Impact of Probiotics on Atopic Dermatitis in Pediatric Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
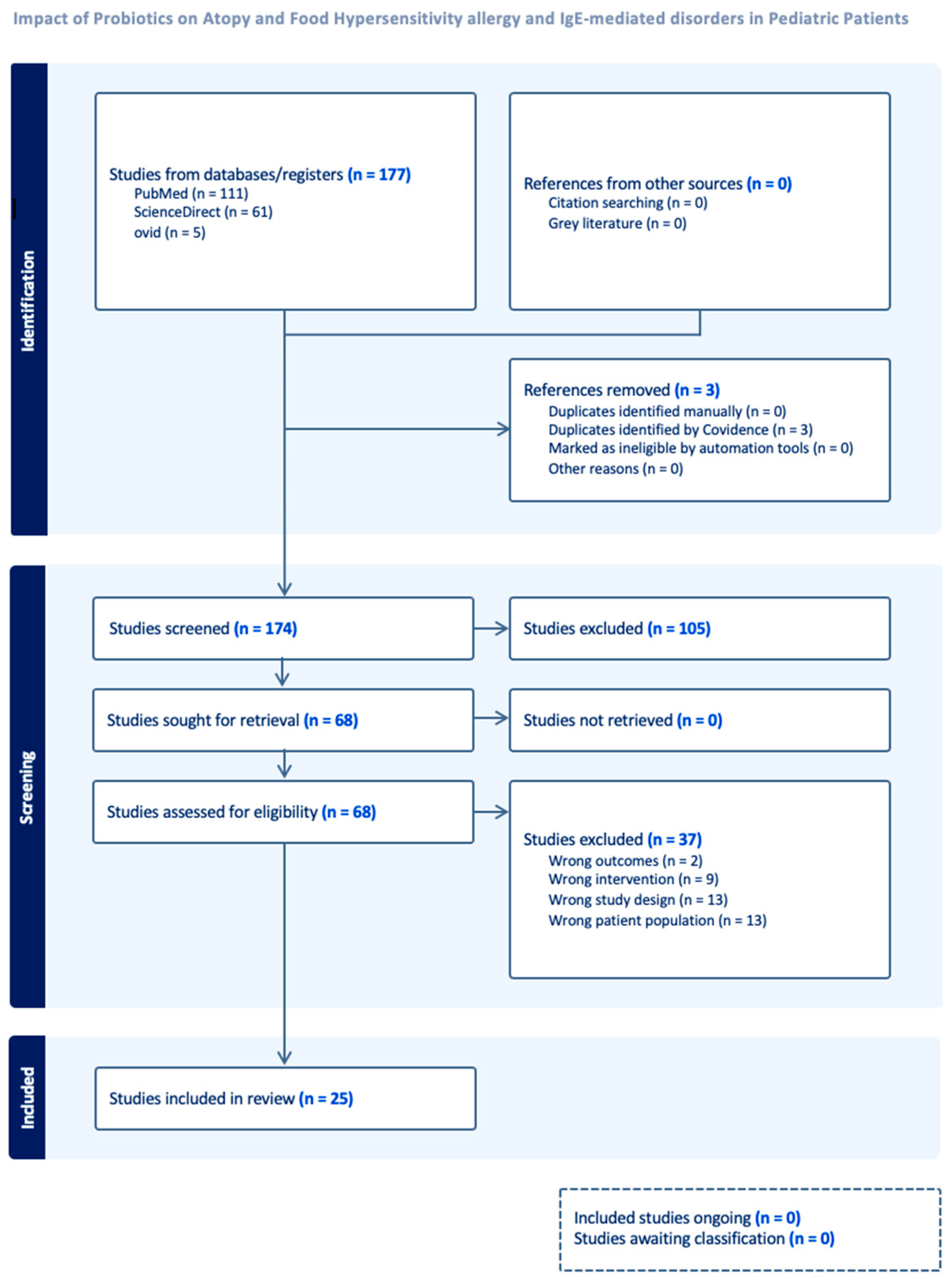
2.1. Study Selection and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Risk of Bias Assessment
3. Results
3.1. Study and Patient Characteristics
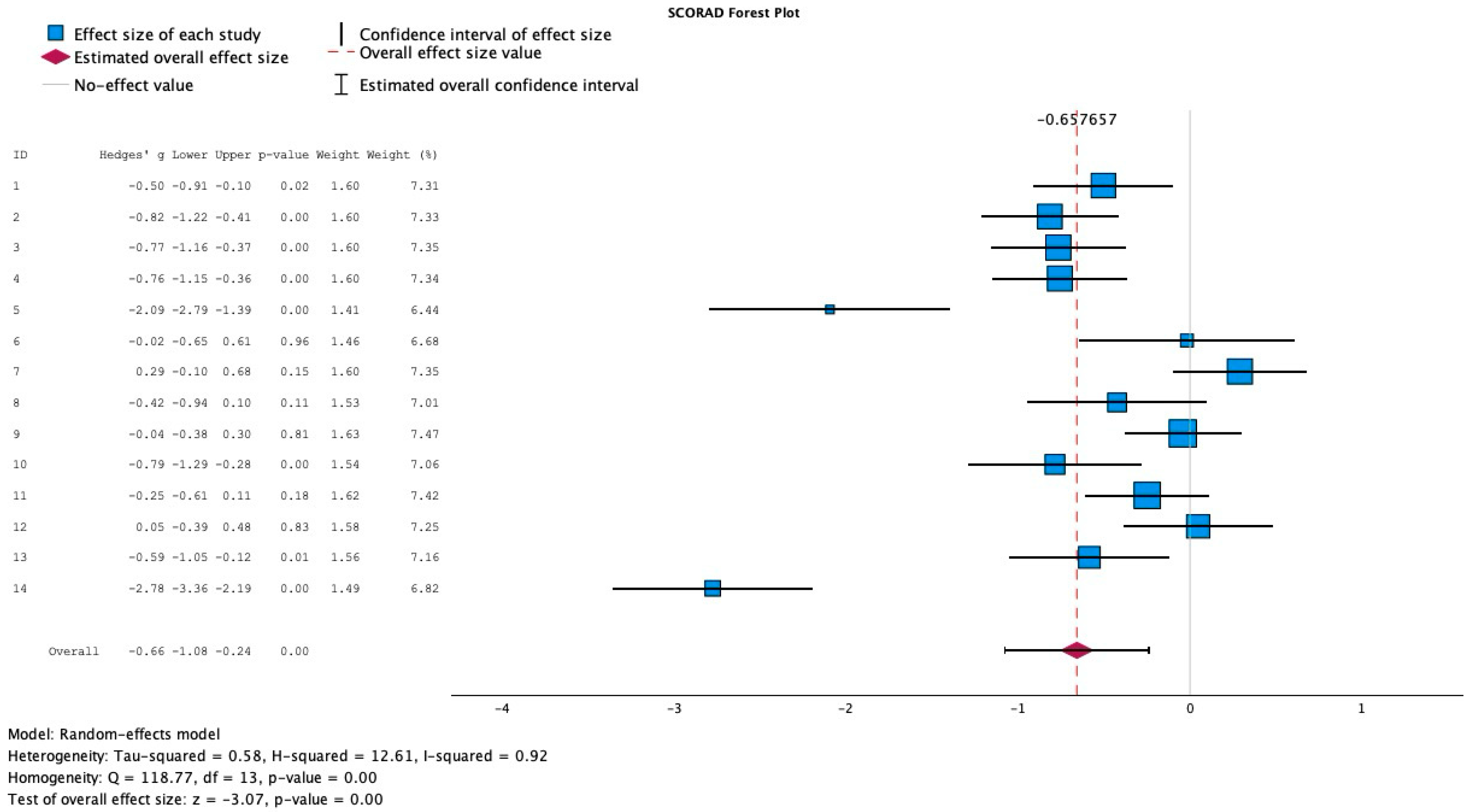
3.2. Effect of Probiotics on the Extent and Severity of AD
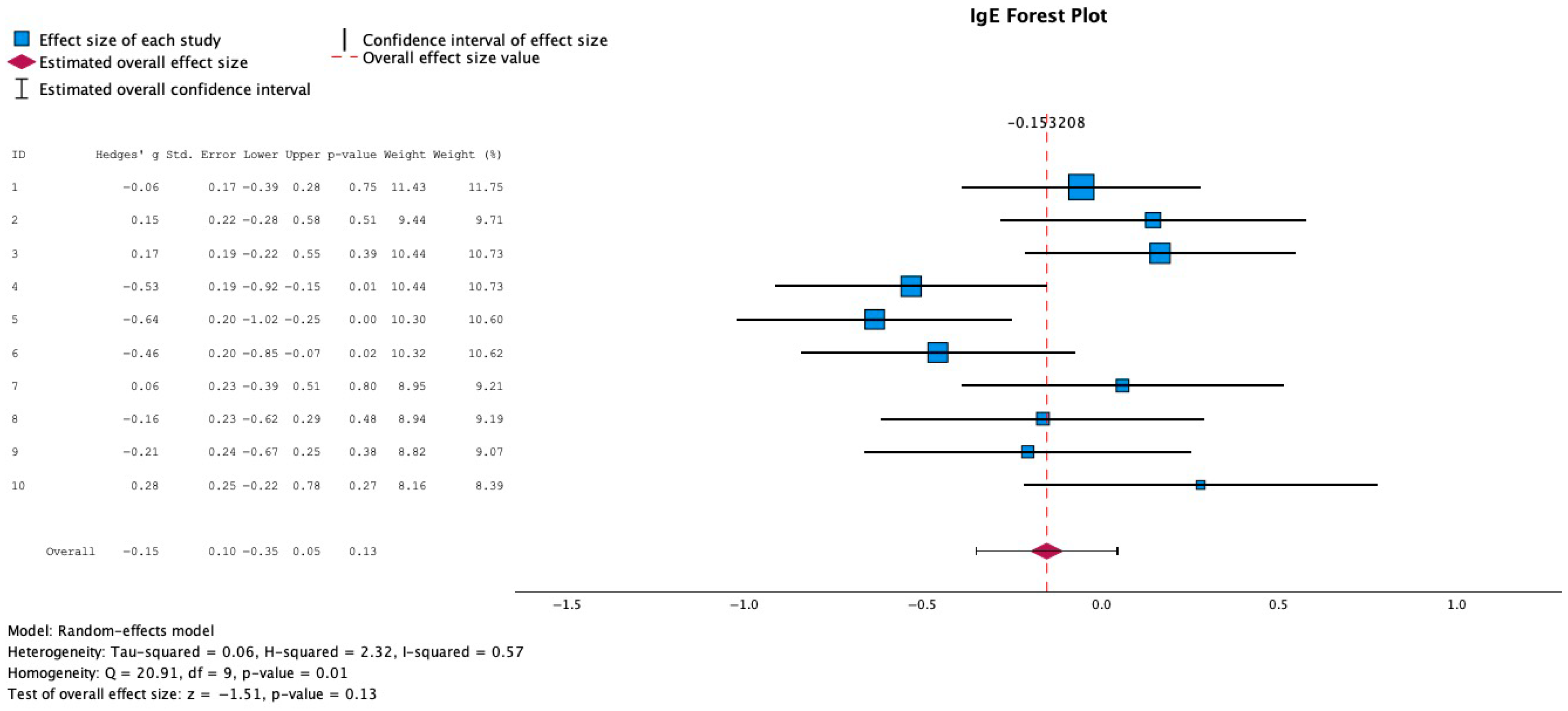
3.3. Effect of Probiotics on IgE Levels
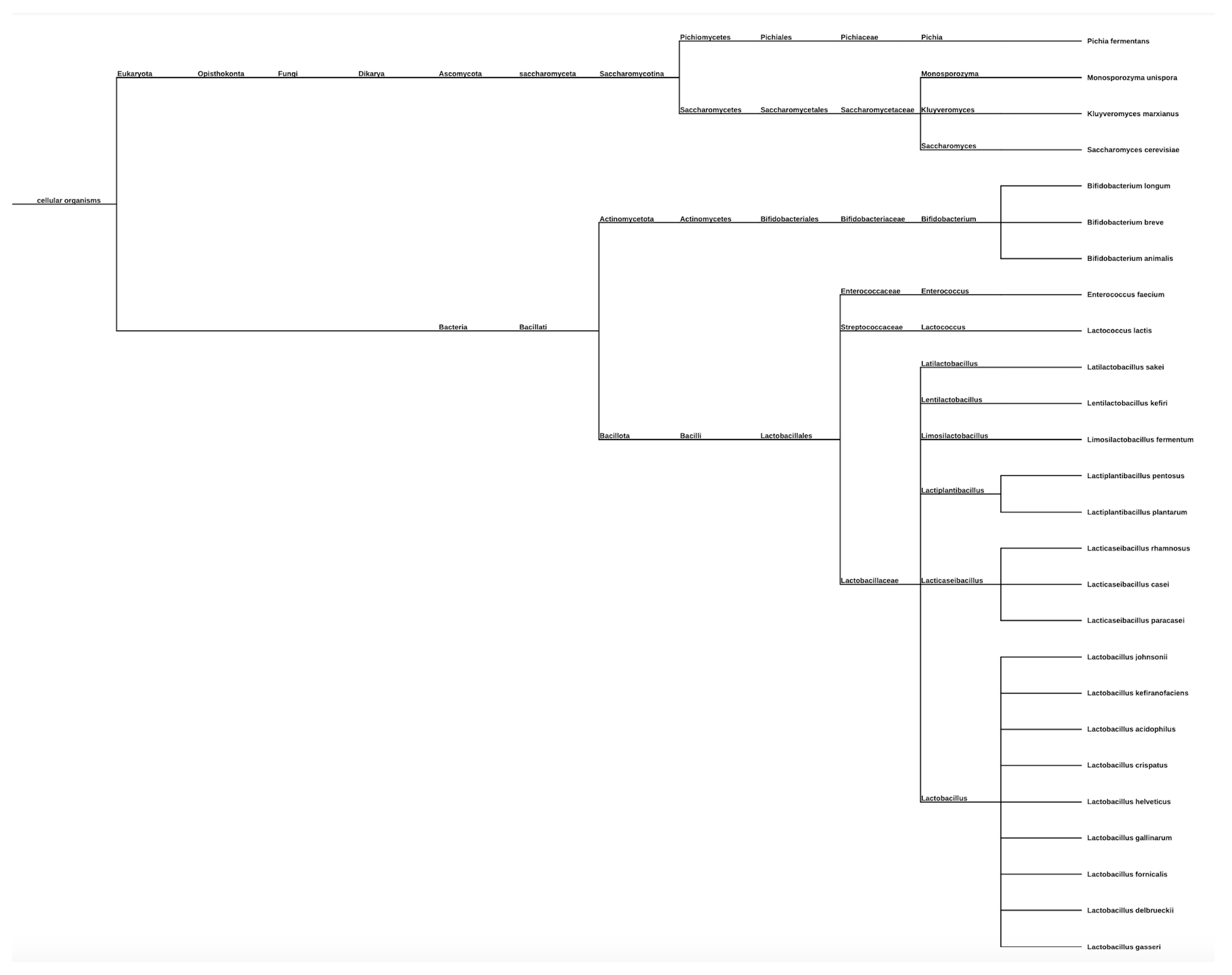
3.4. Probiotic Strain Types: Phylogenetic Tree Representation
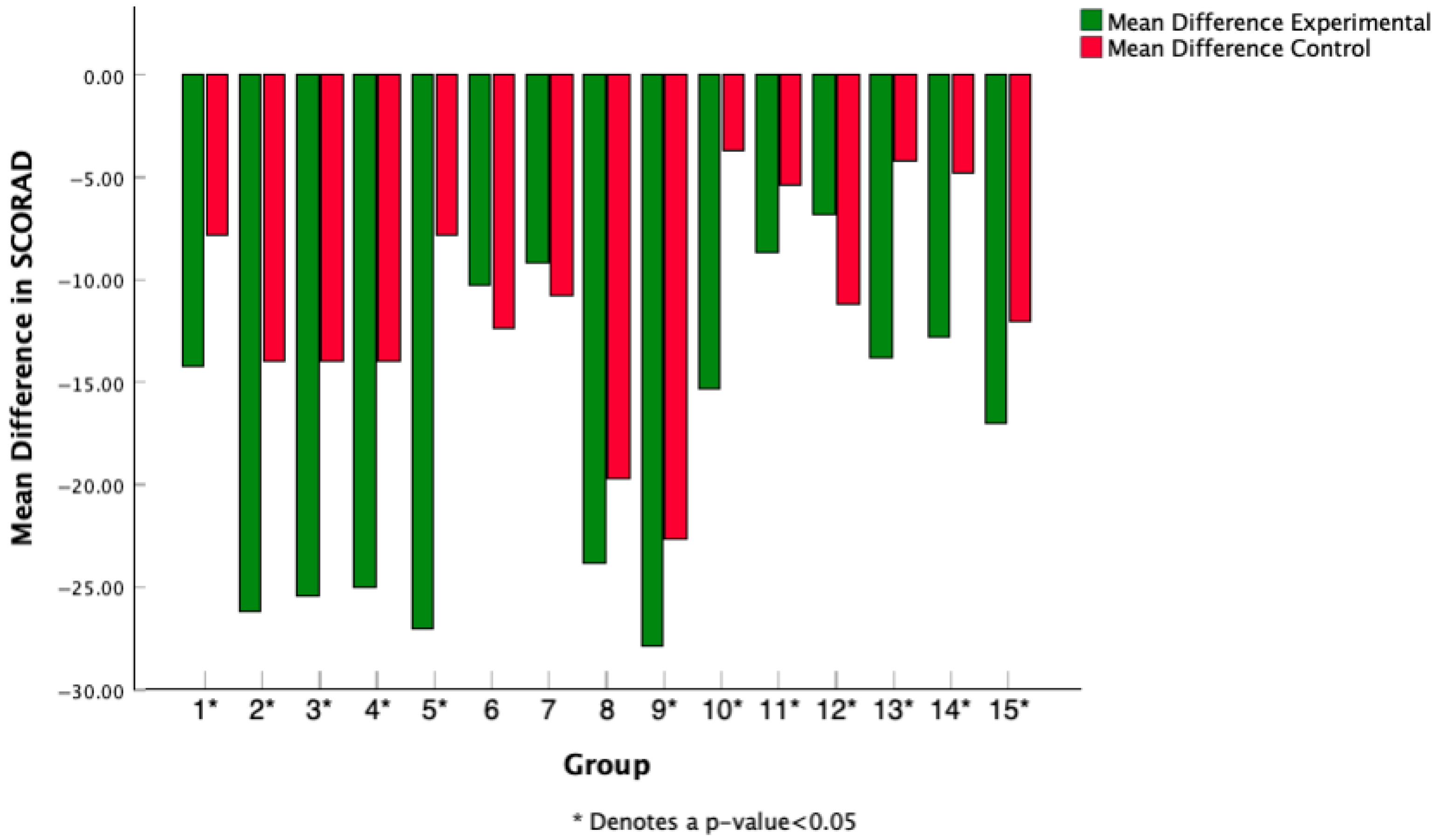
3.5. Mean Difference in SCORAD Change Across Studies
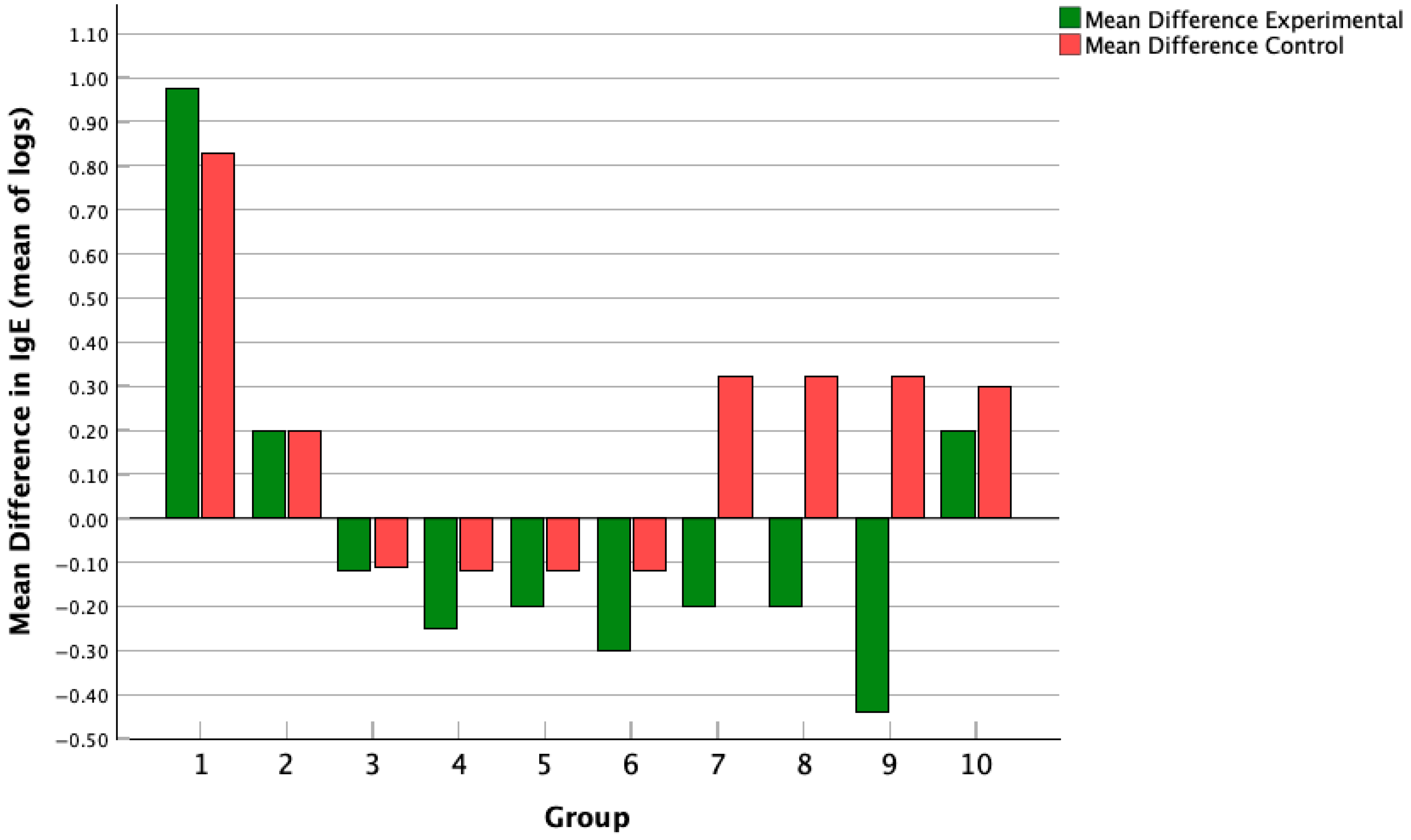
3.6. Mean Difference in IgE Change Across Studies
3.7. Single vs. Combined Probiotic Strain vs. Intervention vs. Placebo SCORAD Scoring
3.8. Risk of Bias Assessment of Results
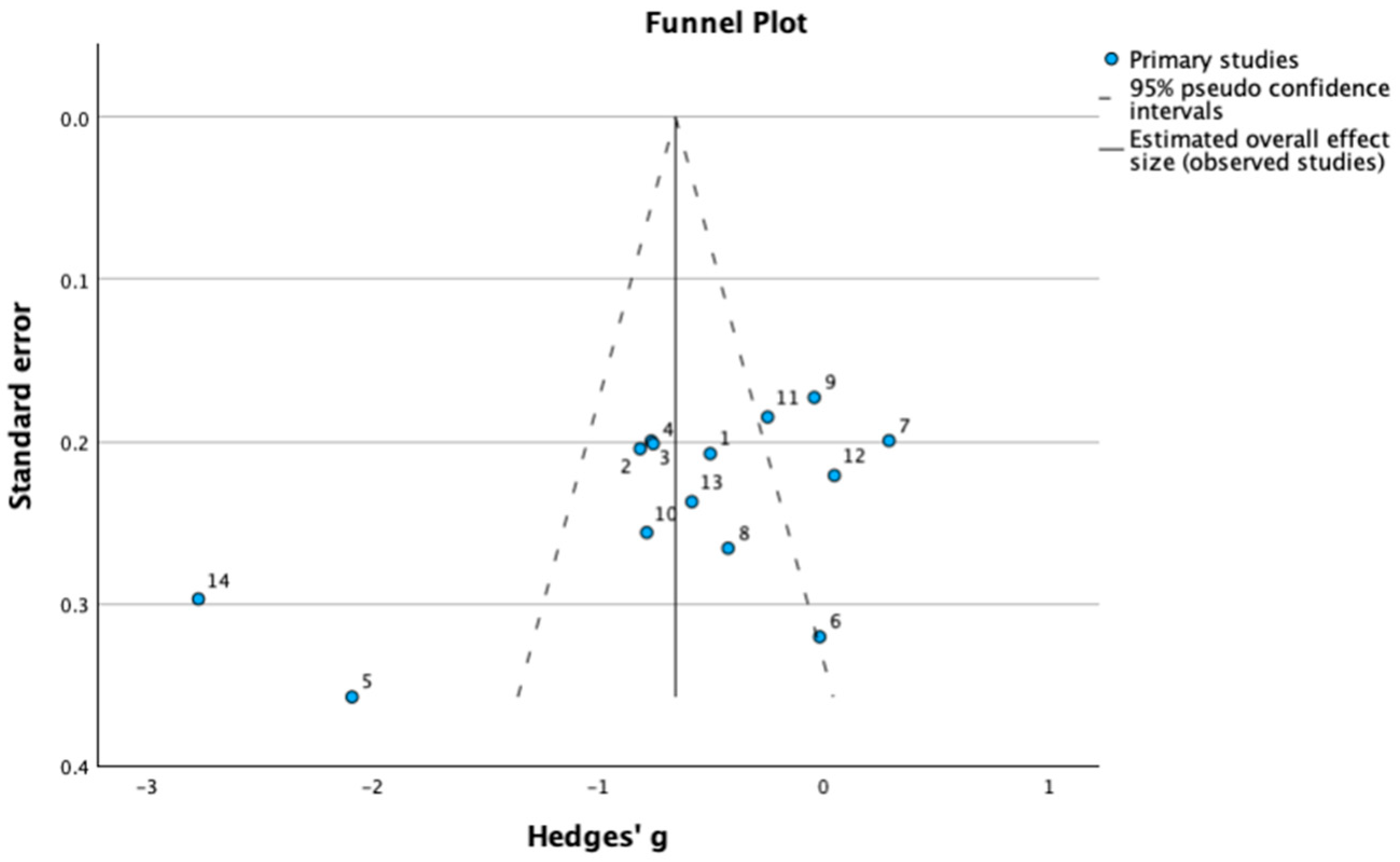
3.9. Assessment of Publication Bias
3.10. Sensitivity Analysis
4. Discussion
Limitations
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.; Bergman, E.A.; Silverberg, J.I. Atopic Dermatitis (Eczema): Pathogenesis, Clinical Manifestations, and Diagnosis; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 2025. [Google Scholar]
- Meledathu, S.; Naidu, M.P.; Brunner, P.M. Update on atopic dermatitis. J. Allergy Clin. Immunol. 2025, 155, 1124–1132. [Google Scholar] [CrossRef]
- Fasseeh, A.N.; Elezbawy, B.; Korra, N.; Tannira, M.; Dalle, H.; Aderian, S.; Abaza, S.; Kaló, Z. Burden of atopic dermatitis in adults and adolescents: A systematic literature review. Dermatol. Ther. 2022, 12, 2653–2668. [Google Scholar] [CrossRef]
- Bai, R.; Zheng, Y.; Dai, X. Atopic dermatitis: Diagnosis, molecular pathogenesis, and therapeutics. Mol. Biomed. 2025, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011, 2, 110. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the skin and gut in atopic dermatitis (AD): Understanding the pathophysiology and finding novel management strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Pallanti, S.; Lotti, T.; Urpe, M. Psychoneuroimmunodermatology of atopic dermatitis: From empiric data to the evolutionary hypothesis. Dermatol. Clin. 2005, 23, 695–701. [Google Scholar] [CrossRef]
