Sex Differences in Autoimmune Multimorbidity Across Eleven Disorders: A Real-World Primary Care Study in Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
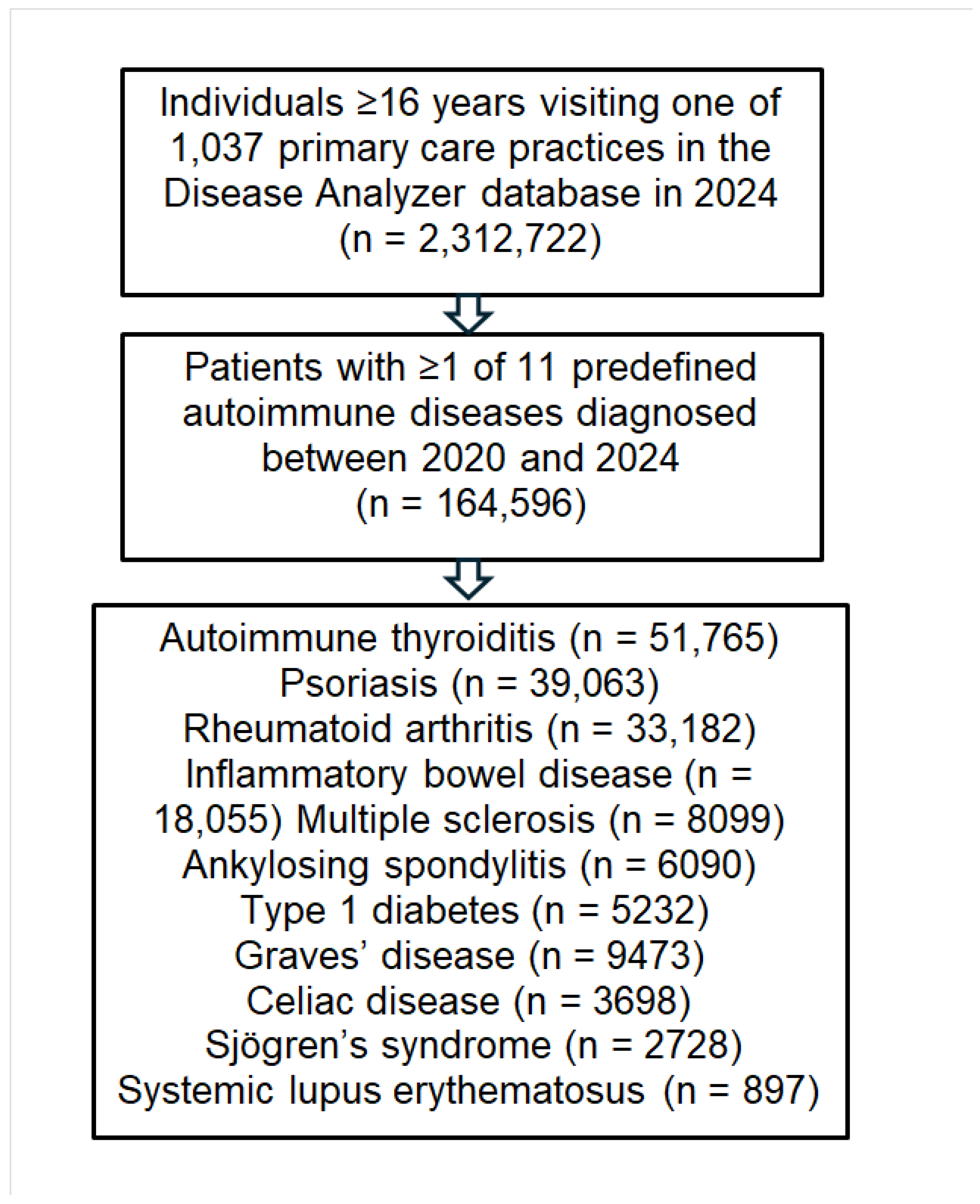
2.2. Study Population
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
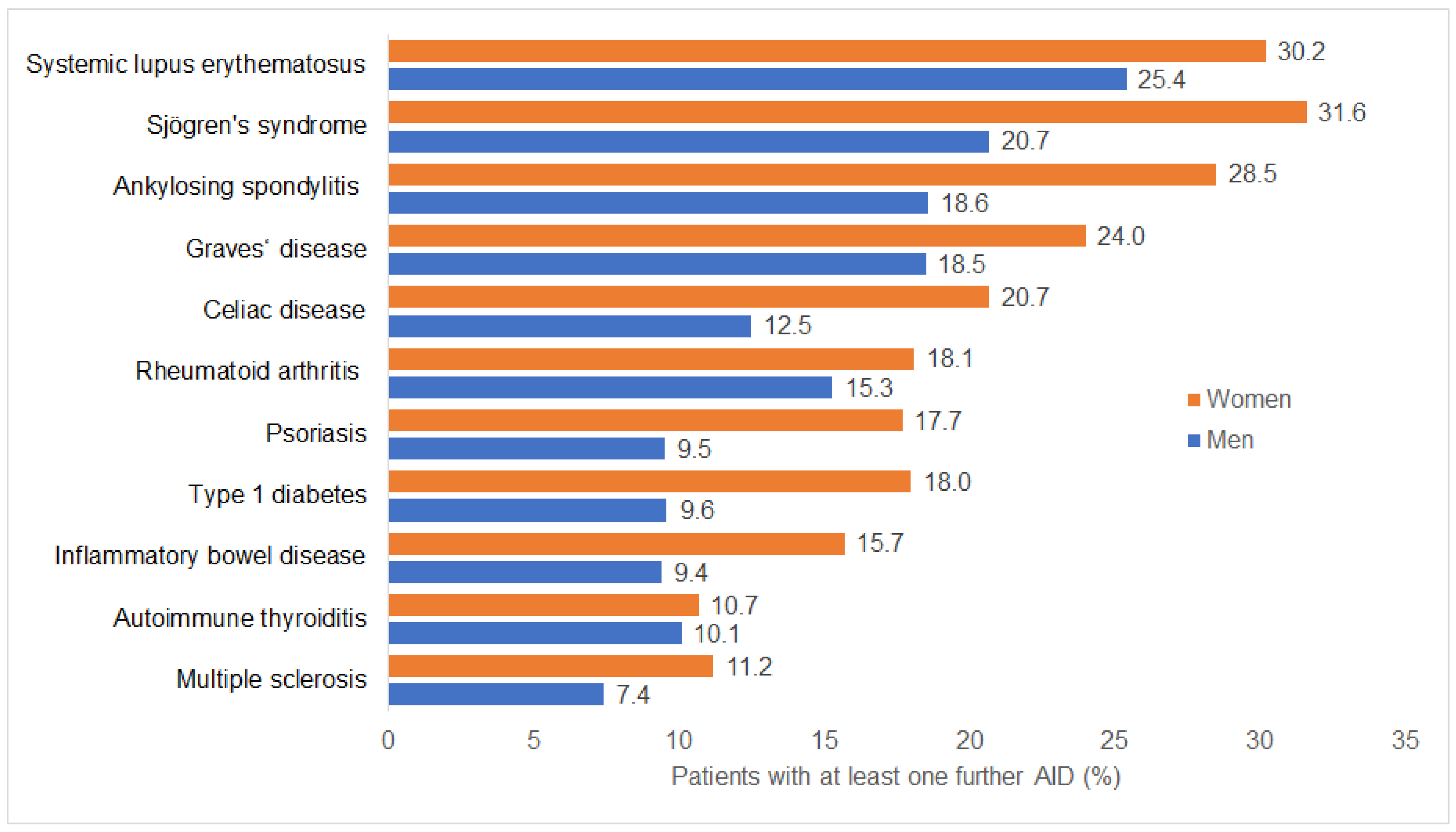
3.2. Autoimmune Multimorbidity by Disease and Sex
3.3. Most Frequent Coexisting Autoimmune Disorders
4. Discussion
4.1. Clinical Implications
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef]
- Miller, F.W. The increasing prevalence of autoimmunity and autoimmune diseases: An urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr. Opin. Immunol. 2023, 80, 102266. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. Lancet 2023, 401, 1878–1890. [Google Scholar] [CrossRef]
- Angum, F.; Khan, T.; Kaler, J.; Siddiqui, L.; Hussain, A. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus 2020, 12, e8094. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, A.; Yalcinkaya, R.; Sardh, F.; Landegren, N. Immune dynamics throughout life in relation to sex hormones and perspectives gained from gender-affirming hormone therapy. Front. Immunol. 2025, 15, 1501364. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, F.; Campolo, F.; Franceschini, E.; Carlomagno, F.; Venneri, M.A. Gender-Specific Impact of Sex Hormones on the Immune System. Int. J. Mol. Sci. 2023, 24, 6302. [Google Scholar] [CrossRef]
- Tripathi, P.; Bhushan, D.; Banerjee, A.; Mahto, M. The Kaleidoscope of Polyautoimmunity: An Odyssey of Diagnostic Dilemmas. Cureus 2024, 16, e57799. [Google Scholar] [CrossRef]
- Sohn, S.Y.; Ahn, J.; Lee, M.K.; Lee, J.H.; Kwon, J.W.; Kweon, J.M.; Lee, J.Y. Risk of non-thyroidal autoimmune diseases in patients with Graves’ disease: A nationwide retrospective cohort study. Rheumatology 2025, 64, 303–309. [Google Scholar] [CrossRef]
