Association of MASLD with Baseline and New-Onset Liver Function Test Elevation in Medical ICU Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
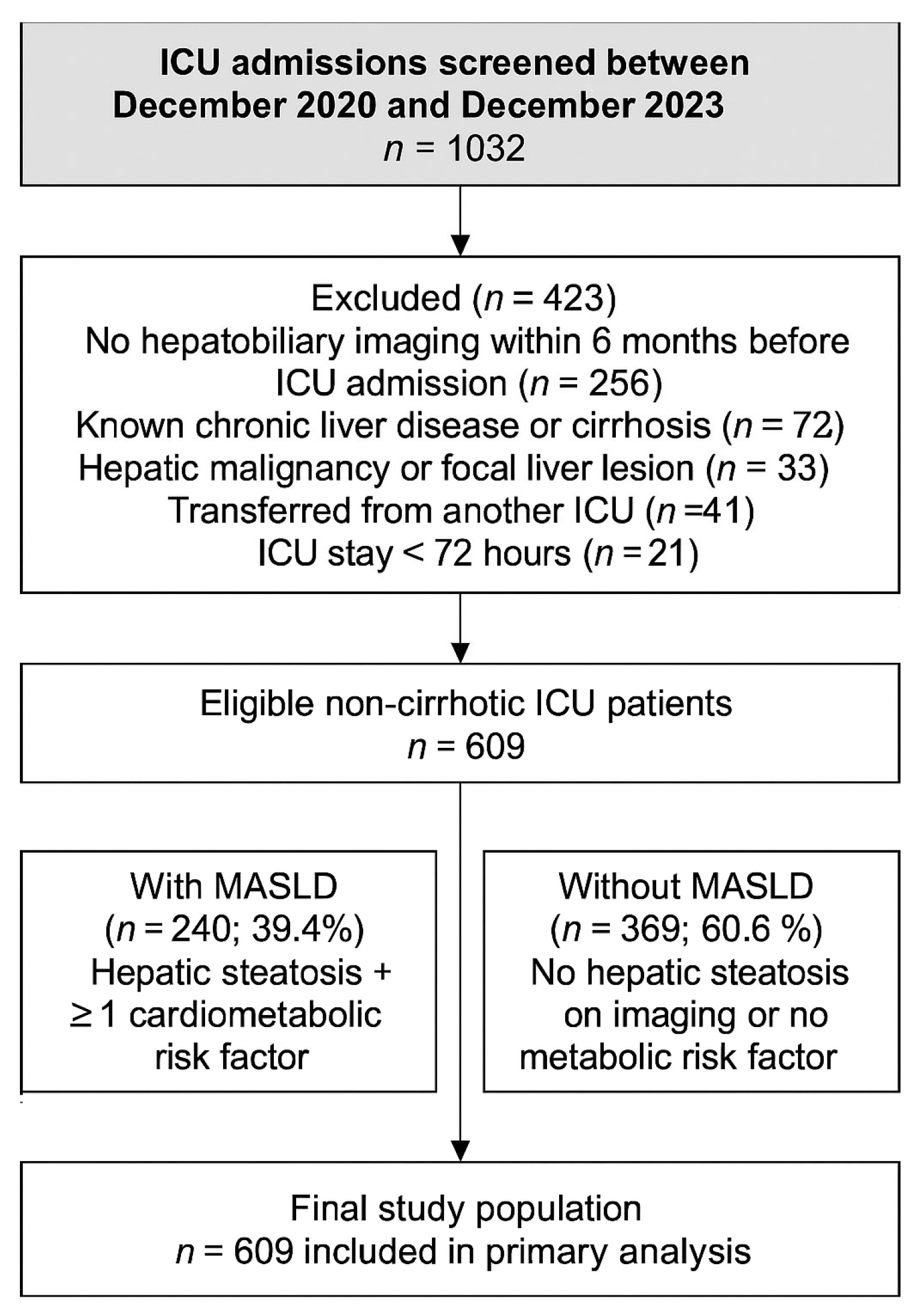
2.2. Patient Selection
2.3. Hepatic Steatosis Classification
- Grade 0 (absent): normal hepatic echotexture and clear visualization of the portal vein walls and diaphragm.
