF Cells: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Proanthocyanidin-Rich Material
2.2. Cell Culture and Growth Conditions
2.3. Evaluation of Cell Viability and Cytotoxicity of PSRE
2.4. Analysis of Cellular Effects of PSRE and Chlorhexidine on HGF-1 Cells
2.5. Evaluation of TGFβ-1 Levels in HGF-1 and HPDLF Cells
2.6. Statistical Analysis
3. Results
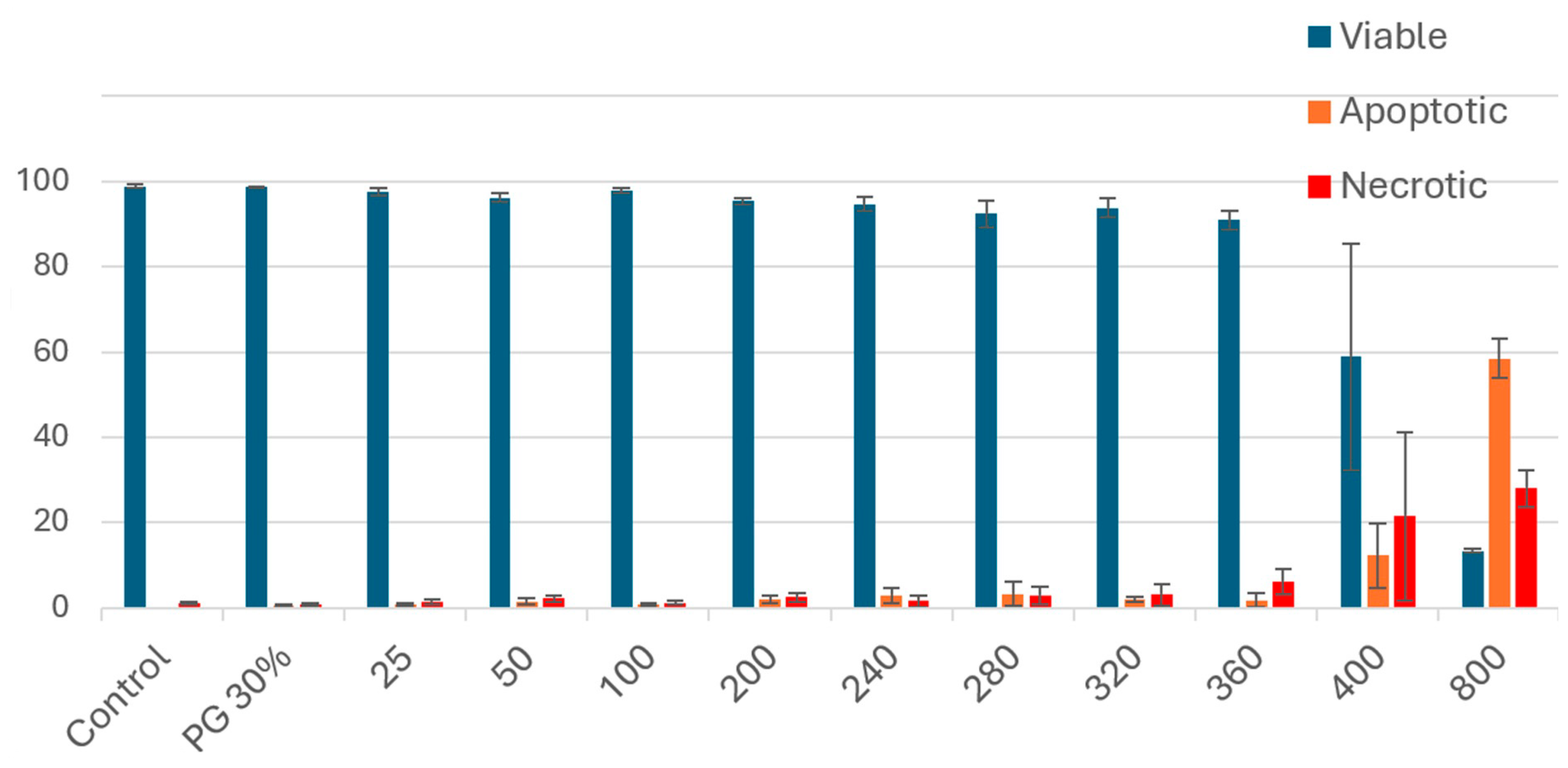
3.1. Cell Viability and Cytotoxicity
3.2. Proliferation Potential of PSRE
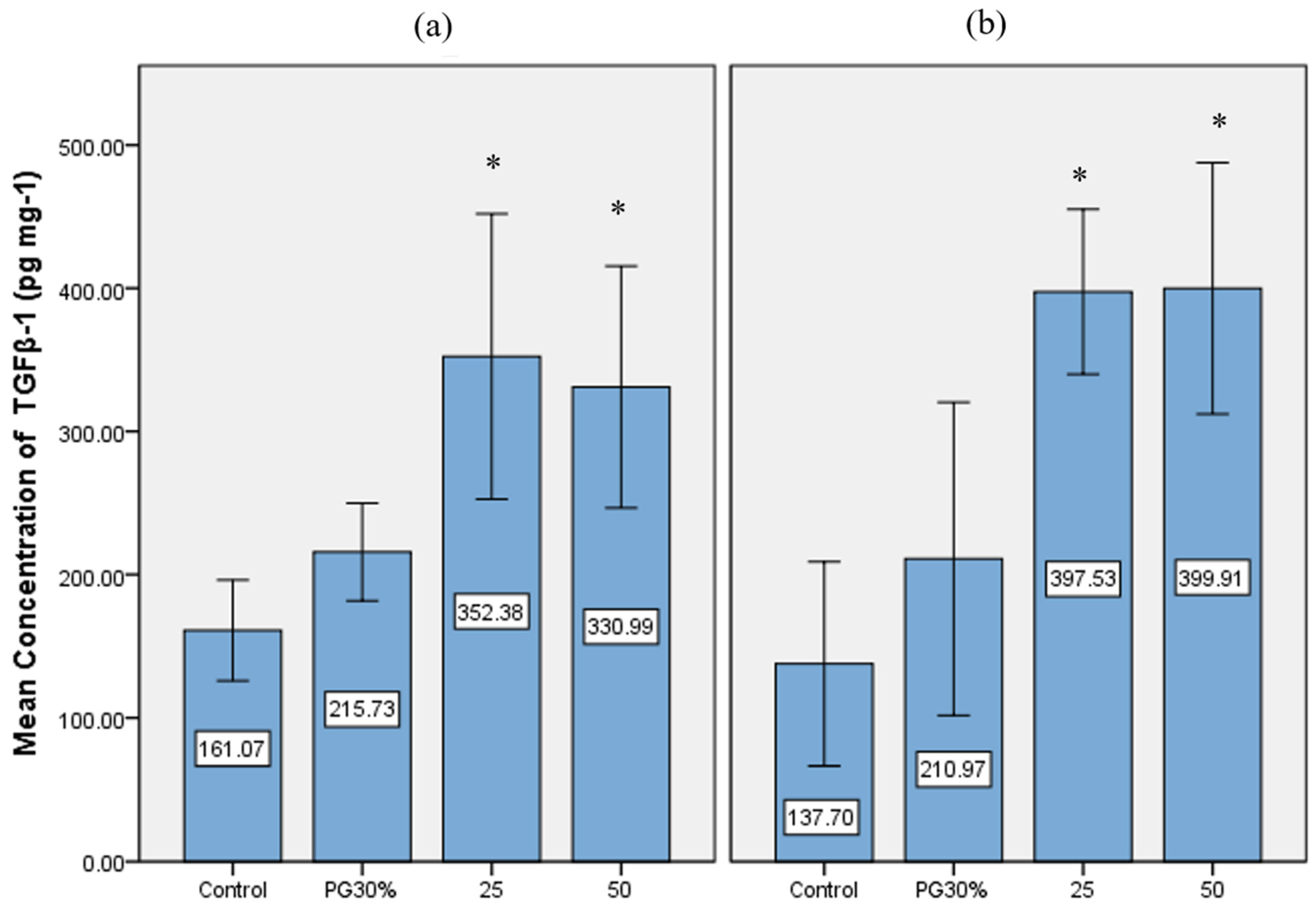
3.3. TGFβ-1 Analysis in PSRE-Treated Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PACN, (also PACNs) | proanthocyanidin (-s) |
| PSRE | Pelargonium sidoides DC. root extract |
| CHX | chlorhexidine digluconate solution |
| HGF-1 | human gingival fibroblasts |
| TGFβ-1 | transforming growth factor beta-1 |
| HPDLF | human periodontal ligament fibroblasts |
| ELISA | enzyme-linked immunosorbent assay |
| SE | standard error |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| IC50 | half-maximal inhibitory concentration |
| PI | propidium iodide |
| MAPK | mitogen-activated protein kinase |
| PI3K/Akt/mTOR | phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin |
| NFR2/HO1 | nuclear factor erythroid 2-related factor 2/heme oxygenase-1 |
| TNF-α | tumor necrosis factor-alpha |
| IL-1β | interleukin 1 beta |
| TGF-βR1 | TGF-β type I receptor |
| TGF-βR2 | TGF-β type II receptor |
| Smad2/3/4 | suppressor of mothers against decapentaplegic |
| PG30% | 30% polyethylene glycol solution |
References
- Özden, F.O.; Sakallioğlu, E.E.; Sakallioğlu, U.; Ayas, B.; Erişgin, Z. Effects of Grape Seed Extract on Periodontal Disease: An Experimental Study in Rats. J. Appl. Oral Sci. 2017, 25, 121–129. [Google Scholar] [CrossRef]
- Toker, H.; Balci Yuce, H.; Lektemur Alpan, A.; Gevrek, F.; Elmastas, M. Morphometric and Histopathological Evaluation of the Effect of Grape Seed Proanthocyanidin on Alveolar Bone Loss in Experimental Diabetes and Periodontitis. J. Periodontal Res. 2018, 53, 478–486. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Schoetz, K.; Erdelmeier, C.; Germer, S.; Hauer, H. A Detailed View on the Constituents of EPs 7630. Planta Med. 2008, 74, 667–674. [Google Scholar] [CrossRef]
- Savickiene, N.; Jekabsone, A.; Raudone, L.; Abdelgeliel, A.S.; Cochis, A.; Rimondini, L.; Makarova, E.; Grinberga, S.; Pugovics, O.; Dambrova, M.; et al. Efficacy of Proanthocyanidins from Pelargonium Sidoides Root Extract in Reducing P. Gingivalis Viability While Preserving Oral Commensal S. Salivarius. Materials 2018, 11, 1499. [Google Scholar] [CrossRef]