- Graven-Nielsen, C.S.; Vittrup, I.V.; Kragh, A.J.; Lund, F.; Bliddal, S.; Kofoed, K.; Kristensen, S.; Stensballe, A.; Nielsen, C.H.; Feldt-Rasmussen, U.; et al. Polyautoimmunity in patients with cutaneous lupus erythematosus: A nationwide sex- and age-matched cohort study from Denmark. JAAD Int. 2023, 13, 126–133. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Vorobyev, A.; Bieber, K.; Kridin, K.; Ludwig, R.J. Risk of comorbid autoimmune diseases in patients with immunobullous disorders: A global large-scale cohort study. J. Am. Acad. Dermatol. 2023, 89, 1269–1271. [Google Scholar] [CrossRef]
- Santos-Moreno, P.; Arias-Aponte, J.; Rodríguez-Vargas, G.S.; Nieto-Zambrano, P.D.; Villarreal, L.; Ibatá, L.; Martinez, S.; Rubio-Rubio, J.A.; Rodríguez, P.; Rojas-Villarraga, A. Polyautoimmunity in systemic lupus erythematosus patients: New insights from a cross-sectional study. J. Transl. Autoimmun. 2023, 6, 100187. [Google Scholar] [CrossRef] [PubMed]
- AlAhmed, O.; Sivaraman, V.; Moore-Clingenpeel, M.; Ardoin, S.P.; Bout-Tabaku, S.; CARRA Registry Investigators. Autoimmune thyroid diseases, autoimmune hepatitis, celiac disease and type 1 diabetes mellitus in pediatric systemic lupus erythematosus: Results from the CARRA Legacy Registry. Lupus 2020, 29, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, W.; Bongaerts, B.; Carius, H.J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef]
- Zingel, R.; Jacob, L.; Smith, L.; Konrad, M.; Kostev, K. Association Between Psoriasis and Dementia: A Retrospective Cohort Study. J. Alzheimers Dis. Rep. 2023, 7, 41–49. [Google Scholar] [CrossRef]
- Loosen, S.H.; Kostev, K.; Schöler, D.; Orth, H.M.; Freise, N.F.; Jensen, B.O.; May, P.; Bode, J.G.; Roderburg, C.; Luedde, T. Infectious mononucleosis is associated with an increased incidence of Crohn’s disease: Results from a cohort study of 31 862 outpatients in Germany. Eur. J. Gastroenterol. Hepatol. 2023, 35, 255–260. [Google Scholar] [CrossRef]