- Grade 1 (mild): Slight, diffuse increase in liver echogenicity with preserved visualization of the portal vein wall and diaphragm.
- Grade 2 (moderate): Moderate increase in echogenicity with partial obscuration of the portal vein walls and diaphragm.
- Grade 3 (severe): Marked echogenicity with poor or absent visualization of the diaphragm, portal vein walls, and posterior aspect of the right hepatic lobe.
2.4. Definition of MASLD
2.4.1. Evidence of Hepatic Steatosis
2.4.2. At Least One Cardiometabolic Risk Factor
- Overweight/obesity (BMI ≥ 25 kg m2; BMI ≥ 23 kg m2 for Asian ancestry).
- Type 2 diabetes mellitus or prediabetes (HbA1c ≥ 5.7% or fasting glucose ≥ 100 mg dL).
- Arterial hypertension (blood pressure ≥ 130/85 mm Hg or antihypertensive therapy).
- Atherogenic dyslipidemia (triglycerides ≥ 150 mg/dL or HDL-C < 40 mg/dL in men and <50 mg/dL in women or lipid-lowering therapy).
- Insulin resistance (HOMA-IR ≥ 2.5) or waist circumference > 94 cm in men and >80 cm in women.
2.5. Further Criteria
2.6. Data Collection
2.7. Definition of Elevated Liver Function Tests
2.7.1. Time Points and Dynamic Assessment
2.7.2. Definition of “Abnormal”
2.7.3. Pattern Classification (Primary Analysis)
2.7.4. Sensitivity (Alternative Pragmatic Rule, Pre-Specified)
2.8. Outcome Measures and Grouping
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the AASLD practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212–1219. [Google Scholar] [CrossRef]
- Zhang, X.; Linden, S.; Levesley, C.R.; He, X.; Yang, Z.; Barnet, S.D.; Cheung, R.; Ji, F.; Nguyen, M.H. Projected trends in metabolic dysfunction-associated steatotic liver disease mortality through 2040. JAMA Netw. Open 2025, 8, e2516367. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Yuan, Y.; Shen, H.; Gao, J.; Kong, X.; Che, Z.; Guo, Y.; Wang, H.; Dong, E.; Xiao, J. Liver diseases: Epidemiology, causes, trends and predictions. Signal Transduct. Target. Ther. 2025, 10, 33. [Google Scholar] [CrossRef]
- Van Eldere, A.; Pirani, T. Liver intensive care for the general intensivist. Anaesthesia 2023, 78, 884–901. [Google Scholar] [CrossRef]
- Lyu, X.; Liu, B.; Li, Y.; Wang, Y.; Miskovsky, J.; Gaitanis, M.; Promrat, K.; Wu, W.-C. Impact of non-alcoholic fatty liver disease on sepsis inpatient outcomes: A nationwide sample analysis (2000–2019). J. Clin. Med. 2024, 13, 5737. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, W.; Chen, H.; Sun, J.; Yin, Y. Mechanisms of sepsis-induced acute liver injury: A comprehensive review. Front. Cell. Infect. Microbiol. 2025, 15, 1504223. [Google Scholar] [CrossRef]
- Ampuero, J.; Aller, R.; Gallego-Durán, R.; Crespo, J.; Calleja, J.L.; García-Monzón, C.; Gómez-Camarero, J.; Caballería, J.; Lo Iacono, O.; Ibañez, L.; et al. The biochemical pattern defines MASLD phenotypes linked to distinct histology and prognosis. J. Gastroenterol. 2024, 59, 586–597. [Google Scholar] [CrossRef]