- Arndt, J.; Smith, N.; Tausk, F. Stress and atopic dermatitis. Curr. Allergy Asthma Rep. 2008, 8, 312–317. [Google Scholar] [CrossRef]
- Rather, I.A.; Kim, B.C.; Lew, L.C.; Cha, S.K.; Lee, J.H.; Nam, G.J.; Majumder, R.; Lim, J.; Lim, S.K.; Seo, Y.J.; et al. Oral administration of live and dead cells of Lactobacillus sakei proBio65 alleviated atopic dermatitis in children and adolescents: A randomized, double-blind, and placebo-controlled study. Probiotics Antimicrob. Proteins 2021, 13, 315–326. [Google Scholar] [CrossRef]
- Brouwer, M.L.; Wolt-Plompen, S.A.; Dubois, A.E.; Van Der Heide, S.; Jansen, D.F.; Hoijer, M.A.; Kauffman, H.F.; Duiverman, E.J. No effects of probiotics on atopic dermatitis in infancy: A randomized placebo-controlled trial. Clin. Exp. Allergy 2006, 36, 899–906. [Google Scholar] [CrossRef]
- Weston, S.; Halbert, A.; Richmond, P.; Prescott, S.L. Effects of probiotics on atopic dermatitis: A randomised controlled trial. Arch. Dis. Child. 2005, 90, 892–897. [Google Scholar] [CrossRef]
- Isolauri, E.; Arvola, T.; Sütas, Y.; Moilanen, E.; Salminen, S. Probiotics in the management of atopic eczema. Clin. Exp. Allergy 2000, 30, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, S.V.; Vasjuta, V.V.; Myhovych, O.O.; Bondarchuk, L.I. Probiotic Supplement Reduces Atopic Dermatitis in Preschool Children. Am. J. Clin. Dermatol. 2010, 11, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.; Wang, J.Y. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clin. Exp. Allergy 2015, 45, 779–787. [Google Scholar] [CrossRef]
- Navarro-López, V.; Ramírez-Boscá, A.; Ramón-Vidal, D.; Ruzafa-Costas, B.; Genovés-Martínez, S.; Chenoll-Cuadros, E.; Carrión-Gutiérrez, M.; de la Parte, J.H.; Prieto-Merino, D.; Codoñer-Cortés, F.M. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD Index and Use of Topical Steroids in Young Patients with Moderate Atopic Dermatitis. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nermes, M.; Kantele, J.M.; Atosuo, T.J.; Salminen, S.; Isolauri, E. Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clin. Exp. Allergy 2010, 41, 370–377. [Google Scholar] [CrossRef]