- Jekabsone, A.; Sile, I.; Cochis, A.; Makrecka-Kuka, M.; Laucaityte, G.; Makarova, E.; Rimondini, L.; Bernotiene, R.; Raudone, L.; Vedlugaite, E.; et al. Investigation of Antibacterial and Antiinflammatory Activities of Proanthocyanidins from Pelargonium Sidoides DC Root Extract. Nutrients 2019, 11, 2829. [Google Scholar] [CrossRef] [PubMed]
- Alkimavičienė, E.; Pušinskaitė, R.; Basevičienė, N.; Banienė, R.; Savickienė, N.; Pacauskienė, I.M. Efficacy of Proanthocyanidins in Nonsurgical Periodontal Therapy. Int. Dent. J. 2023, 73, 195–204. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant Activity of Natural Flavonoids Is Governed by Number and Location of Their Aromatic Hydroxyl Groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Comp. Rev. Food Sci. Food Safe 2019, 18, 971–985. [Google Scholar] [CrossRef]
- Salinas-Sánchez, D.; Jiménez-Ferrer, E.; Sánchez-Sánchez, V.; Zamilpa, A.; González-Cortazar, M.; Tortoriello, J.; Herrera-Ruiz, M. Anti-Inflammatory Activity of a Polymeric Proanthocyanidin from Serjania Schiedeana. Molecules 2017, 22, 863. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.G.M.; Haenen, G.R.M.M.; Van Acker, F.A.A.; Van Der Vijgh, W.J.F.; Bast, A. Flavonoids as Peroxynitrite Scavengers: The Role of the Hydroxyl Groups. Toxicol. In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, R.; Yu, S.; Lu, G.; Yu, Y.; Jiang, C. Anti-Inflammatory Activity of Oligomeric Proanthocyanidins Via Inhibition of NF-κB and MAPK in LPS-Stimulated MAC-T Cells. J. Microbiol. Biotechnol. 2020, 30, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Kong, X.; Yang, X.; Wang, Y.; Ma, L.; Luo, M.; Xu, D. Molecular Modification of Proanthocyanidins. Bioengineered 2016, 7, 274–281. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Matkowski, A.; Kubasiewicz-Ross, P.; Hadzik, J. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis—Immunomodulatory Effects, Animal and Clinical Studies. Nutrients 2021, 13, 239. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Dohle, E.; Ramanauskaite, A.; Al-Maawi, S.; Obreja, K.; Magini, R.; Sader, R.; Ghanaati, S.; Schwarz, F. Anti-Inflammatory and Macrophage Polarization Effects of Cranberry Proanthocyanidins (PACs) for Periodontal and Peri-Implant Disease Therapy. J. Periodontal Res. 2020, 55, 821–829. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Matkowski, A.; Hadzik, J.; Dobrowolska-Czopor, B.; Olchowy, C.; Dominiak, M.; Kubasiewicz-Ross, P. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis-Antibacterial Effects. Nutrients 2021, 13, 165. [Google Scholar] [CrossRef]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen Free Radical Scavenging Abilities of Vitamins C and E, and a Grape Seed Proanthocyanidin Extract In Vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Larrauri, M.; Zunino, M.P.; Zygadlo, J.A.; Grosso, N.R.; Nepote, V. Chemical Characterization and Antioxidant Properties of Fractions Separated from Extract of Peanut Skin Derived from Different Industrial Processes. Ind. Crops Prod. 2016, 94, 964–971. [Google Scholar] [CrossRef]