- Bozic, D.; Podrug, K.; Mikolasevic, I.; Grgurevic, I. Ultrasound methods for the assessment of liver steatosis: A critical appraisal. Diagnostics 2022, 12, 2287. [Google Scholar] [CrossRef]
- Arab, J.P.; Díaz, L.A.; Rehm, J.; Im, G.; Arrese, M.; Kamath, P.S.; Lucey, M.R.; Mellinger, J.; Thiele, M.; Thursz, M.; et al. Metabolic dysfunction and alcohol-related liver disease (MetALD): Position statement by an expert panel. J. Hepatol. 2025, 82, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.J.; Cowan, M.L.; Johnston, I.; Musa, S.; Grounds, M.; Rahman, T.M. ‘Liver function tests’ on the intensive care unit: A prospective, observational study. Intensive Care Med. 2009, 35, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Ghenu, M.I.; Dragoş, D.; Manea, M.M.; Ionescu, D.; Negreanu, L. Pathophysiology of sepsis-induced cholestasis: A review. JGH Open 2022, 6, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Kneidinger, N.; Herkner, H.; Heinz, G.; Nikfardjam, M.; Bojic, A.; Schellongowski, P.; Angermayr, B.; Schöniger-Hekele, M.; Madl, C. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011, 37, 1302–1310. [Google Scholar] [CrossRef]
- Beyer, D.; Hoff, J.; Sommerfeld, O.; Zipprich, A.; Gaßler, N.; Press, A.T. The liver in sepsis: Molecular mechanism of liver failure and their potential for clinical translation. Mol. Med. 2022, 28, 84. [Google Scholar] [CrossRef]
- Prasad, B.R.; Mahadevan, B.; Verghese, J.; Venkataraman, J. Deranged liver biochemistry in a non-liver intensive care unit: A prospective study. Gastroenterol. Hepatol. Endosc. Pract. 2024, 4, 147–150. [Google Scholar] [CrossRef]
- Krznaric, J.; Papic, N.; Vrsaljko, N.; Gjurasin, B.; Kutlesa, M.; Vince, A. Steatotic liver disease and sepsis outcomes—A prospective cohort study (SepsisFAT). J. Clin. Med. 2024, 13, 798. [Google Scholar] [CrossRef]
- Mannino, C.B.; Ajmera, A.; Judge, T. Influence of fatty liver and cirrhosis on survival in septic shock: 342. Am. J. Gastroenterol. 2011, 106, S134–S135. [Google Scholar] [CrossRef]
- Yin, H.; Xiong, B.; Yu, J.; Fan, Y.; Zhou, B.; Sun, Y.; Wang, L.; Xu, H.; Zhu, Y. Interoperator reproducibility of quantitative ultrasound analysis of hepatic steatosis in participants with suspected MASLD: A prospective study. Eur. J. Radiol. 2024, 175, 111427. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Raghani, N.; Chorawala, M.; Bhattacharya, S.; Prajapati, B.G.; Elossaily, G.M.; Chaiyasut, C. NF-κB Pathway and Its Inhibitors: A Promising Frontier in the Management of Alzheimer’s Disease. Biomedicines 2023, 11, 2587. [Google Scholar] [CrossRef]
- Iacobazzi, D.; Convertini, P.; Todisco, S.; Santarsiero, A.; Iacobazzi, V.; Infantino, V. New Insights into NF-κB Signaling in Innate Immunity: Focus on Immunometabolic Crosstalks. Biology 2023, 12, 776. [Google Scholar] [CrossRef]