- Grüber, C.; Wendt, M.; Sulser, C.; Lau, S.; Kulig, M.; Wahn, U.; Werfel, T.; Niggemann, B. Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 2007, 62, 1270–1276. [Google Scholar] [CrossRef]
- D’Auria, E.; Panelli, S.; Lunardon, L.; Pajoro, M.; Paradiso, L.; Beretta, S.; Loretelli, C.; Tosi, D.; Perini, M.; Bedogni, G.; et al. Rice flour fermented with Lactobacillus paracasei CBA L74 and atopic dermatitis in infants: A randomized controlled trial. Pharmacol Res. 2021, 167, 105284. [Google Scholar] [CrossRef]
- Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients 2021, 13, 1169. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, M.; Jeon, S.A.; Kim, Y.-H.; Lee, S. A randomized trial of Lactobacillus rhamnosus IDCC 3201 tyndallizate (RHT3201) for treating atopic dermatitis. Pediatr. Allergy Immunol. 2020, 31, 783–792. [Google Scholar] [CrossRef]
- Han, Y.; Kim, B.; Ban, J.; Lee, J.; Kim, B.J.; Choi, B.S.; Hwang, S.; Ahn, K.; Kim, J. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2012, 23, 667–673. [Google Scholar] [CrossRef]
- Ahn, S.H.; Yoon, W.; Lee, S.Y.; Shin, H.S.; Lim, M.Y.; Nam, Y.-D.; Yoo, Y. Effects of Lactobacillus pentosus in Children with Allergen-Sensitized Atopic Dermatitis. J. Korean Med Sci. 2020, 35, e128. [Google Scholar] [CrossRef]
- Woo, S.I.; Kim, J.Y.; Lee, Y.J.; Kim, N.S.; Hahn, Y.S. Effect of Lactobacillus sakei supplementation in children with atopic eczema–dermatitis syndrome. Ann. Allergy Asthma Immunol. 2010, 104, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Carucci, L.; Nocerino, R.; Paparo, L.; De Filippis, F.; Coppola, S.; Giglio, V.; Cozzolino, T.; Valentino, V.; Sequino, G.; Bedogni, G.; et al. Therapeutic effects elicited by the probiotic Lacticaseibacillusrhamnosus GG in children with atopic dermatitis. The results of the ProPAD trial. Pediatr. Allergy Immunol. 2022, 33, e13836. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chie, W.