- Li, S.; Xu, M.; Niu, Q.; Xu, S.; Ding, Y.; Yan, Y.; Guo, S.; Li, F. Efficacy of Procyanidins against In Vivo Cellular Oxidative Damage: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0139455. [Google Scholar] [CrossRef]
- Yun, J.-H.; Pang, E.-K.; Kim, C.-S.; Yoo, Y.-J.; Cho, K.-S.; Chai, J.-K.; Kim, C.-K.; Choi, S.-H. Inhibitory Effects of Green Tea Polyphenol (-)-Epigallocatechin Gallate on the Expression of Matrix Metalloproteinase-9 and on the Formation of Osteoclasts. J. Periodontal Res. 2004, 39, 300–307. [Google Scholar] [CrossRef]
- Tanabe, S.; Santos, J.; La, V.D.; Howell, A.B.; Grenier, D. A-Type Cranberry Proanthocyanidins Inhibit the RANKL-Dependent Differentiation and Function of Human Osteoclasts. Molecules 2011, 16, 2365–2374. [Google Scholar] [CrossRef]
- La, V.D.; Howell, A.B.; Grenier, D. Anti- Porphyromonas gingivalis and Anti-Inflammatory Activities of A-Type Cranberry Proanthocyanidins. Antimicrob. Agents Chemother. 2010, 54, 1778–1784. [Google Scholar] [CrossRef]
- Bodet, C.; Piché, M.; Chandad, F.; Grenier, D. Inhibition of Periodontopathogen-Derived Proteolytic Enzymes by a High-Molecular-Weight Fraction Isolated from Cranberry. J. Antimicrob. Chemother. 2006, 57, 685–690. [Google Scholar] [CrossRef]
- Huang, J.; Liu, L.; Jin, S.; Zhang, Y.; Zhang, L.; Li, S.; Song, A.; Yang, P. Proanthocyanidins Promote Osteogenic Differentiation of Human Periodontal Ligament Fibroblasts in Inflammatory Environment Via Suppressing NF-κB Signal Pathway. Inflammation 2020, 43, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, W.-C.; Liu, X.; Li, Y.; Lin, C.; Lin, Y.-M.; Wang, A.-N.; Nguyen, T.-T.; Lin, Y.-C.; Chung, R.-J. Study on Proanthocyanidins Crosslinked Collagen Membrane for Guided Bone Tissue Regeneration. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211005379. [Google Scholar] [CrossRef] [PubMed]
- Tenkumo, T.; Aobulikasimu, A.; Asou, Y.; Shirato, M.; Shishido, S.; Kanno, T.; Niwano, Y.; Sasaki, K.; Nakamura, K. Proanthocyanidin-Rich Grape Seed Extract Improves Bone Loss, Bone Healing, and Implant Osseointegration in Ovariectomized Animals. Sci. Rep. 2020, 10, 8812. [Google Scholar] [CrossRef] [PubMed]
- Niwano, Y.; Shishido, S.; Shirato, M.; Kohzaki, H.; Nakamura, K. Therapeutic Potential of Proanthocyanidins in Dentistry: A Focus on Periodontal Disease and on Dental Implants in Osteoporotic Patients. Antioxidants 2025, 14, 850. [Google Scholar] [CrossRef]