- Lousa, I.; Reis, F.; Santos-Silva, A.; Belo, L. The Signaling Pathway of TNF Receptors: Linking Animal Models of Renal Disease to Human CKD. Int. J. Mol. Sci. 2022, 23, 3284. [Google Scholar] [CrossRef]
| Characteristics | All Patients n = 609 | MASLD (+) n = 240 (39.4%) | MASLD (−) n = 369 (60.6%) | p Value |
|---|---|---|---|---|
| Age (years) | 73 (64–81) | 73 (64–81) | 73 (63–81) | 0.69 |
| Female, n (%) | 273(45%) | 97(40%) | 176(44%) | 0.04 |
| Admission source, n (%) Emergency Department Inpatient Wards | 343 (56%) 266 (44%) | 110 (46%) 116 (48%) | 233 (59%) 150 (38%) | 0.03 |
| Comorbidities, n (%) Chronic renal disease Pulmonary disease Hypertension Neurological Rheumatological Diabetes Mellitus Malignancies | 200 (33%) 222 (36%) 351 (58%) 130 (21%) 40 (7%) 218 (36%) 183 (30%) | 82 (34%) 94 (39%) 150 (63%) 43 (18%) 18 (8%) 92 (38%) 84 (35%) | 118 (30%) 128 (32%) 201 (51%) 87 (22%) 22 (6%) 126 (32%) 99 (25%) | 0.33 0.11 0.12 0.05 0.18 0.44 0.28 |
| BMI (kg/m2) | 24.2 (22.5–28.2) | 24.6 (22.5–28.9) | 24.2 (22.5–27.8) | 0.46 |
| APACHE-II Score | 22 (16–29) | 22 (16–30) | 21 (16–29) | 0.06 |
| SOFA Score | 6 (3–9) | 6 (3–10) | 5 (3–8) | 0.16 |
| Glasgow Coma Scale | 14 (10–15) | 14 (10–15] | 15 (10–15) | 0.11 |
| RIFLE stage, n (%) Risk Injury Failure Loss ESRD | 125 (21%) 88 (14%) 117 (19%) 26 (4%) 62 (10%) | 47 (20%) 39 (16%) 48 (20%) 11 (5%) 31 (13%) | 78 (20%) 49 (12%) 69 (17%) 15 (4%) 31 (8%) | 0.36 0.18 0.38 0.45 0.04 |
| Reason of ICU Admission, n (%) Respiratory failure Sepsis Renal failure Cardiac decompensation Acute neurological disorders Metabolic disturbances Surgery | 346 (57%) 339 (56%) 273 (45%) 95 (16%) 68 (11%) 31 (5%) 24 (4%) | 147 (61%) 108 (45%) 141 (59%) 42 (18%) 26 (11%) 11 (5%) 9 (4%) | 192 (48%) 65 (16%) 205 (52%) 53 (13%) 42 (11%) 20 (5%) 15 (4%) | 0.06 0.07 0.28 0.18 0.47 0.39 0.51 |
| AKI at ICU Admission, n (%) | 345 (57%) | 141 (59%) | 204 (52%) | 0.06 |
| Shock at ICU Admission, n (%) | 144 (24%) | 90 (38%) | 54 (14%) | <0.01 |
| Laboratory Tests on ICU Admission ALT (>35 IU/L) AST (>35 IU/L) ALP (>104 IU/L) GGT (>42 IU/L) TB (>1.2 mg/dL) DB (>0.3 mg/dL) Albumin (<3.5 g/dL) Creatinine (>0.9 mg/dL) PT (>14.5 s) aPTT (aPTT > 32 s) INR (INR > 1.2) | 24 (14–57) 33 (21–71) 93 (67–144) 43 (29–100) 0.78 (0.48–1.31) 0.29 (0.16–0.62) 2.9 ± 0.6 1.51 (0.89–2.49) 14.6 (13–18) 29 (24.6–32.2) 1.24 (1.11–1.49) | 27 (14–61) 33 (22–65) 103 (69–148) 46 [26–118) 0.8 (0.48–1.47) 0.3 (0.15–0.69) 2.9 ± 0.7 1.43 (0.9–3.26) 14.6 (13–18.4) 29.6 (24.7–36.9)] 1.24 (1.09–1.55) | 23 (13–53) 32 (20–74) 89 (65–139) 40 (22–95) 0.74 (0.47–1.22) 0.28 (0.16–0.60) 2.9 ± 0.6 1.52 (0.89–2.81) 14.6 (13–17.4) 28.8 (23.6–33.2) 1.24 (1.11–1.50) | 0.91 0.97 0.03 0.11 0.23 0.68 0.19 0.08 0.21 0.26 0.06 |
| LFT Elevation on ICU Admission Hepatocellular injury Cholestatic pattern Mixed injury | 282 (46%) 101 (17%) 81 (13%) 100 (16%) | 124 (52%) 45 (19%) 35 (15%) 44 (18%) | 158 (39%) 56 (14%) 46 (12%) 56 (14%) | 0.03 0.11 0.26 0.18 |