-C.; Wang, I.-J. Efficacy of Lactobacillus Administration in School-Age Children with Asthma: A Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 1678. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, G.V.; de Lorena, V.M.B.; Montenegro, S.M.L.; de Albuquerque, E.C.; Peixoto, D.M.; Sarinho, E.S.C. Probiotics as an adjunct for the treatment of recurrent wheezing in infants and effects on expression of T-helper 1 and regulatory T cytokines. J. Funct. Foods 2017, 35, 398–407. [Google Scholar] [CrossRef]
- Basturk, A.; Isik, İ.; Atalay, A.; Yılmaz, A. Investigation of the Efficacy of Lactobacillus rhamnosus GG in Infants with Cow’s Milk Protein Allergy: A Randomised Double-Blind Placebo-Controlled Trial. Probiotics Antimicrob. Prot. 2020, 12, 138–143. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, Y.L.; Jan, R.L.; Chen, H.H.; Wang, J.Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010, 45, 1111–1120. [Google Scholar] [CrossRef]
- Torii, S.; Torii, A.; Itoh, K.; Urisu, A.; Terada, A.; Fujisawa, T.; Yamada, K.; Suzuki, H.; Ishida, Y.; Nakamura, F.; et al. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum markers of atopic dermatitis in children. Int. Arch. Allergy Immunol. 2011, 154, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Jerzyńska, J.; Stelmach, W.; Balcerak, J.; Woicka-Kolejwa, K.; Rychlik, B.; Blauz, A.; Wachulec, M.; Stelmach, P.; Majak, P.; Stelmach, I. Effect of Lactobacillus rhamnosus GG and vitamin D supplementation on the immunologic effectiveness of grass-specific sublingual immunotherapy in children with allergy. Allergy Asthma Proc. 2016, 37, 324–334. [Google Scholar] [CrossRef]
- Hol, J.; van Leer, E.H.; Elink Schuurman, B.E.; de Ruiter, L.F.; Samsom, J.N.; Hop, W.; Neijens, H.J.; Kimpen, J.L.L.; Penders, J.; Dobrindt, U.; et al. The acquisition of tolerance toward cow’s milk through probiotic supplementation: A randomized, controlled trial. J. Allergy Clin. Immunol. 2008, 121, 1448–1454. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef]