- Guo, C.; Rizkalla, A.S.; Hamilton, D.W. FGF and TGF-β Growth Factor Isoform Modulation of Human Gingival and Periodontal Ligament Fibroblast Wound Healing Phenotype. Matrix Biol. 2025, 136, 9–21. [Google Scholar] [CrossRef]
- Dereka, X.E.; Markopoulou, C.E.; Vrotsos, I.A. Role of Growth Factors on Periodontal Repair. Growth Factors 2006, 24, 260–267. [Google Scholar] [CrossRef]
- Kaigler, D.; Cirelli, J.A.; Giannobile, W.V. Growth Factor Delivery for Oral and Periodontal Tissue Engineering. Expert. Opin. Drug Deliv. 2006, 3, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G.; Lopreiato, M.; Lollobrigida, M.; Lamazza, L.; Mazzucchi, G.; Fortunato, L.; Mariano, A.; Scotto d’Abusco, A.; Fontana, M.; De Biase, A. Platelet Rich Fibrin (PRF) and Its Related Products: Biomolecular Characterization of the Liquid Fibrinogen. J. Clin. Med. 2020, 9, 1099. [Google Scholar] [CrossRef]
- Bodet, C.; Chandad, F.; Grenier, D. Cranberry Components Inhibit Interleukin-6, Interleukin-8, and Prostaglandin E2 Production by Lipopolysaccharide-activated Gingival Fibroblasts. Eur. J. Oral Sci. 2007, 115, 64–70. [Google Scholar] [CrossRef]
- Déziel, B.A.; Patel, K.; Neto, C.; Gottschall-Pass, K.; Hurta, R.A.R. Proanthocyanidins from the American Cranberry (Vaccinium macrocarpon) Inhibit Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Activity in Human Prostate Cancer Cells via Alterations in Multiple Cellular Signalling Pathways. J. Cell. Biochem. 2010, 111, 742–754. [Google Scholar] [CrossRef]
- Feldman, M.; Grenier, D. Cranberry Proanthocyanidins Act in Synergy with Licochalcone A to Reduce Porphyromonas Gingivalis Growth and Virulence Properties, and to Suppress Cytokine Secretion by Macrophages: Synergy between Proanthocyanidins and Licochalcone A. J. Appl. Microbiol. 2012, 113, 438–447. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Liao, H.; Li, C.; Chen, M. Anti-Inflammatory Effect of Lipophilic Grape Seed Proanthocyanidin in RAW 264.7 Cells and a Zebrafish Model. J. Funct. Foods 2020, 75, 104217. [Google Scholar] [CrossRef]
- Han, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.-J.; Li, J.; Luo, Y.-Y.; Li, J.-X.; Zhao, L.-C.; et al. Procyanidin A1 Alleviates Inflammatory Response Induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 Cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef] [PubMed]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/mTOR Pathway by Different Flavonoids: A Cancer Chemopreventive Approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Satyshur, K.A.; Syed, D.N.; Dodwad, S.-J.M.; Chaves-Rodriquez, M.