| Characteristics | All Patients n = 609 | MASLD (+) n = 240(39.4%) | MASLD (-) n = 369(60.6%) | p Value |
|---|---|---|---|---|
| Laboratory Tests on 72nd Hour ALT (>35 IU/L) AST (>35 IU/L) ALP (>104 IU/L) GGT (>42 IU/L) TB (>1.2 mg/dL) DB (>0.3 mg/dL) | 26 (15–52) 31 (19–62) 87 (63–138) 44 (22–106) 0.69 (0.46–1.25) 0.27 (0.15–0.56) | 27 (16–48) 34 (21–68) 96 (67–146) 50 (27–123) 0.69 (0.49–1.31) 0.28 (0.16–0.6) | 25 (14–53) 28 (18–56) 85 (60–137) 42 (20–100) 0.69 (0.45–1.21) 0.27 (0.14–0.56) | 0.65 0.25 0.01 0.02 0.56 0.33 |
| LFT Elevation on 72nd Hour Hepatocellular injury Cholestatic pattern Mixed injury | 333 (55%) 153 (25%) 93 (15%) 87 (14%) | 137 (57%) 67 (28%) 35 (15%) 35 (15%) | 196 (49%) 86 (22%) 58 (15%) 52 (13%) | 0.06 0.18 0.39 0.47 |
| New-Onset LFT Elevation on 72nd Hour (n = 327 Patients with no LFT Elevation on ICU Admission) | 101 (17%) | 39 (16%) | 62 (16%) | 0.25 |
| Parenteral or Enteral Nutrition, n (%) | 510 (88%) | 202 (84%) | 308 (78%) | 0.42 |
| Requirement of respiratory support, n (%) Invasive mechanical ventilation Non-invasive mechanical ventilation High-flow nasal oxygen therapy | 284 (47%) 145 (24%) 55 (10%) | 153 (64%) 44 (18%) 18 (8%) | 131 (33%) 101 (26%) 37 (9%) | <0.01 <0.01 0.16 |
| Development of ARDS, n (%) | 24 (4%) | 14 (6%) | 10 (3%) | 0.12 |
| Central Venous Catheterization, n (%) | 343 (56%) | 169 (70%) | 174 (44%) | <0.01 |
| Nosocomial Infection | 219 (36%) | 105 (44%) | 114 (29%) | <0.01 |
| Requirement of RRT, n (%) Hemodialysis Continuous renal replacement therapy | 158 (26%) 107 (18%) | 72 (30%) 68 (28%) | 86 (22%) 39 (10%) | 0.06 <0.01 |
| Blood Product Replacement, n (%) | 229 (38%) | 119 (50%) | 110 (28%) | <0.01 |
| ICU Mortality | 216 (35%) | 147 (61%) | 69 (17%) | <0.01 |
| Length of ICU Stay | 6 (4–13) | 7 (4–17) | 6 (4–12) | 0.04 |
| Characteristics | All Patients n = 327 | New Onset Elevation (+) n = 101 (31%) | New Onset Elevation (-) n = 226 (69%) | p Value |
|---|---|---|---|---|
| Age (years) | 73 (66–81) | 72 (66–81) | 74 (65–82) | 0.15 |
| Female, n (%) | 149 (47%) | 42 (60%) | 107 (47%) | 0.2 |
| Admission source, n (%) Emergency Department Inpatient Wards | 185 (57%) 142 (43%) | 55 (79%) 46 (66%) | 130 (58%) 96 (42%) | 0.32 0.42 |
| Comorbidities, n (%) Hypertension Pulmonary disease Diabetes Mellitus Chronic renal disease Malignancies Neurological Rheumatological | 199 (44%) 135 (41%) 117 (36%) 111 (34%) 93 (28%) 84 (26%) 21 (6%) | 61 (87%) 41 (59%) 35 (50%) 36 (51%) 24 (34%) 33 (47%) 9 (13%) | 138 (61%) 94 (42%) 82 (36%) 75 (33%) 69 (31%) 51 (23%) 12 (5%) | 0.17 0.04 0.42 0.48 0.32 0.26 0.18 |
| BMI (kg/m2) | 24.8 (23.5–29.4) | 24.3 (22.5–28) | 0.45 | |
| APACHE-II Score | 20 (15–27) | 22 (15–27) | 20 (14–27) | 0.26 |
| SOFA Score | 5 (3–8) | 6 (4–10) | 4 (2–8) | <0.01 |