- Wang, M.F.; Lin, H.C.; Wang, Y.Y.; Hsu, C.H. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr. Allergy Immunol. 2004, 15, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Di Marino, V.P.; Olivero, F.; De Canditiis, D.; Brindisi, G.; Iannilli, F.; De Castro, G.; Zicari, A.M.; Duse, M. Treatment with a Probiotic Mixture Containing Bifidobacterium animalis Subsp. Lactis BB12 and Enterococcus faecium L3 for the Prevention of Allergic Rhinitis Symptoms in Children: A Randomized Controlled Trial. Nutrients 2021, 13, 1315. [Google Scholar] [PubMed]
- Sestito, S.; D’Auria, E.; Baldassarre, M.E.; Salvatore, S.; Tallarico, V.; Stefanelli, E.; Tarsitano, F.; Concolino, D.; Pensabene, L. The role of prebiotics and probiotics in prevention of allergic diseases in infants. Front. Pediatr. 2020, 8, 583946. [Google Scholar] [CrossRef]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Malaty, H.M. Epidemiology of Helicobacter pylori infection. Best. Pract. Res. Clin. Gastroenterol. 2007, 21, 205–214. [Google Scholar] [CrossRef]
- National Institutes of Health, Office of Dietary Supplements. Probiotics—Health Professional Fact Sheet. Updated 25 March 2025. Available online: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/ (accessed on 20 October 2024).
- Chin-Lee, B.; Curry, W.J.; Fetterman, J.; Graybill, M.A.; Karpa, K. Patient experience and use of probiotics in community-based health care settings. Patient Prefer. Adherence 2014, 8, 1513–1520. [Google Scholar] [CrossRef]
- Wang, Y.; Florez, I.D.; Morgan, R.L.; Foroutan, F.; Chang, Y.; Crandon, H.N.; Zeraatkar, D.; Bala, M.M.; Mao, R.Q.; Tao, B.; et al. Probiotics, Prebiotics, Lactoferrin, and Combination Products for Prevention of Mortality and Morbidity in Preterm Infants: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023, 177, 1158–1167. [Google Scholar] [CrossRef]
- Fijan, S.; Kolč, N.; Hrašovec, M.; Jamtvedt, G.; Pogačar, M.Š.; Turk, D.M.; Maver, U. Single-Strain Probiotic Lactobacilli for the Treatment of Atopic Dermatitis in Children: A Systematic Review and Meta-Analysis. Pharmaceutics 2023, 15, 1256. [Google Scholar] [CrossRef]