-I.; Longley, B.J.; et al. Dual Inhibition of PI3K/Akt and mTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features In Vitro and in an Imiquimod-Induced Psoriasis-Like Disease in Mice. Antioxid. Redox Signal 2017, 26, 49–69. [Google Scholar] [CrossRef]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-Seed Proanthocyanidins Inhibit the Lipopolysaccharide-Induced Inflammatory Mediator Expression in RAW264.7 Macrophages by Suppressing MAPK and NF-Κb Signal Pathways. Environ. Toxicol. Pharmacol. 2016, 41, 159–166. [Google Scholar] [CrossRef]
- Shuhua, Y.; Lingqi, M.; Yunlong, D.; He, T.; Yao, S.; Peng, L. Proanthocyanidins Activate Nrf2/ARE Signaling Pathway in Intestinal Epithelial Cells by Inhibiting the Ubiquitinated Degradation of Nrf2. BioMed Res. Int. 2022, 2022, 8562795. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Lenardo, M.J. The Nuclear Signaling of NF-κB: Current Knowledge, New Insights, and Future Perspectives. Cell Res. 2010, 20, 24–33. [Google Scholar] [CrossRef]
- Scheidereit, C. IκB Kinase Complexes: Gateways to NF-κB Activation and Transcription. Oncogene 2006, 25, 6685–6705. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardèvol, A.; Pinent, M.; Blay, M.T. Procyanidins and Inflammation: Molecular Targets and Health Implications. BioFactors 2012, 38, 257–265. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Sun, J.; He, L.; Ai, C.; Jin, W.; Abd El-Aty, A.M. Beneficial Effects of Proanthocyanidins on Skin Aging: A Review. Front. Nutr. 2025, 12, 1650328. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Fan, J.; Jia, H.; Li, J. Interrelation of Natural Polyphenol and Fibrosis in Diabetic Nephropathy. Molecules 2024, 30, 20. [Google Scholar] [CrossRef]
- Meng, X.-M.; Tang, P.M.-K.; Li, J.; Lan, H.Y. TGF-Î2/Smad Signaling in Renal Fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Mao, T.K.; Water, J.V.D.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Cocoa Flavonols and Procyanidins Promote Transforming Growth Factor-β1 Homeostasis in Peripheral Blood Mononuclear Cells. Exp. Biol. Med. 2003, 228, 93–99. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Y.; Zhang, N.; Wang, D.; Cheng, X.; Li, K.; Huang, R.; Lu, Y.; Wang, H.; Han, D.; et al. The Phosphorylation of the Smad2/3 Linker Region by Nemo-like Kinase Regulates TGF-β Signaling. J. Biol. Chem. 2021, 296, 100512. [Google Scholar] [CrossRef] [PubMed]
- Battegay, E.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F.; Ross, R. TGF-β Induces Bimodal Proliferation of Connective Tissue Cells via Complex Control of an Autocrine PDGF Loop. Cell 1990, 63, 515–524. [Google Scholar] [CrossRef]
- Strutz, F.; Zeisberg, M.; Renziehausen, A.; Raschke, B.; Becker, V.; Van Kooten, C.; Müller, G.A. TGF-Β1 Induces Proliferation in Human Renal Fibroblasts via Induction of Basic Fibroblast Growth Factor (FGF-2). Kidney Int. 2001, 59, 579–592. [Google Scholar] [CrossRef]