- Fornaro, M.; Venerito, V.; Pellico, M.R.; Iannone, F.; Joshi, M.; Chen, Y.M.; Tan, A.L.; Saha, S.; Chatterjee, T.; Agarwal, V.; et al. The impact of multimorbidity on Quality of Life in inflammatory myopathies: A cluster analysis from the COVAD dataset. Rheumatology 2025, 64, 2133–2142. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Wei, M.Y.; Kim, C.; Lee, J.M. Sex Differences in Autoimmune Multimorbidity in Type 1 Diabetes Mellitus and the Risk of Cardiovascular and Renal Disease: A Longitudinal Study in the United States, 2001–2017. J. Womens Health 2020, 29, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.K.; Brinton, R.D. Autoimmune Disease in Women: Endocrine Transition and Risk Across the Lifespan. Front. Endocrinol. 2019, 10, 265. [Google Scholar] [CrossRef]
- Coates, L.C.; FitzGerald, O.; Helliwell, P.S.; Paul, C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same? Semin. Arthritis Rheum. 2016, 46, 291–304. [Google Scholar] [CrossRef]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef] [PubMed]
- Icen, M.; Nicola, P.J.; Maradit-Kremers, H.; Crowson, C.S.; Therneau, T.M.; Matteson, E.L.; Gabriel, S.E. Systemic lupus erythematosus features in rheumatoid arthritis and their effect on overall mortality. J. Rheumatol. 2009, 36, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.A.; Kawabata, H.; Ray, N.; Baheti, A.; Suissa, S.; Esdaile, J.M. Prevalence of Co-existing Autoimmune Disease in Rheumatoid Arthritis: A Cross-Sectional Study. Adv. Ther. 2017, 34, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Naha, K.; Hande, M.; Vivek, G. Hot and cold: Coexistent Graves’ disease and Hashimoto’s thyroiditis in a patient with Schmidt’s syndrome. BMJ Case Rep. 2014, 2014, bcr2013010432. [Google Scholar] [CrossRef]
- Tamai, H.; Kasagi, K.; Takaichi, Y.; Takamatsu, J.; Komaki, G.; Matsubayashi, S.; Konishi, J.; Kuma, K.; Kumagai, L.F.; Nagataki, S. Development of spontaneous hypothyroidism in patients with Graves’ disease treated with antithyroidal drugs: Clinical, immunological, and histological findings in 26 patients. J. Clin. Endocrinol. Metab. 1989, 69, 49–53. [Google Scholar] [CrossRef]