- Xue, X.; Yang, X.; Shi, X.; Deng, Z. Efficacy of probiotics in pediatric atopic dermatitis: A systematic review and meta-analysis. Clin. Transl. Allergy 2023, 13, e12283. [Google Scholar] [CrossRef] [PubMed]
- Louis, R.; Pilette, C.; Michel, O.; Michils, A.; Brusselle, G.; Poskin, A.; Van Schoor, J.; Denhaerynck, K.; Vancayzeele, S.; Abraham, I.; et al. Variability in total serum IgE over 1 year in severe asthmatics. Allergy Asthma Clin. Immunol. 2019, 15, 20. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Study # | Probiotic Used | Sample Size (n) | Measured SCORAD (Yes/No) | SCORAD Mean (SD) Pre; Post Intervention | End of Study Period | Follow-Ups (Weeks) |
|---|---|---|---|---|---|---|---|
| Gerasimov 2010 [14] | 1 | L. acidophilus DDS-1, B. lactis UABLA-12 with fructo-oligosaccharide | 96 | YES | 42.1 (12.6); 27.9 (10.5) | Week 8 | Week 2, 4, 8 |
| Wang 2015 [15] | 2, 3, 4 | LP—Lactobacillus paracasei GMNL-133 (LP), 2 × 109 colony-forming units (cfu) qd.; LF—Lactobacillus fermentum GM090 (LF), 2 × 109 colony forming units (cfu) qd. | 104 | YES | LP: 50.93 (19.42); 25.48 (21.73) LF: 52.25 (16.85); 27.23 (18.1) LP + LF: 51.9 (18.9); 25.76 (19.23) | Week 12 | Week 16 |
| Navarro-López 2018 [16] | 5 | Lactobacillus johnsonii EM1 (Lj EM1) | 50 | YES | 33.8; 6.8 | Week 12 | Week 4 |
| Nermes 2011 [17] | 6 | Lactobacillus rhamnosus GG (LGG) | 39 | YES | 27.9; 17.6 | Week 12 | None reported |
| Grüber 2007 [18] | 7 | Lactobacillus rhamnosus GG (LGG) | 102 | YES | 34.5 (4.76); 25.35(15.13) | Week 12 | Week 4, 8, 12 |
| D’Auria 2021 [19] | 8 | Lactobacillus paracasei CBA L74 | 58 | YES | 42.5; 18.7 | Week 12 | Week 4, 8, 12 |
| Cukrowska 2021 [20] | 9 | 50% of Lactobacillus casei ŁOCK 0919, 25% of Lactobacillus rhamnosus ŁOCK 0908, 25% of Lactobacillus rhamnosus ŁOCK 0900 (Latopic®, Biomed S.A., Cracow, Poland). | 134 | YES | 40.4 (20); 17.6 (14.8) | Week 12 | Week 36 |
| Jeong 2020 [21] | 10 | Lactobacillus rhamnosus (IDCC 3201, isolated from the feces of a Korean breast-fed infant, repeated heat-treated and incubated, RHT3201 | 66 | YES | 38.15; 24.25 | Week 12 | Week 6 |
| Han 2012 [22] | 11 | Lactobacillus plantarum CJLP133 | 118 | YES | 30.6 (7.7); | Week 16 | Week 14 |