- Li, A.G.; Wang, D.; Feng, X.-H.; Wang, X.-J. Latent TGFβ1 Overexpression in Keratinocytes Results in a Severe Psoriasis-like Skin Disorder. EMBO J. 2004, 23, 1770–1781. [Google Scholar] [CrossRef]
- Wilkes, M.C.; Mitchell, H.; Penheiter, S.G.; Doré, J.J.; Suzuki, K.; Edens, M.; Sharma, D.K.; Pagano, R.E.; Leof, E.B. Transforming Growth Factor-β Activation of Phosphatidylinositol 3-Kinase Is Independent of Smad2 and Smad3 and Regulates Fibroblast Responses via P21-Activated Kinase-2. Cancer Res. 2005, 65, 10431–10440. [Google Scholar] [CrossRef]
- Shi, X.; Young, C.D.; Zhou, H.; Wang, X.-J. Transforming Growth Factor-β Signaling in Fibrotic Diseases and Cancer-Associated Fibroblasts. Biomolecules 2020, 10, 1666. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease and Therapeutics. Sig. Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent Advances in Periodontal Regeneration: A Biomaterial Perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wielento, A.; Lagosz-Cwik, K.B.; Potempa, J.; Grabiec, A.M. The Role of Gingival Fibroblasts in the Pathogenesis of Periodontitis. J. Dent. Res. 2023, 102, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Nawrot-Hadzik, I.; Matkowski, A.; Pitułaj, A.; Sterczała, B.; Olchowy, C.; Szewczyk, A.; Choromańska, A. In Vitro Gingival Wound Healing Activity of Extracts from Reynoutria Japonica Houtt Rhizomes. Pharmaceutics 2021, 13, 1764. [Google Scholar] [CrossRef] [PubMed]
- Kurauchi, M.; Niwano, Y.; Shirato, M.; Kanno, T.; Nakamura, K.; Egusa, H.; Sasaki, K. Cytoprotective Effect of Short-Term Pretreatment with Proanthocyanidin on Human Gingival Fibroblasts Exposed to Harsh Environmental Conditions. PLoS ONE 2014, 9, e113403. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.-J.; Min, K.-S. Effects of Proanthocyanidin, a Crosslinking Agent, on Physical and Biological Properties of Collagen Hydrogel Scaffold. Restor. Dent. Endod. 2016, 41, 296–303. [Google Scholar] [CrossRef]
- Kwak, S.C.; Cheon, Y.-H.; Lee, C.H.; Jun, H.Y.; Yoon, K.-H.; Lee, M.S.; Kim, J.-Y. Grape Seed Proanthocyanidin Extract Prevents Bone Loss via Regulation of Osteoclast Differentiation, Apoptosis, and Proliferation. Nutrients 2020, 12, 3164. [Google Scholar] [CrossRef]
- Tofani, I.; Maki, K.; Kojima, K.; Kimura, M. Beneficial Effects of Grape Seed Proanthocyanidins Extract on Formation of Tibia Bone in Low-Calcium Feeding Rats. Pediatr. Dent. J. 2004, 14, 47–53. [Google Scholar] [CrossRef]