- Rubtsov, A.V.; Rubtsova, K.; Kappler, J.W.; Marrack, P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun. Rev. 2010, 9, 494–498. [Google Scholar] [CrossRef] [PubMed]
| Disease | Number of Patients | Age (Mean, SD) | Female (N, %) | Male (N, %) |
|---|---|---|---|---|
| Psoriasis | 39,063 | 59.2 (16.9) | 20,194 (51.7) | 18,869 (48.3) |
| Rheumatoid arthritis | 33,182 | 65.3 (15.4) | 22,649 (68.3) | 10,533 (31.7) |
| Systemic lupus erythematosus | 897 | 54.1 (16.6) | 771 (86.0) | 126 (14.0) |
| Autoimmune thyroiditis | 51,765 | 54.1 (16.5) | 44,039 (85.1) | 7726 (14.9) |
| Inflammatory bowel disease | 18,055 | 53.2 (18.0) | 9757 (54.0) | 8298 (46.0) |
| Multiple sclerosis | 8099 | 52.7 (15.7) | 5653 (69.8) | 2446 (30.2) |
| Celiac disease | 3698 | 46.5 (18.2) | 2689 (72.7) | 1009 (27.3) |
| Ankylosing spondylitis | 6090 | 57.2 (16.1) | 2706 (44.4) | 3384 (55.6) |
| Type 1 diabetes | 5232 | 58.9 (19.3) | 2142 (40.9) | 3090 (59.1) |
| Graves’ disease | 9473 | 57.7 (16.8) | 7550 (79.7) | 1923 (20.3) |
| Sjögren’s syndrome | 2728 | 64.8 (16.2) | 2106 (77.2) | 622 (22.8) |
| Disease Affected by Autoimmune Multimorbidity | Most Frequent Coexisting AID | Proportion of Patients with a Co-Diagnosis (%) | |
|---|---|---|---|
| Women | Men | ||
| Psoriasis | Rheumatoid arthritis | 7.9 | 4.7 |
| Rheumatoid arthritis | Psoriasis | 7.1 | 8.4 |
| Systemic lupus erythematosus | Rheumatoid arthritis | 17.5 | 10.3 |
| Autoimmune thyroiditis | Rheumatoid arthritis | 3.1 | 1.9 |
| Inflammatory bowel disease | Rheumatoid arthritis | 5.0 | 2.8 |
| Multiple sclerosis | Autoimmune thyroiditis | 4.5 | 0.9 |
| Celiac disease | Autoimmune thyroiditis | 11.0 | 2.9 |
| Ankylosing spondylitis | Rheumatoid arthritis | 14.5 | 8.7 |
| Type 1 diabetes | Autoimmune thyroiditis | 7.7 | 2.2 |