| 20.4 (12.6) | |||||||
| Ahn 2020 [23] | 12 | Lactobacillus pentosus | 82 | YES | 30.4(8.6); | Week 12 | None reported |
| 23.6 (11) | |||||||
| Woo 2010 [24] | 13 | Lactobacillus sakei KCTC 10755BP supplementation | 75 | YES | 42.6; 28.8 | Week 12 | Week 6 |
| Carucci 2022 [25] | 14 | Lacticaseibacillus rhamnosus GG | 100 | YES | Group A | Week 16 | Week 16 |
| 29.8 (12.1);25.0 (2.5) | |||||||
| Group B | |||||||
| 30.8 (12.8);18.0 (2.5) | |||||||
| * Weston 2005 [12] | 15 | Lactobacillus fermentum VRI-033 PCC | 56 | YES | 40.8; 23.8 | Week 8 | Week 2, 4, 8, 16 |
| ** Isolauri 2000 [13] | 16 | Bifdobacterium lactis Bb-12 or Lactobacillus strain GG (ATCC 53103) | 18 | YES | Not reported. | Week 8 | None reported |
| Huang 2018 [26] | 17 | Lactobacillus paracasei (LP), Lactobacillus fermentum (LF), both (LP + LF) | 147 | NO | Not reported. | Week 12 | Week 4, 8, 12, 16 |
| de Araujo 2017 [27] | 18 | Lb. helveticus, Lb. kefiranofaciens, Lb. para-casei, Lb. kefiri, Lb. delbruecki, Lb. acidophilus. Lb. crispatus, Lb. gallinarum, Lb. fornicalis, Bifidobacterium breve, Lactococcus lactis, Enterococcus faecium, Kluyveromyces marxianus, Kazachstania unispora, Pichia fermentans and Sach. cerevisiae. | 60 | NO | Not reported. | Week 16 | Week 8 |
| Basturk 2020 [28] | 19 | Lactobacillus rhamnosus GG (LGG) | 106 | NO | Not reported. | Week 4 | None reported |
| Chen 2010 [29] | 20 | Lactobacillus gasseri A5 | 105 | NO | Not reported. | Week 8 | None reported |
| Torii 2011 [30] | 21 | Lactobacillus acidophilus | 50 | NO | Not reported. | Week 8 | Week 4 |
| Jerzyńska 2016 [31] | 22 | Lactobacillus rhamnosus GG (LGG) | 50 | NO | Not reported. | Week 20 | None reported |
| Hol 2008 [32] | 23 | Lactobacillus casei CRL431 and Bifidobacterium lactis Bb-12 | 119 | NO | Not reported. | Week 24 | Week 48 |
| Miraglia Del Giudice 2017 [33] | 24 | Bifidobacterium mixture (B. longum BB536, B. infantis M-63, B. breve M-16V) | 40 | NO | Not reported. | Week 8 | None reported |
| Wang 2004 [34] | 25 | Lactobacillus paracasei-33 (LP-33) | 80 | NO | Not reported. | Week 4 | None reported |