- Ming, P.; Liu, Y.; Yu, P.; Jiang, X.; Yuan, L.; Cai, S.; Rao, P.; Cai, R.; Lan, X.; Tao, G.; et al. A Biomimetic Se-nHA/PC Composite Microsphere with Synergistic Immunomodulatory and Osteogenic Ability to Activate Bone Regeneration in Periodontitis. Small 2024, 20, e2305490. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Yu, S.; Zhao, C.; Yang, Y.; Yan, J.; Wang, Y.; Liu, D.; Liu, Y.; Zhang, X. Proanthocyanidins Modification of the Mineralized Collagen Scaffold Based on Synchronous Self-Assembly/Mineralization for Bone Regeneration. Colloids Surf. B Biointerfaces 2025, 245, 114290. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Maki, K.; Tofani, I.; Kimura, K.; Kimura, M. Grape Seed Proanthocyanidins Extract Promotes Bone Formation in Rat’s Mandibular Condyle. Eur. J. Oral Sci. 2005, 113, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, X.; Zhang, Y.; Wen, Z.; Li, Y.; Zhang, K.; Gosar, N.; Li, Q.; Mao, J.; Gong, S. Proanthocyanidins Ameliorate LPS-Inhibited Osteogenesis of PDLSCs by Restoring Lysine Lactylation. Int. J. Mol. Sci. 2024, 25, 2947. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Wang, X.; Wu, Y.; Zhang, Z.; Mao, J.; Gong, S. Proanthocyanidin Enhances the Endogenous Regeneration of Alveolar Bone by Elevating the Autophagy of PDLSCs. J. Periodontal Res. 2023, 58, 1300–1314. [Google Scholar] [CrossRef]
- Zhao, J.; Jiao, J.; Chen, X.; Zhang, Y.; Chen, T.; Xie, J.; Ou, X. Procyanidin B2 Targeted CCR7 Expression to Inhibit the Senescence-Associated Secretory Phenotype through the NF-κB Pathway to Promote Osteogenic Differentiation of Periodontal Ligament Stem Cells in Periodontitis. Int. Immunopharmacol. 2024, 143, 113435. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, M.-K.; Oh, H.-J.; Woo, Y.-J.; Lim, M.-A.; Lee, J.-H.; Ju, J.H.; Jung, Y.O.; Lee, Z.H.; Park, S.-H.; et al. Grape-Seed Proanthocyanidin Extract as Suppressors of Bone Destruction in Inflammatory Autoimmune Arthritis. PLoS ONE 2012, 7, e51377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yin, Z.; Zhang, Q.; Guo, S.; Shen, Y.; Liu, T.; Liu, B.; Wan, L.; Li, S.; Chen, X.; et al. Proanthocyanidins Inhibit Osteoclast Formation and Function by Inhibiting the NF-κB and JNK Signaling Pathways during Osteoporosis Treatment. Biochem. Biophys. Res. Commun. 2019, 509, 294–300. [Google Scholar] [CrossRef]
- Fiorillo, L.; D’Amico, C.; Mehta, V.; Cicciù, M.; Cervino, G. Chlorhexidine Cytotoxicity on Oral Behaviors: Last 20 Years Systematic Review. Oral. Oncol. Rep. 2024, 9, 100245. [Google Scholar] [CrossRef]
- Azzimonti, B.; Cochis, A.; Beyrouthy, M.E.; Iriti, M.; Uberti, F.; Sorrentino, R.; Landini, M.M.; Rimondini, L.; Varoni, E.M. Essential Oil from Berries of Lebanese Juniperus Excelsa M. Bieb Displays Similar Antibacterial Activity to Chlorhexidine but Higher Cytocompatibility with Human Oral Primary Cells. Molecules 2015, 20, 9344–9357. [Google Scholar] [CrossRef]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity Evaluation of Chlorhexidine Gluconate on Human Fibroblasts, Myoblasts, and Osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef]