| Graves’ disease | Autoimmune thyroiditis | 15.7 | 10.3 |
| Sjögren’s syndrome | Rheumatoid arthritis | 14.0 | 8.8 |
| Disease | OR (Females vs. Males (95% CI)) | p Value, FDR |
|---|---|---|
| Psoriasis | 2.10 (1.97–2.24) | <0.001 |
| Rheumatoid arthritis | 1.29 (1.21–1.39) | <0.001 |
| Systemic lupus erythematosus | 1.43 (0.93–2.17) | 0.1001 |
| Autoimmune thyroiditis | 1.09 (1.00–1.19) | 0.0515 |
| Inflammatory bowel disease | 1.79 (1.62–1.98) | <0.001 |
| Multiple sclerosis | 1.56 (1.31–1.86) | <0.001 |
| Celiac disease | 1.88 (1.51–2.34) | <0.001 |
| Ankylosing spondylitis | 1.74 (1.54–1.95) | <0.001 |
| Type 1 diabetes | 2.15 (1.80–2.57) | <0.001 |
| Graves’ disease | 1.40 (1.24–1.58) | <0.001 |
| Sjögren’s syndrome | 1.76 (1.35–2.29) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostev, K.; Kaur, N.; Höfle, J.; Konrad, M.; Hammed, A.; Tanislav, C.; Rodemer, I. Sex Differences in Autoimmune Multimorbidity Across Eleven Disorders: A Real-World Primary Care Study in Germany. Medicina 2025, 61, 2091. https://doi.org/10.3390/medicina61122091
Kostev K, Kaur N, Höfle J, Konrad M, Hammed A, Tanislav C, Rodemer I. Sex Differences in Autoimmune Multimorbidity Across Eleven Disorders: A Real-World Primary Care Study in Germany. Medicina. 2025; 61(12):2091. https://doi.org/10.3390/medicina61122091
Chicago/Turabian StyleKostev, Karel, Nimran Kaur, Judith Höfle, Marcel Konrad, Ali Hammed, Christian Tanislav, and Ira Rodemer. 2025. "Sex Differences in Autoimmune Multimorbidity Across Eleven Disorders: A Real-World Primary Care Study in Germany" Medicina 61, no. 12: 2091. https://doi.org/10.3390/medicina61122091
APA StyleKostev, K., Kaur, N., Höfle, J., Konrad, M., Hammed, A., Tanislav, C., & Rodemer, I. (2025). Sex Differences in Autoimmune Multimorbidity Across Eleven Disorders: A Real-World Primary Care Study in Germany. Medicina, 61(12), 2091. https://doi.org/10.3390/medicina61122091