| Brouwer 2006 [11] | 26 | Lactobacillus rhamnosis (NP-Lrh) or Lactobacillus GG (NP-LGG) | 33 | NO | Not reported. | Week 12 | Week 4, 8, 12 |
| Anania 2021 [35] | 27 | mixture of Bifidobacterium animalis subspecies of Lactis BB12 and Enterococcus faecium L. | 203 | NO | Not reported. | Week 12 | None reported |
| Egger’s Regression-Based Test a | ||||||
|---|---|---|---|---|---|---|
| Parameter | Coefficient | Std. Error | t | Sig. (2-Tailed) | 95% Confidence Interval | |
| Lower | Upper | |||||
| (Intercept) | 1.378 | 0.8404 | 1.640 | 0.127 | −0.453 | 3.209 |
| SE b | −8.651 | 3.5014 | −2.471 | 0.029 | −16.280 | −1.022 |
| Leave-One-Out Sensitivity Analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excluding Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Hedge’s g | −0.67 | −0.65 | −0.65 | −0.65 | −0.56 | −0.70 | −0.73 | −0.68 | −0.71 | −0.65 | −0.69 | −0.71 | −0.66 | −0.49 |
| p-value | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaikwad, R.; Kondle, S.; Chang, S.; Barnes, C.; Kubba, R.; Lane, C.; Uppu, S.; Frezza, E. Impact of Probiotics on Atopic Dermatitis in Pediatric Patients: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 2090. https://doi.org/10.3390/medicina61122090
Gaikwad R, Kondle S, Chang S, Barnes C, Kubba R, Lane C, Uppu S, Frezza E. Impact of Probiotics on Atopic Dermatitis in Pediatric Patients: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(12):2090. https://doi.org/10.3390/medicina61122090
Chicago/Turabian StyleGaikwad, Ritu, Soham Kondle, Sean Chang, Chris Barnes, Rohan Kubba, Christopher Lane, Snigdha Uppu, and Eldo Frezza. 2025. "Impact of Probiotics on Atopic Dermatitis in Pediatric Patients: A Systematic Review and Meta-Analysis" Medicina 61, no. 12: 2090. https://doi.org/10.3390/medicina61122090
APA StyleGaikwad, R., Kondle, S., Chang, S., Barnes, C., Kubba, R., Lane, C., Uppu, S., & Frezza, E. (2025). Impact of Probiotics on Atopic Dermatitis in Pediatric Patients: A Systematic Review and Meta-Analysis. Medicina, 61(12), 2090. https://doi.org/10.3390/medicina61122090