- Cunha, G.; D’Angieri Saugo, G.; Gabrielli, M.A.C.; Barbeiro, C.d.O.; de Almeida, L.Y.; Bufalino, A.; Pereira-Filho, V.A. Cytotoxicity Evaluation of Chlorhexidine and Blue®M Applied to a Human Gingival Fibroblast (HGF-1) and Keratinocytes (NOK-SI): In Vitro Study. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101923. [Google Scholar] [CrossRef]
- Dinu, S.; Matichescu, A.; Buzatu, R.; Marcovici, I.; Geamantan-Sirbu, A.; Semenescu, A.D.; Bratu, R.C.; Bratu, D.-C. Insights into the Cytotoxicity and Irritant Potential of Chlorhexidine Digluconate: An In Vitro and In Ovo Safety Screening. Dent. J. 2024, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, K.; Mukai, Y.; Saito, M.; Watanabe, K.; Kumada, H.; Nihei, T.; Hamada, N.; Teranaka, T. Antibacterial Action of a Condensed Tannin Extracted from Astringent Persimmon as a Component of Food Addictive Pancil PS-M on Oral Polymicrobial Biofilms. BioMed Res. Int. 2016, 2016, 5730748. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, J.; Xue, B.; Zhang, C.; Shi, L.; Wu, C.; Su, Y.; Jin, X.; Liu, Y.; Zhu, X. Molecular Insights for the Biological Interactions between Polyethylene Glycol and Cells. Biomaterials 2017, 147, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, X.; Qiang, Q.; Zhang, Z. Ultrasound-assisted Extraction and Preliminary Purification of Proanthocyanidins and Chlorogenic Acid from Almond (Prunus dulcis) Skin. J. Sep. Sci. 2014, 37, 1834–1841. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkimavičienė, E.; Basevičienė, N.; Strazdauskas, A.; Banienė, R.; Savickienė, N. Proliferative Effect of Proanthocyanidins on HGF-1 and HPDLF Cells: An In Vitro Study. Medicina 2025, 61, 2098. https://doi.org/10.3390/medicina61122098
Alkimavičienė E, Basevičienė N, Strazdauskas A, Banienė R, Savickienė N. Proliferative Effect of Proanthocyanidins on HGF-1 and HPDLF Cells: An In Vitro Study. Medicina. 2025; 61(12):2098. https://doi.org/10.3390/medicina61122098
Chicago/Turabian StyleAlkimavičienė, Evelina, Nomeda Basevičienė, Arvydas Strazdauskas, Rasa Banienė, and Nijolė Savickienė. 2025. "Proliferative Effect of Proanthocyanidins on HGF-1 and HPDLF Cells: An In Vitro Study" Medicina 61, no. 12: 2098. https://doi.org/10.3390/medicina61122098
APA StyleAlkimavičienė, E., Basevičienė, N., Strazdauskas, A., Banienė, R., & Savickienė, N. (2025). Proliferative Effect of Proanthocyanidins on HGF-1 and HPDLF Cells: An In Vitro Study. Medicina, 61(12), 2098. https://doi.org/10.3390/medicina61122098