| Glasgow Coma Scale | 15 (12–15) | 14 (10–15) | 15 (12–15) | <0.01 |
| RIFLE stage, n (%) Risk Injury Failure Loss ESRD | 73 (22%) 43 (13%) 53 (16%) 13 (4%) 30 (9%) | 17 (24%) 15 (21%) 15 (21%) 8 (11%) 9 (13%) | 56 (25%) 28 (12%) 38 (17%) 5 (2%) 21 (9%) | 0.07 0.33 0.38 0.02 0.55 |
| Reason for ICU Admission, n (%) Respiratory failure Sepsis Renal failure Cardiac decompensation Acute neurological disorders Metabolic disturbances Surgery | 187 (57%) 153 (47%) 135 (41%) 47 (14%) 43 (13%) 13 (4%) 9 (3%) | 52 (74%) 55 (79%) 44 (63%) 14 (20%) 19 (27%) 6 (9%) 4 (6%) | 135 (60%) 98 (43%) 89 (39%) 33 (15%) 24 (11%) 7 (3%) 5 (2%) | 0.09 0.02 0.04 0.49 0.03 0.18 0.29 |
| AKI at ICU Admission, n (%) | 178 (54%) | 53 (76%) | 125 (55%) | 0.35 |
| Shock at ICU Admission, n (%) | 67 (20%) | 23 (33%) | 44 (19%) | 0.01 |
| Requirement of mechanical ventilation, n (%) | ||||
| Invasive mechanical ventilation | 126 (39%) | 54 (54%) | 72 (32%) | 0.01 |
| Non-invasive mechanical ventilation | 91 (28%) | 23 (23%) | 68 (30%) | 0.18 |
| Presence of MASLD | 116 (35%) | 39 (56%) | 77 (34%) | 0.25 |
| Hepatic Steatosis Grade Grade 1 Grade 2 Grade 3 | 69 (21%) 39 (12%) 20 (6%) | 24 (34%) 12 (17%) 5 (7%) | 45 (20%) 27 (12%) 15 (7%) | 0.18 0.39 0.47 |
| ICU Mortality | 94 (29%) | 40 (57%) | 54 (24%) | <0.01 |
| Adjusted OR [95% CI] | p Value | |
|---|---|---|
| Mortality (n = 609) | ||
| Invasive Mechanical Ventilation | 21.6 [9.7–48.7] | <0.01 |
| Shock | 4.3 [2.2–8.4] | <0.01 |
| APACHE-II Score | 1.06 [1.02–1.12] | 0.01 |
| New-Onset LFT Elevation (n = 327) | ||
| SOFA Score on ICU Admission | 1.09 [1.03–1.17] | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karataş, A.; İnci, K.; Dündar, N.B.; Aygencel, G.; Türkoğlu, M.; Taş, A.O.; Avcı, B.; Gedik, C.; Cindoruk, M. Association of MASLD with Baseline and New-Onset Liver Function Test Elevation in Medical ICU Patients. Medicina 2025, 61, 2092. https://doi.org/10.3390/medicina61122092
Karataş A, İnci K, Dündar NB, Aygencel G, Türkoğlu M, Taş AO, Avcı B, Gedik C, Cindoruk M. Association of MASLD with Baseline and New-Onset Liver Function Test Elevation in Medical ICU Patients. Medicina. 2025; 61(12):2092. https://doi.org/10.3390/medicina61122092
Chicago/Turabian StyleKarataş, Ali, Kamil İnci, Nazlıhan Boyacı Dündar, Gülbin Aygencel, Melda Türkoğlu, Ali Osman Taş, Beril Avcı, Cansu Gedik, and Mehmet Cindoruk. 2025. "Association of MASLD with Baseline and New-Onset Liver Function Test Elevation in Medical ICU Patients" Medicina 61, no. 12: 2092. https://doi.org/10.3390/medicina61122092
APA StyleKarataş, A., İnci, K., Dündar, N. B., Aygencel, G., Türkoğlu, M., Taş, A. O., Avcı, B., Gedik, C., & Cindoruk, M. (2025). Association of MASLD with Baseline and New-Onset Liver Function Test Elevation in Medical ICU Patients. Medicina, 61(12), 2092. https://doi.org/10.3390/medicina61122092






